Abstract
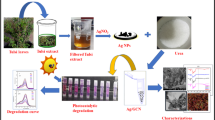
A simple and easy green synthesis of silver nanoparticles (Ag NPs) was prepared using Sapindus emarginatus stem bark extract and anchored over graphitic carbon nitride (g-C3N4) surface. The synthesized materials characterized by UV–Visible, XRD, FESEM, FETEM, FT-IR and Raman spectroscopy ensured the development of Ag NPs anchored over the g-C3N4 surface. The prepared catalyst was utilized for Methylene Blue (MB) dye degradation through visible light photocatalysis. The optimization of operational parameters, like the effect of catalyst dosage, H2O2 concentration and slurry pH, was performed. The results exhibited that compared to pristine g-C3N4, photocatalytic efficacy was enhanced two-fold in Ag@g-C3N4. Also, the prepared Ag@g-C3N4 showed a faster catalytic MB dye reduction (25 s) in the presence of NaBH4. The catalyst was recovered from the reaction solution by centrifugation and reused for five consecutive cycles. The results showed that there was no significant loss in the efficiency of the catalyst. These results confirmed that the green synthesized Ag@g-C3N4 is likely advantageous for organic pollutants degradation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Presence of toxic organic compound in water is a severe threat to global human health. Photocatalytic mediated degradation of those harmful recalcitrant organic compound is becoming a promising treatment method [1]. Photocatalyst such as TiO2, ZnO, CdS, WO and C3N4 have been employed for organic compounds degradation previously. Among them, graphitic carbon nitride (g-C3N4) mediated photocatalysis is gaining significant consideration because it has narrow bandgap, low cost, high stability and non-toxicity. However, due to its higher electron (e−) and hole (h+) recombination rate and lower utilization of visible light, limits it practical photocatalytic applications [2]. These limitations can be overcome by anchoring with noble metal nanoparticles such as Au, Pt, Pd and Ag, which traps the photogenerated electron and also enhances the visible light utilization [3]. Among these noble metals, silver is gaining much attention due to facile synthesis, tuning its size, shape and morphology, cost-effective when compared to other noble metals and activate the catalyst under visible region by its Localized Surface Plasmon Resonance (LSPR) property [4,5,6]. Synthesis of silver nanoparticles and anchoring over various semiconductor by chemical reduction, photo deposition, Wet impregnation, evaporation–condensation, laser ablation and biogenic method were demonstrated previously [7,8,9,10,11].
Biogenic mediated preparation of Ag NPs with the help of plant extracts has gained ample consideration than other methods because the phytochemicals act as reducing and stabilizing agents, easy availability and preparation, low cost and low toxic [12,13,14,15]. Recently, Kumar et al. [16] synthesized Ag@TiO2 by using Rambutan peel extract and utilized it for enhanced photocatalytic degradation of MB dye. Likewise, Tahir et al. 2016 [17] developed Ag/TiO2 using Cestrum nocturnum leaf extract and used it for enhanced photocatalytic MB Dye. Sohrabnezhad and Seifi [18] utilized Urtica dioica leaf extract for the synthesis of Ag/ZnO to improve its photocatalytic activity towards MB dye degradation under visible light. Similarly, Sorbiun et al. 2018 [19] green synthesized Ag/ZnO using oak fruit hull to increase its photocatalytic activity towards basic violet 3 dye degradation. The green synthesis of Ag@g-C3N4 using plant extract and its application on photocatalytic degradation was limited [20, 21]. Hence the present study was aimed to utilize the phytochemicals present in the Sapindus emarginatus stem bark for the preparation of Ag NPs, and it was anchored over the g-C3N4 surface. The prepared particles were utilized for enhanced photocatalytic and catalytic degradation of MB dye.
Materials and Methods
Materials
Analytical grade (AR) Melamine, silver nitrate, sodium hydroxide, sulphuric acid, methylene blue, sodium borohydride, and hydrogen peroxide were purchased from Merck India Ltd and utilized. Double distilled water was used for reagent preparation and experiment purposes.
Preparation of Bark Extract
Sapindus emarginatus stem bark was collected from IIT Guwahati campus and shade dried for 1 week. The bark was grounded into a fine powder using a mechanical grinder. For the preparation of aqueous bark extract, stem bark powder of about 10 g was added with 100 ml of double-distilled water and refluxed at 65 °C for half an hour. The sample was cooled, filtered and preserved at 4 °C for further purpose.
Preparation of Pristine g-C3N4
By simple polymerization of melamine, g-C3N4 was prepared by heating in a muffle furnace for 2 h at 550 °C with a ramp increment of 5 °C min−1 under Nitrogen condition. The obtained yellow powder was ground well and exfoliated by the ultra-sonication process [22].
Green Synthesis of Ag@g-C3N4
Sapindus emarginatus bark extract was utilized for the formation of Ag NPs anchored over g-C3N4. In a typical procedure, 500 mg of g-C3N4 was added with 50 ml of AgNO3 solution (100 ppm Ag) and sonicated for 30 min for the dispersion of particles. The above solution was mixed with 10 ml of bark extract as a reducing agent, and the solution was stirred at 500 rpm for 2 h for the formation of Ag NPs. The resultant solution was dried at 120 °C in a hot air oven for 48 h and sintered at 450 °C for 2 h. The powder was cooled and grounded well and stored for further usage.
Characterization Studies
The preliminary confirmation of silver NPs formation was measured by using UV–Visible spectrophotometer (Shimadzu UV 1900). The diffused reflectance spectra of pristine and Ag anchored g-C3N4 was measured using UV- Vis. NIR. Spectrophotometer (Varian, 5000). The crystalline structure and phase of the prepared particles were measured using powder X-ray diffractometer (Rigaku, SmartLab). The surface morphology, size and shape of NPs were measured using FESEM (Zeiss, Sigma 300), and high-resolution TEM image was obtained using FETEM (JEOL, 2100F). The elemental mapping of Ag@g-C3N4 was analyzed using EDX (Thermofisher) attached in the FESEM. The functional group present in the plant extract and prepared particles were analyzed using FTIR (PerkinElmer, Spectrum Two). The Raman scattering of prepared samples was measured using Raman Spectrophotometer (Horiba, LABRAMAN). The photoluminescence spectra were obtained using a spectrofluorometer (JY Fluorolog—FL3-11) by exciting the sample at 360 nm.
Photocatalytic Activity
The prepared particles were tested for Methylene blue (MB) dye degradation. For photocatalytic studies, 100 ml of MB solution (10 ppm) was taken in 250 ml beaker and mixed with 50 mg of prepared particles and sonicated for 30 min for dispersion of particles. The samples were then exposed under 350 W Xenon lamp with 420 nm cutoff filter with constant stirring. The samples were withdrawn at constant intervals, and the catalyst was removed by centrifugation. The resultant supernatant was examined by spectrometry to estimate the degradation percentage of MB dye.
Catalytic Dehydrogenation
The catalytic efficacy of the synthesized particles was assessed by MB dye dehydrogenation reaction with the existence of NaBH4. The catalytic reaction was performed in a 3 cm cuvette by mixing 2 ml of MB dye (50 ppm) along with 1 ml of NaBH4 solution (0.1 M). To initiate the catalytic reaction, 2.0 mg of the Ag@ g-C3N4 was added and mixed well. The absorbance was recorded with UV–Vis. Spectrophotometer, after removing the catalyst from the solution by centrifugation.
Reuse and Recyclability Test
The reuse and recyclability test was performed up to five successive cycles by recovering the catalyst from the solution by centrifugation, washed and dried at 90 °C in a hot air oven.
Results
Characterization Results
The development of Ag NPs with the help of bark extract of Sapindus emarginatus was examined preliminary by using UV–Vis. Spectroscopy. Figure 1a ensures the development of Ag NPs by exhibiting a strong absorption peak at 450 nm ascribed to surface Plasmon resonance (SPR) properties of Ag NPs [23, 24]. The optical properties of pristine g-C3N4 and Ag@ g-C3N4 was measured by diffused reflectance spectra (Fig. 1b). Pristine g-C3N4 showed absorption edge at 460 nm ascribed to 2.7 eV of optical bandgap energy.
In contrast, the Ag@g-C3N4 exhibited extended wavelength with redshift in the visible region due to the SPR effect of nano-sized Ag anchored over the surface of g-C3N4. The UV- Vis. Spectra confirm that Ag@g-C3N4 can utilize visible light better than pristine [25].
Figure 2 illustrated the crystalline nature of pure g-C3N4 and green synthesized Ag@g-C3N4 were analyzed using PXRD. From the result, it was clear that both the samples exhibited hexagonal phase of g-C3N4 with a significant peak at 27° allotted to (002) plane of aromatic interlayer stacking segments and another minor peak at 13° consigned to crystal (100) plane of tris-s-triazine. Compared to pristine, Ag@g-C3N4 (Fig. 2 inset) showed a shift from 27.53° to 27.72°, which denoted a decrease in the gallery distance between pristine g-C3N4, and Ag@g-C3N4. There were no diffraction peaks of Ag observed due to lower loading percentage (1wt %) and well dispersion of Ag NPs in the Ag@g-C3N4 [26].
The structural, shape and morphological characteristic of pristine and Ag@g-C3N4 were obtained using FESEM and FETEM analysis. The morphology of pristine g-C3N4 and Ag@g-C3N4 was measured by FESEM (Fig. S1) reveals that both the samples are irregular in shape with strong agglomeration with other particles. There were not many morphological modifications observed between the pristine and Ag@g-C3N4 particles. Whereas, the FETEM image of Ag@g-C3N4 (Fig. 3) displayed the existence of spherical Ag NPs with an average size of 16 nm, which was uniformly anchored over the g-C3N4 surface. The TEM image (Fig. S2) of pristine g-C3N4 confirmed that the sheet-like structure was formed. The elemental mapping of Ag@g-C3N4 was illustrated in Fig. 4 ensures the existence of silver beside with nitrogen, carbon and a small percentage of oxygen. The TEM image and EDX mapping clearly display that Ag NPs was distributed uniformly on the g-C3N4 surface.
The FTIR spectra of bark extract, pristine g-C3N4 and Ag@g-C3N4 are illustrated in Fig S3. The FTIR spectra of bark extract exhibit peak at 3330 cm−1 and 2903 cm−1 corresponds to O–H bond stretching of the phenolic group and C-H stretching of alkene bond respectively. The intense peak at 1606 cm−1 and 1033 cm−1 were ascribed to N=H and C–O of secondary amide and ether groups, respectively [27, 28]. The FTIR peak of both pristine and Ag anchored g-C3N4 with similar peaks confirmed that anchoring of Ag NPs did not alter the g-C3N4 original structure. The FTIR spectrum of gC3N4 showed a broad peak at 3000 cm−1 to 3300 cm−1 related to stretching vibration of the N–H group. In contrast, many peaks situated in the range of 1200 cm−1 to 1650 cm−1 corresponded to CN heterocycles, while the sharp peak at 808 cm−1 ascribed to s-triazine breathing mode [29]. From the FTIR spectrum, it was clear that phytochemicals such as polyphenols, flavonoids and other organic compounds play a pivotal role in the synthesis of Ag NPs, which acts as reducing, stabilizing and capping agents.
Raman spectra of g-C3N4 and Ag@g-C3N4 (Fig. S4) illustrated that pristine g-C3N4 did not display a significant Raman signal, whereas the Ag NPs anchored g-C3N4 showed a surface-enhanced Raman scattering spectrum. The strong Raman spectral band seen at 1570 cm−1 matches with vibrational stretching of C-N bond and peak at 550 cm−1 relates to heptazine heterocyclic vibrational stretch. The peak at 1240 cm−1 denoted the CN heterocyclic vibrational mode. The intensity of Raman spectra was significantly enhanced in Ag@g-C3N4 due to the transferring of charge between Ag and g-C3N4 molecule [26]. This result ensures that there is a strong interface existed with g-C3N4 and Ag NPs, which facilitate the charge transfer between them.
The photoluminescence (PL) spectra of pristine and Ag@g-C3N4 was measured with an excitation at 360 nm to study the transfer, migration and photogenerated e− and h+ recombination rate. From Fig. 5, it was clear that the PL intensity of Ag@g-C3N4 was quenched notably than pristine g-C3N4. The results suggested that Ag NPs acted as an electron trap thereby it decreased the e− and h+ recombination process leads to increased the formation of more radicles leads to boosted photocatalytic efficiency of Ag@g-C3N4 [30].
Photocatalytic Degradation of MB Dye
The photocatalytic efficacy of the prepared Ag@g-C3N4 was assessed towards MB dye degradation. The experiments were executed with pristine, Ag@g-C3N4, and by photolysis was shown in Fig. 6. The results reveal that pristine g-C3N4 has less efficacy (42%) to degrade MB dye, due to fast electron–hole recombination rate. Whereas, Ag@g-C3N4 showed a nearly two-fold increase in photocatalytic MB dye (99%) degradation exposed under visible light for 1 h. The adsorption capacity of the catalyst was performed under the dark condition. The results showed that nearly 14% of dye was adsorbed over the catalyst surface after 2 h contact between dye and the catalyst. The increased photocatalytic activity of Ag@g-C3N4 was due to electron trapping ability of Ag NPs, in turn, reduces electron and hole rejoining rate, which increases the formation of ROS [31]. Only negligible degradation of MB dye observed when the dye was exposed under direct light without any catalyst. Ag@g-C3N4 has been selected to optimize the reaction parameters since it showed higher degradation towards MB dye.
Effect of Catalyst Concentration
To optimize the catalyst dosage on MB dye degradation, the experiment was performed with different catalyst concentration from 100 ppm to 1000 ppm. Figure 7 depicts the influence of the different catalyst dosages on the percentage degradation of MB dye after 60 min exposed under 350 W Xenon lamp. The results reveal that a gradual increment in the efficiency of the MB dye degradation was observed from 100 ppm to 500 ppm, and additional increment in the catalyst dosage led to a reduction in the degradation rate. The initial increment in dye degradation because of increment in the active surface with increasing catalyst dosage. Whereas, a decrease in the degradation rate with further increment in catalyst concentration was observed due to the screening effect of suspended particles which hampers the light penetration [32].
Effect of Initial Slurry pH
To study the effect of initial slurry pH on dye degradation, the testing was performed with different pH from 2 to 10 (Fig. 8). The results illustrated that there was a gradual increment in the MB dye degradation seen when the pH of the slurry was adjusted from acidic to alkaline. Under the alkaline condition, the catalyst surface becomes more positively charged, which attracts more dye molecules towards it and thereby increasing the rate of degradation under alkaline compared to the acidic condition [33].
Effect of H2O2 Concentration
The effect of the oxidizing agent on MB dye degradation was conducted with various concentration of H2O2 ranging from 0 mM to 5 mM. Figure 9 infers that the amount of dye degradation was improved with increasing the peroxide dosage because H2O2 was acting as electron scavenger thereby sinking the e− and h+ recombination rate which increases the formation of more radicals in the reaction system. Also, H2O2 may dissociate photocatalytic into οOH radicals, thus enhancing the photocatalytic efficiency [34].
Possible Mechanism Of Photocatalytic Degradation of MB Dye
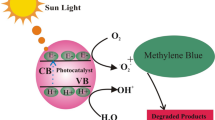
The possible mechanism behind the photocatalytic MB dye degradation using Ag@g-C3N4 was illustrated in Fig. 10. When the sample was irradiated with visible light, it activates the charge separation process, which leads to the development of condition band e− and h+ in the valance band. The Ag NPs anchored over the g-C3N4 traps the conduction band electron decrease the e− and h+ recombination rate resulted in enhanced photocatalytic degradation which was observed with Ag@ g-C3N4 comparted to pristine. The electron trapped by the Ag NPs reduces the molecular O2 into O2−superoxide radical anion, and h+ can react directly with dye solution or split the water into °OH radicals. These photogenerated radicals and h+ plays a vital role in the degradation of MB dye [35].
Catalytic Reduction of MB Dye
The catalytic ability of Ag@g-C3N4 was evaluated for the reduction of MB dye with the existence of NaBH4. Figure 11 shows the fast reduction of MB to Leuco methylene blue (LMB), which was observed within 25 s confirmed by decreased in the intensity of adsorption peak at 664 nm. No reduction in the adsorption peak of MB dye was observed when the reaction is conducted with pristine g-C3N4 [36, 37].
Recovery and Reuse of the Catalyst
The catalyst was recovered by centrifuging the sample at 6000 rpm, and the recovered catalyst was reused up to 5 successive cycles to check its photocatalytic and catalytic efficiencies (Fig. 12). The results exhibited that the Ag@ g-C3N4 showed improved photocatalytic degradation (~ 84%) and catalytic reduction of MB (~ 94%) after five cycles which confirmed that the prepared particles were effective and stable for the reduction and degradation of MB dye. The stability of the Ag@ g-C3N4 catalyst after the 5th cycle was verified using TEM (Fig. S5) and XRD (Fig. S6) analysis. The results revealed that there is no morphological, and crystalline changes observed even after five cycles, which ensured that the catalyst is more stable and can be reused several times.
Conclusion
A facile and simple biogenic mediated Ag NPs was prepared and anchored over g-C3N4 using the aqueous stem bark extract of Sapindus emarginatus. The phytochemicals compound in the bark extracts act as reducing and stabilizing agent for Ag NPs synthesis. The characterization results ensured the anchoring of Ag NPs over the g-C3N4 surface. The synthesized catalyst was utilized for the visible light active catalyst for MB dye degradation. Compared to pristine, Ag@ g-C3N4 showed better photocatalytic activity under visible region and good catalytic reduction of MB dye. Addition of oxidizing agent (H2O2) and alkaline slurry pH enhance the rate of photocatalytic degradation. The prepared Ag@g-C3N4 catalyst also showed excellent stability and recyclability even after 5 successive cycles. This study provided an easy, and greener method for the preparation of Ag anchored g-C3N4 for efficient organic pollutants degradation.
References
N. Cheng, J. Tian, Q. Liu, C. Ge, A. H. Qusti, A. M. Asiri, A. O. Al-Youbi, and X. Sun (2013). ACS Appl. Mater. Inter. 5, 6815.
X. Han, L. An, Y. Li, C. Hou, H. Wang, and Q. Zhang (2020). Appl. Cat. B: Environ. 265, 118539.
A. Mohammad, M. R. Karim, M. E. Khan, M. M. Khan, and M. H. Cho (2019). J. Phys. Chem. C 123, 20936.
A. Hatamifard, M. Nasrollahzadeh, and S. M. Sajadi (2016). New J. Chem. 40, 2501.
S. Saran, P. Arunkumar, G. Manjari, and S. P. Devipriya (2019). Environ. Technol. 40, 3190.
M. Maryami, M. Nasrollahzadeh, E. Mehdipour, and S. M. Sajadi (2016). Int. J. Hydrog. Energy 41, 21236.
S. Saran, P. G. Kamalraj, G. Arunkumar, and S. P. Devipriya (2016). Environ. Sci. Pollut. Res. 23, 17730.
L. Tang, C. Feng, Y. Deng, G. Zeng, J. Wang, Y. Liu, H. Feng, and J. Wang (2018). Appl. Cat. B: Environ. 230, 102.
C. H. Liu, M. H. Hong, Y. Zhou, G. X. Chen, M. M. Saw, and A. T. S. Hor (2007). Physica Scripta T129, 326.
L. G. Devi and R. Kavitha (2016). Appl. Surf. Sci. 360, 601.
G. Manjari, S. Saran, S. P. Devipriya, and A. V. B. Rao (2018). Catal. Lett. 148, 2561.
G. Manjari, S. Saran, T. Arun, S. P. Devipriya, and A. V. B. Rao (2017). J. Clust. Sci. 28, 2041.
M. Nasrollahzadeh, S. Mahmoudi-Gom Yek, N. Motahharifar, and M. Ghafori Gorab (2019). Chem. Rec. 19, 2436.
M. Sajjadi, M. Nasrollahzadeh, and S. M. Sajadi (2017). J. Colloid Interface Sci. 497, 1.
B. Khodadadi, M. Bordbar, and M. Nasrollahzadeh (2017). J. Colloid Interface Sci. 493, 85.
B. Kumar, K. Smita, Y. Angulo, and L. Cumbal (2016). Green Process. Synth. 5, 371.
K. Tahir, A. Ahmad, B. Li, S. Nazir, A. U. Khan, T. Nasir, Z. U. H. Khan, R. Naz, and M. Raza (2016). J. Photochem. Photobiol. b. Biol. 162, 189.
S. Sohrabnezhad and A. Seifi (2016). Appl. Surf. Sci. 386, 33.
M. Sorbiun, E. S. Mehr, A. Ramazani, and S. T. Fardood (2018). Mater. Electron. 29, 2806.
K. Tian, W. J. Liu, and H. Jiang (2015). ACS Sustain. Chem. Eng. 3, 269.
P. C. Nagajyothi, M. Pandurangan, S. V. P. Vattikuti, C. O. Tettey, T. V. M. Sreekanth, and J. Shim (2017). Sep. Purif. Technol. 188, 228.
S. Tonda, S. Kumar, M. Bhardwaj, P. Yadav, and S. Ogale (2018). ACS Appl. Mater. Inter. 10, 2667.
Y. K. Mohanta, S. K. Panda, R. Jayabalan, N. Sharma, A. K. Bastia, and T. K. Mohanta (2017). Front. Mol. Biosci. 4, 14.
G. Manjari, A. Parthiban, and S. Saran (2019). J. Clust. Sci.. https://doi.org/10.1007/s10876-019-01721-6.
K. Qi, Y. Li, Y. Xie, S. Y. Liu, K. Zheng, Z. Chen, and R. Wang (2019). Front. Chem. 7, 91.
H. Li, Y. Jing, X. Ma, T. Liu, L. Yang, B. Liu, S. Yin, Y. Wei, and Y. Wang (2017). RSC Adv. 7, 8688.
N. Mir, M. Bahrami, E. Safari, and S. M. Hosseinpour-Mashkani (2015). J. Clust. Sci. 26, 1113.
B. Khodadadi, M. Bordbar, and M. Nasrollahzadeh (2017). J. Colloid Interface Sci. 490, 1.
M. Induja, K. Sivaprakash, and S. Karthikeyan (2019). Mater. Res. Bull. 112, 331.
Y. Sun, T. Xiong, Z. Ni, J. Liu, F. Dong, W. Zhang, and W. K. Ho (2015). Appl. Surf. Sci. 358, 356.
L. Ge, C. Han, J. Liu, and Y. Li (2011). Appl. Cat. A: Gen. 409, 215.
M. S. Dorraji, A. R. Amani-Ghadim, M. H. Rasoulifard, H. Daneshvar, B. S. Z. Aghdam, A. R. Tarighati, and S. F. Hosseini (2017). Chem. Eng. Res. Des. 127, 113.
P. Murugesan, N. Girichandran, S. Narayanan, and M. Manickam (2018). Opt. Mater. 75, 431.
A. Kumar, A. Kumar, G. Sharma, H. Ala’a, M. Naushad, A. A. Ghfar, C. Guo, and F. J. Stadler (2018). Chem. Eng. J. 339, 393.
M. E. Khan, T. H. Han, M. M. Khan, M. R. Karim, and M. H. Cho (2018). ACS Appl. Nano Mater. 1, 2912.
Y. Fu, T. Huang, L. Zhang, J. Zhu, and X. Wang (2015). Nanoscale 7, 13723.
M. Nasrollahzadeh, M. Bagherzadeh, and H. Karimi (2016). J. Colloid Interface Sci. 465, 271.
Acknowledgement
The authors acknowledge Central Instrumental Facility (CIF), Department of Chemical Engineering and Center for the Environment, Indian Institute of Technology Guwahati for characterization studies. The first author is thankful to IIT Guwahati for providing Institutional Post-Doctoral fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sarangapany, S., Mohanty, K. Facile Green Synthesis of Ag@g-C3N4 for Enhanced Photocatalytic and Catalytic Degradation of Organic Pollutant. J Clust Sci 32, 585–592 (2021). https://doi.org/10.1007/s10876-020-01816-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-020-01816-5