Abstract
In this study, the electric spark discharge method was used to prepare tungsten (W) and tungsten-trioxide (WO3) nanocomposite colloid. A high-energy arc was used to melt a W rod, and the W ions were then ionized with a medium arc to prepare W and WO3 nanocomposite colloid at a high temperature. This preparation method was simple, fast, and effective. The particle size and zeta potential of the samples were measured with a Zetasizer and the shape, size, distribution, and crystal lattice of the W (0.225 nm) and WO3 (0.355 nm) nanoparticles were observed and compared using a Transmission Electron Microscope. The optical properties of the nanocomposite colloids were measured with a UV–visible spectrophotometer (UV–Vis). X-ray diffraction and a Raman Spectrometer were used to detect the crystal properties of the W and WO3 nanoparticles. The results showed that the components of the nanocomposite colloid were W and WO3. The average size of the nanoparticles was 79.65 nm and the zeta potential was − 52.5 mV; thus, the W and WO3 nanoparticles had the suspension effect. There was a peak at 320 nm according to UV–Vis, which is the characteristic absorbance of W and WO3.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The thermophysical properties of W are excellent and can be operated at high temperatures. With its excellent heat resistance and high melting point, tungsten is generally used in high-temperature processes. This is due to the low coefficient of thermal expansion and high conductivity [1]. Even under extremely high temperatures, components made of W can continue to work for a very long time.
In recent years, environmental pollution has continued to worsen; thus, technologies that are non-toxic and have high energy-efficiency have drawn the attention of scientists. For example, in the application of photocatalytic technology, WO3 can degrade organic pollutants. Due to its narrow band gap, excellent electron transport, and photoelectrochemical properties, WO3 can be excited by visible light and catalyze degradations. In addition, it can efficiently and selectively catalyze chemical reactions that do not occur easily under normal conditions [2]. As photocatalytic materials [3], not only can W and WO3 degrade organic pollutants [4, 5], they can also break down gases such as hydrogen sulfide, ozone, and nitrogen oxide into harmless substances such as water and carbon dioxide, for the purpose of purifying the air.
W and WO3 exhibit excellent gas sensing properties for both oxidizing and reducing gases, thereby enabling the rapid development of W and WO3 oxide gas sensors for the detection of gases such as hydrogen sulfide, nitric oxide, nitric dioxide, and ammonia [6,7,8,9]. Electrochromism is a phenomenon in which materials change color due to a chemical reaction under an applied electric field. W and WO3 nanomaterials have good electrochromic properties [10], making them the most common electrochromic semiconductor materials. Amorphous W and WO3 films were the first electrochromic materials to be discovered and have been widely studied. With the advantages of a long cycle life, an obvious discoloration response, a long memory time, and high stability, they have been widely used in smart windows and display devices in the automobile and aerospace industries [11].
In terms of previous studies using ESDM to prepare nanocolloids, Stability Analysis of Platinum Nanoparticles [12], TiO2 Nanocolloids [13], and gold ethanol colloid [14], the research directions include nanocolloid, parameter control, and suspension stability analysis. In this study, EDM was used to generate a DC arc in dielectric fluid medium, which produced temperatures of 5000 ~ 6000 K between the two poles [15] that were used to melt and cut W rods and condense them into W and WO3 nano-colloid. After preparation, the size distribution [16] and zeta potential [17] were measured by a Zetasizer (NanoZS90, Malvern). The actual shape and distribution were observed using TEM (JEM- 2100F) [18]. The optical properties of the W and WO3 nanocolloid were measured by UV–Vis (9423UV–VISA1002E, Helios Alpha) [19]. Then, XRD (PANalytical, Empyrean) [20] and a Raman Spectrometer (Ramboss 500i) [21] were used to confirm the properties of the WO3 crystal. The results showed that the proposed method could prepare nano-scale W and WO3 and could be suspended in deionized water. According to later studies [22, 23], the Nano-Ag colloid prepared by ESDM maintains good suspensibility and the characteristics of the silver ions in the Nano-Ag colloid are used for antibacterial purposes. ESDM only uses silver metal and deionized water for preparing Nano-Ag colloids without chemical agents and dispersants. The preparation process is rapid, simple, and safe; the nanocolloid can be fabricated at normal temperature and pressure, and it will not harm the human body or pollute the environment.
Experiment
Materials and Equipment
The WO3 nanoparticles were prepared using EDM and the experimental steps were as follows:
-
1.
The tungsten consisted of 99.97% W rods with diameters of 1 mm and 2 mm, respectively.
-
2.
Deionized water with a conductivity of 1.94 μs/cm was used as the dielectric fluid.
-
3.
A magnet was rotated in the beaker using a digital electromagnetic stirrer (Thermo, CIMAREC Digital, SP131325) to disperse the nanoparticles evenly.
-
4.
The W rod electrodes were discharged in deionized water through EDM (TOPEDM, MANTIS) [24].
Experimental Steps
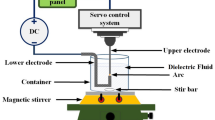
A beaker containing 200 mL of deionized water was placed on a digital electromagnetic stirrer and a magnet was placed in the beaker. A 1 mm diameter W rod was used as an anode, and a 2 mm W rod was used as a cathode with a fixing tool. The cathode was immersed in deionized water and grounded via a fixing tool. Finally, the beaker was placed in the EDM table adjustment and a servo motor controlled the distance between the anode and cathode at 30 μm [25]. According to the parameters shown in Table 1, the EDM was started. During the preparation, the electric arc ionized the dielectric liquid [26] and melted the electrodes with a temperature of 5000–6000 K. After a 20-min discharge, the preparation of W and WO3 nanocomposite colloid was completed. The nanocolloid sample is shown in Fig. 1, and the preparation process is shown in Fig. 2. The ESDM process was as follows: (a) waiting for discharge: adjust the distance between two electrodes to above 30 μm and align them for the preparation of discharge; (b) discharge phenomenon: when a small bump electric field occurs between two electrodes, when the strength is greater than the dielectric strength the electric field force will spurt out electrons from the electrode; (c) ionization: when the dielectric insulation is completely destroyed, the electrons will bump into the neutral atom of the electrode, causing the detachment of the outlying electrons. Lack of electronics, electrode reduce into cations, and quickly flow to the anode, and the ions will line up as ion tunnels generating a gap current; (d) arc effect: when the anions and cations hit the metal surface, the kinetic energy is transferred into a heat producing discharge arc and the heat melts the surface of the two electrodes into metal nanoparticles which splash and evaporate into dielectrics; (e) discharge cut-off: when the discharge pulse is turned to Toff, the electrode stops providing electrons. The ion tunnels gradually dissipate with the decrease of current, and the gap voltage and current decrease. The nanoparticles are then coagulated with dielectric fluid and are were suspended in the dielectric liquid; (f) restoring the insulation: as the surface temperature of the electrodes decreases, nanoparticles are dispersed in the dielectric fluid. The electrode surface processing marks are formed, and the dielectric breakdown voltage is restored to the insulation state until the next discharge.
Synthetic Mechanism of W and WO3 Nanocomposite
As shown in Fig. 3, under the special preparation conditions of ESDM, in the discharge process, the electrode gap generates high temperature due to the high energy. The magnitude of the electric field of ionization is 25–50 kV/cm, and the liquid tungsten exfoliated from the tungsten wire is vaporized to form tungsten atoms. The dielectric fluid used in this study was deionized water, and the water molecules were decomposed due to the high temperature and electric field into oxygen atoms and oxygen ions. In the preparation process, in the state of high temperature and a high electric field, the tungsten atoms generated tungsten ions by enhanced ionization continuously, and the tungsten ions combined with the oxygen ions to form WO3. If the tungsten atoms received an insufficient electric field intensity or departed from the range of the electric field during ionization, the tungsten atoms would not lose electrons, and the tungsten atoms would maintain the original morphology to become nano tungsten. Therefore, the nanocolloid contained nano tungsten and WO3 simultaneously.
Results and Discussion
Zetasizer and UV–Vis Test Results
As shown in Fig. 4, the results of the Zetasizer showed that the W and WO3 nanocomposite particles were evenly distributed at 79.65 nm. This result showed the average size distribution of the nanoparticles in the sample. The result of the size distribution was calculated using an instrument after the laser light penetrated through the sample. As some nanoparticles would agglomerate into cluster due to the Van der Waals force, the size distribution could be larger. Therefore, the detection result was not the actual size of nanoparticles, and the actual particle size needed to be observed from TEM image. As the size of the nanoparticles was below 100 nm, the result proved that the W and WO3 nanoparticles prepared in this study met the nanoscale. The Zeta potential was tested according to electrophoresis. A test tube with a sample was placed in the Zetasizer, the sheet metals on both sides of test tube supplied a voltage, the charged particles migrated towards the opposite polar end, and the migration velocity was measured. Finally, the zeta potential was calculated by the Henry equation. As shown in Fig. 5, the zeta potential was − 52.5 mV. When the zeta potential is greater than − 30 mV, it is difficult for particle settlement to occur. The result of UV–Vis showed that there was a wavelength of 320 nm. The characteristic absorption peak at 320 nm belonged to WO3, as shown in Fig. 6. The UV–Vis analysis result was coincident with Ref. [27], meaning the optical properties were identical.
Raman Test Result
The result of the Raman test showed that there were four peaks in the band of 247 cm−1 to 807 cm−1. The (O–W–O) bending mode was present at 247 cm−1 and 313 cm−1, and the (O–W–O) stretching mode was present at 707 cm−1 and 807 cm−1, respectively. These results indicated the existence of WO3, as shown in Fig. 7. According to the Raman research findings of other Ref. [28], the Raman shift positions in the Raman spectra of the nano complex prepared by ESDM were approximately the same, meaning the two vibration modes were identical.
XRD and TEM Test Results
Figure 8 shows the results of the W and WO3 XRD patterns. It was obvious that W and WO3 crystals coexisted in the nanocomposite. The characteristic peaks of W (110) and WO3 (200) had two properties, which were high intensity and a narrow peak, meaning the W and WO3 contained in the sample had good crystallinity and there were no other impurity peaks compared with the chemical method. According to the results, when a W rod was used to prepare nanocolloid by ESDM, W and WO3 nanocomposite colloid was prepared and the prepared colloid was free of other impurities, indicating that ESDM was a clean and environmentally friendly preparation method. The nanocomposite of W and WO3 is quite scarce in references. It may because this nanocomposite is not easy to fabricate under the traditional process, whereas the mechanism of ESDM can smoothly synthesize this composite. According to Refs. [29, 30], W and WO3 have respectively representative diffraction peak positions, and the diffraction peak position in the XRD pattern of the nano complex prepared by ESDM matched the references. The TEM result showed that the W and WO3 nanoparticles were in shape of a rectangle, as shown in Figs. 9a, b. Figure 9a shows that the crystal mesh line width of WO3 was 0.355 nm and the crystal face phase was (200), proving that the cube was a crystal made of WO3 nanoparticles with a crystal size of about 15 nm. Figure 9b shows that the crystal mesh line width of W was 0.225 nm, the crystal face phase was (110), and that the composite state of the W and WO3 crystals could be observed. According to the results, the TEM result matched that of XRD. In terms of TEM, the particle size in the image can be measured using Gatan DigitalMicrograph software. The measurement results showed that the length and width of the nano WO3 prepared by ESDM were about 15.5 nm, and the length and width of the nano tungsten were about 12.5 nm.
Conclusion
In this study, W and WO3 nanocomposite colloid was successfully prepared by EDM. This study therefore reached the following conclusions:
-
1.
When a W rod was used as an electrode, the experiment showed that W and WO3 nanocomposite colloid could be prepared successfully using this novel preparation method (ESDM).
-
2.
The average particle size of the W and WO3 nanoparticles prepared by EDM was 79.65 nm, which was confirmed to be nano-scale. The Zeta potential was − 52.5 mV, indicating that the W and WO3 nanoparticles could be suspended in deionized water without sedimentation. The W and WO3 nanoparticles were in shape of a rectangle, as observed by TEM.
-
3.
The W and WO3 nanocomposite colloid prepared by EDM was composed of W and WO3, as found by XRD. The optical properties of W and WO3 were measured by UV–Vis. With the verification of these instruments, the component of the nanocomposite colloid was confirmed as W and WO3 nanoparticles.
-
4.
This preparation was found to be a simple, fast, and environment-friendly method to produce W and WO3 nanocomposite colloid. It is expected to have numerous applications in the future.
References
D. Lin, J. S. Han, Y. S. Kwon, et al. (2015). Int. J. Refract. Met. Hard Mater. 53, 87–91.
S. Jing, X. Wang, and Y. Tan (2018). F. Appl. Surf. Sci. 441, 654–662.
H. Kim, K. Senthil, and K. Yong (2010). Mater. Chem. Phys. 120, 452–455.
R. Abe, H. Takami, N. Murakami, and B. Ohtani (2008). J. Am. Chem. Soc. 130, 7780–7781.
M. Sadakane, K. Sasaki, H. Kunioku, B. Ohtani, W. Ueda, and R. Abe (2008). Chem. Commun. 48, 6552–6554.
V. V. Malyshev and A. V. Pislyakov (2014). J. Anal. Chem. 69, 123–135.
W. H. Tao and C. H. Tsai (2002). Sens. Actuators B: Chem. 81, 237–247.
F. Fang, J. Kennedy, J. Futter, T. Hopf, A. Markwitz, E. Manikandan, and G. Henshaw (2011). Nanotechnology. 22, 335702.
J. Kennedy, F. Fang, J. Futter, J. Leveneur, P. P. Murmu, G. N. Panin, et al. (2017). Diam. Relat. Mater. 71, 79–84.
S. Adhikari and D. Sarkar (2014). ElectrochimicaActa 138, 115–123.
N. Oka, M. Watanabe, K. Sugie, et al. (2013). Thin Solid Films 532, 1–6.
K. H. Tseng, Y. H. Lin, D. C. Tien, H. C. Ku, and L. Stobinski (2018). Micro Nano Lett. 13, 1545–1549.
K. H. Tseng, C. Y. Chang, M. Y. Chung, and T. S. Cheng (2017). Nanotechnology 28, 465701.
K. H. Tseng and J. C. Huang (2011). J. Nanoparticle Res. 13, 2963–2972.
J. Baizán, A. Navarro-Crespín, R. Casanueva, et al. (2014). IEEE Trans. Ind. Appl. 50, 4356–4362.
W. Anderson, D. Kozak, V. A. Coleman, et al. (2013). J.colloid Interface Sci. 405, 322–330.
D. E. Haines, D. C. O’Hanlon, J. Manna, et al. (2013). Inorg. Chem. 52, 9650–9658.
D. Malakpur, M. Devi, and R. B. Sashidhar (2017). IET Nanobiotechnol. 11, 866–873.
S. Bhople, S. Gaikwad, S. Deshmukh, et al. (2016). IET Nanobiotechnol. 10, 389–394.
A. Gajović, D. Gracin, I. Djerdj, et al. (2008). Appl. Surf. Sci. 254, 2748–2754.
L. M. Malard, M. A. A. Pimenta, G. Dresselhaus, et al. (2009). Phys. Rep. 473, 51–87.
K. H. Tseng, M. Y. Chung, and J. L. Chiu (2018). J.Clust. Sci. 29, 215–224.
K. H. Tseng, M. Y. Chung, and J. L. Chiu (2017). J.Clust. Sci. 28, 2653–2668.
K. Ponappa, S. Aravindan, P. V. Rao, et al. (2010). Int. J. Adv. Manuf. Technol. 46, 1035–1042.
K. H. Tseng, C. J. Chou, S. H. Shih, D. C. Tien, H. C. Ku, and L. Stobinski (2018). Nanomater. Nanotechnol. 8, 184798041775284.
T. O. Hockenberry (1968). The Role of the Dielectric Fluid in Electrical Discharge Machining, SAE Technical Paper, 680635. https://doi.org/10.4271/680635.
F. Zheng, M. Guo, and M. Zhang (2013). CrystEngComm 15, 277–284.
L. Xu, M. L. Yin, and S. F. Liu (2014). Sci. Rep. 4, 6745.
W. H. Hu, G. Q. Han, B. Dong, and C. G. Liu (2015). J.Nanomater. 16, 23.
S. Kanpara, S. Khirwadkar, S. Belsare, K. Bhope, R. Swamy, Y. Patil, and K. Galodiya (2016). Mater. Today Proc. 3, 3055–3063.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author(s) declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tseng, KH., Ku, HC., Lee, HL. et al. Fabricating Tungsten and Tungsten-Trioxide Nanocomposite Colloid in Deionized Water by Electric Spark Discharge Method. J Clust Sci 30, 477–482 (2019). https://doi.org/10.1007/s10876-019-01511-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-019-01511-0