Abstract
Using the density functional theory calculations with the PBE exchange–correlation energy functional, we have studied the magnetic property and electronic properties such as binding energy, embedding energy, charge transfer, ionization potential and electron affinity of the NinGe (n = 19–29) neutral and ionic clusters. The addition of Ge atom can decrease the magnetic moments of Nin clusters except Ni28 and Ni +/−28 . The charge is transferred from Ge atom to Ni clusters. And the local maxima value has appeared at Ni25Ge cluster. Both the calculated ionization potential and electron affinity exhibit an oscillating behavior as the cluster size increases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The study of the electronic structure and properties of atomic clusters is an extremely active area of research due to its importance in nanoscience and nanotechnology. In the past three decades, considerable theoretical and experimental effort has been devoted to the understanding of pure transition metal clusters. However, in recent years, more and more attention has been paid to the study of the physical and chemical properties of bimetallic clusters. Because the bimetallic clusters have potential application in many fields such as catalyst, corrosion, heat resistance, magnetism and so forth. Ni clusters have been widespread concerned in catalytic reaction and magnetic materials because of their unique geometrical structures and delocalized d electrons. Therefore the bimetallic systems of many metals such as Ge [1, 2], Al [3], Fe [4], Rh [5], Pd [6], Ti [7], Cu [8], Mn [9] or others adsorbed Ni clusters have been reported. It is well known that Ge plays an important role in the development of the semiconductor industry. So Ni-Ge clusters are the primary target because of their wide applications in advanced material technology. Unfortunately, as regards Ni-Ge clusters, to the best of our knowledge, only a few studies have reported. To give some examples, Feng et al. studied ab initio calculations of the structures, binding energy and total spin moment of NinGe (n = 2–12) clusters using all electron density-functional method. Their results showed that the doped Ge made both stability and magnetism of NinGe clusters weakened as compared with pure Nin+1 clusters [1]. Dhaka et al. [2] calculated the geometry, electronic structures, growth behavior and stability of neutral and ionized Ni encapsulated Ge clusters within the framework of a linear combination of atomic orbital density functional theory (DFT) under a spin polarized generalized gradient approximation. Moreover there are several studies which have been performed to analyze the structure, electronic and magnetic properties of transition metal impurities in Ge clusters [10, 11]. Thus the study of Ge on the configurations and electronic properties of Ni clusters has very important significance. Such clusters enhance stability because the semiconductor atom absorbs the dangling bonds present on the transition metal cages. Simultaneously, the cluster exhibits a wide range of electronic properties by varying the doping elements. If clusters are to be used for synthesizing materials, one must understand how sensitive their tailored properties are to size and topology. Unfortunately, there are very few experimental techniques can determine cluster structure, which is mainly because nanoclusters are too small to be detected using diffraction techniques and too large to be explored using spectroscopy. Therefore, theoretical calculations are particularly important. Usually the properties of clusters are sensitive to the size and the constituent element types for clusters, so the study of structures and properties for clusters on the one hand can help us to understand some of the features and laws condensed matter, and on the other hand play a guiding role in experimental synthesis.
The purpose of the present work is to perform the equilibrium structures of the NinGe (n = 19–29) clusters theoretically by applying the DFT method implemented in the VASP code with the spin polarized PBE (Perdew, Burke, and Ernzerhof) gradient-corrected exchange–correlation functional method. Based on the neutral and ionic NinGe clusters structures optimized from our DFT-PBE calculations, the magnetic moment, binding energy, embedding energy, charge transfer, adiabatic ionization potential and adiabatic electron affinity of the clusters as the cluster size increases have been systematically analyzed, which can provide useful information for the stability and electronic properties of Ni-Ge clusters in this range. The purpose of this study is to provide the fundamental data for the future estimation of the magnetic alloy materials.
Small size range Ni-Ge alloy clusters have been studied the theoretically before [12,13,14], but due to the smaller size range study can not provide clear growth behavior, structural and electronic properties of the series. Therefore, a study of the range is important from a realistic point of view and would be helpful to compare with the experimentally obtained data over a wide size range in future. The main focus of the present study is to explain the thermodynamic stability of clusters in neutral and charged states along with their chemical properties in detail.
Computational Methods
In the present theoretical work, we have verified the lowest-energy structures of neutral and ionic Nin (n = 19–29) clusters derived from Refs. [15,16,17,18] using density functional theory (DFT) calculations firstly. Next according to the lowest-energy structures of Ni clusters, the stable structures of neutral and ionic NinGe (n = 19–29) clusters have been obtained. DFT has evolved into a widely applicable computational technique, while requiring less computational effort than convergent quantum mechanical methods such as coupled cluster theory. And our calculations are carried out using VASP code [19, 20] with the Perdew, Burke, and Ernzerhof (PBE) gradient-corrected exchange–correlation energy functional. The cutoff energy of plane wave (PW) in the calculations is taken to be 269.5 eV. A simple cubic supercell of size of 20 Å is used. The geometry optimization of each isomer was carried out till the energy is converged to an accuracy of 10−5 eV.
In order to understand the relative stability of the NinGe (n = 19–29) clusters, we have analyzed the binding energy (BE) for neutral and ionic NinGe compared to Nin clusters. BE is defined as the energy gained in assembling a cluster from its isolated constituents. The BE of Nin and NinGe clusters are calculated according to the following definition [21,22,23,24,25,26,27],
where Etotal (Nin) and Etotal (NinGe) are the total energy of Nin and NinGe clusters. Etotal(Ni) and Etotal(Ge) are the energy of a free Ni and Ge atom, respectively.
Next, we also define the BE of Nin and NinGe ionic clusters
in which Etotal (Ni +/−n ) and Etotal (NinGe+/−) are the total energy of the Ni +/−n and NinGe+/− clusters, Etotal(Ni+/−) and Etotal (Ge+/−) are the energy of a free Ni+/− and Ge+/− ion.
In addition, we have calculated the embedding energy (EE), which is the energy gain in incorporating a Ge atom in the lowest energy isomer of the pure Nin cluster [21,22,23,24,25,26,27]. In the case of NinGe clusters, this is defined as
The configurations of Ni and Ge atoms are 3d84s2 and 4s24p2, respectively. We set the spin in our calculation process, and the total spin value is one, so the spin multiplicity of the ground state structures for NinGe clusters are three, which follow the Wigner-Witmer (WW) spin conservation [21,22,23,24,25,26,27].
For charged clusters, there are two possibilities,
or
either the charged Ge atom can be embedded in a neutral Nin cluster or the other way round. We take the lower of these two numbers as the EE with enforcement of the WW spin conservation rule.
Results and Discussion
Structures of the Neutral and Ionic NinGe (n = 19–29) Alloy Clusters
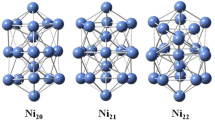
In this section, we have discussed the structures of NinGe (n = 19–29) clusters. And a number of isomers are calculated at each size. Only selected isomers with energy close to the supposed ground state structures are presented in Fig. 1. It has been shown by previous experimental and theoretical studies that the lowest-energy structure of Ni19 is a perfect double-interpenetrating icosahedron, which has three parallel pentagonal rings stacked in a 1-5-1-5-1-5-1 sequence. The ground-state structures, the first and the second adsorption structures (wherever applicable) are marked (a), (b) and (c), respectively. The structures and relative energies presented in this work are all resulted from the DFT-PBE calculations. The lowest-energy structure of Ni19Ge can be viewed as adding one Ge atom to the cluster side face in the waist of Ni19. And the Ni-Ge average bond length is calculated to be 2.365 Å, which is shortened compared to the optimized Ni19 clusters (2.484 Å). The other two low-energy adsorption structures (Ni19Geb and Ni19Gec) are lying 0.409 and 0.682 eV higher above Ni19Gea. The Ni20Gea is formed by adding one Ni atom and Ge atom on the adjacent waist sites of Ni19, which is similar to the lowest-energy structure of Ni21 [17]. Next, in a similar fashion, the Ni21Gea is obtained by adding one Ge atom above the adjacent centre of equilateral triangle in the Ni21. The Ni-Ge average bond lengths of Ni20Gea and Ni21Gea are 2.389 and 2.372 Å, which are shorter than the optimized Ni20 and Ni21 clusters (2.416 and 2.434 Å). It is found to be 0.054 and 0.430 eV (Ni20Geb and Ni20Gec), 0.029 and 0.147 eV (Ni21Geb and Ni21Gec) higher in energy than Ni20Gea and Ni21Gea, respectively. The Ni23 can be seen as three interpenetrating 13-atom icosahedrons. The Ni22Gea is viewed as substitution of one Ni atom above centre of equilateral triangle by one Ge atom of Ni23 [17]. And the average bond lengths of the Ni-Ge (2.511 Å) for Ni22Gea can increase compared to the Ni–Ni (2.431 Å) for Ni22. The low-energy adsorbed structures Ni22Geb and Ni22Gec, 0.029 and 0.135 eV less stable than Ni22Gea. The Ni23Gea and Ni24Gea are found to be one Ge atom located at the adjacent centre of equilateral triangle. The lowest-energy structures of Ni26 and Ni29 are considered to be composed of two interpenetrating 19-atom double-icosahedrons, and two interpenetrating double-icosahedrons and double-icositetrahedrons (with three parallel hexagonal rings), respectively [17]. And the average bond lengths are shortened from 2.471 to 2.443 Å for Ni–Ni (Ni23 and Ni24) to 2.379 and 2.389 Å for Ni-Ge (Ni23Gea and Ni24Gea). The energies of adsorption structures (Ni23Geb and Ni23Gec, Ni24Geb and Ni24Gec) are more than 0.172 and 0.476 eV, 0.071 and 0.205 eV above Ni23Gea and Ni24Gea. The energetically most favorable structure of Ni25Gea from our present study can be viewed as substitution of one Ni atom by one Ge atom to the waist site of Ni26. The average bond lengths of the Ni-Ge (2.571 Å) for Ni25Gea can increase compared to the Ni–Ni (2.491 Å) for Ni25. And the Ni25Geb and Ni25Gec are 0.148 and 0.334 eV higher in energy than Ni25Gea, respectively. The structures of Ni26Gea and Ni29Gea are viewed as adding one Ge atom at an adjacent hollow rhombus site in the waist of double-icosahedral structures for Ni26 and Ni29. The Ni27Gea can be viewed as adding one Ni atom to the cluster side face in the waist of Ni26Gea. We can remove one Ni atom on the top site of pentagonal pyramid of Ni29Gea to form the Ni28Gea. The average bond lengths of Ni-Ge for Ni26Gea, Ni27Gea, Ni28Gea and Ni29Gea are 2.384, 2.386, 2.577 and 2.375 Å. The average bond length of Ni-Ge is increasing and decreasing for Ni28Gea and Ni26,27,29Gea, respectively. The relative energies of the Ni26Geb, Ni27Geb(Ni27Gec) Ni28Geb(Ni28Gec) and Ni29Geb(Ni29Gec) are 0.438, 0.074(0.115), 0.071(0.281) and 0.108(0.478) eV higher compared to Ni26Gea, Ni27Gea, Ni28Gea and Ni29Gea, respectively. Because of the high symmetry, there is only one kind of adsorption isomer for Ni26 cluster. It has been found that the lowest-energy structures of the cluster ions are almost identical with the neutral ones.
Relative Stabilities
The calculated Binding energies (BEs) for neutral and ionic NinGea compared to Nin clusters are shown in Fig. 2a–c. It is found from observing Fig. 2a–c that the BEs of neutral and ionic NinGea clusters is higher than the corresponding Nin clusters, showing that Ni-Ge alloy clusters are relatively more stable. This is mainly because Ge is a relatively stable semiconductor material. When the Ge atom is adsorbed to the active transition-metal Nin clusters, the stability of NinGea clusters is relatively enhanced. The curve of BEs is seen to increase monotonically as a function of cluster size, which shows that the cluster can continue to gain energy during the growth process, and the adsorption of Ge atom is beneficial to improve the stability of the cluster. We also find that the BEs of cationic clusters are significantly higher than of neutral clusters, whereas anionic clusters are just the opposite. It can be seen from that the neutral clusters transit to an ionic clusters, the stability of the cluster structures change to some extent due to the change in the number of electrons.
The relative stability of the clusters can be also estimated through the second difference in energy which is defined by Δ2E(n) = E(n + 1) + E(n − 1) − 2E(n) in which E(n) represents the total energy of cluster of size n. According to this definition, the clusters with positive Δ2E are more stable than those with negative Δ2E. The second difference in energy is a sensitive quantity that can reflect the relative stability of clusters and can be directly compared with the experimental relative abundance. The calculated Δ2E for the Nin and NinGea clusters are plotted in Fig. 2d, showing an oscillating behavior. NinGea at n = 20, 23 and 26 correspond to the local maxima on the curve of Δ2E, indicating that they are relatively more stable than the others. For the ionic clusters, there are several peaks on Δ2E curve at n = 21 and 26 for the cationic clusters, and at n = 20, 23 and 26 for the anionic clusters, showing these cluster ions are relatively more stable in Fig. 2e, f.
Figure 3 shows the embedding energies (EEs) of NinGea neutral, cationic and anionic clusters as a function of cluster size n. It is clear that there is not significant change in the EEs with the increase in cluster size. Similar to the BEs, the EEs of neutral clusters have lower and higher than cationic and anionic clusters, respectively.
Magnetic Properties
Ni clusters are typically ferromagnetic transition-metal clusters in the 3d group. The configuration of the Ni atom is 3d84s2. The spin of the electron follows the Pauli exclusion principle and the Hund’s rules, and the two unpaired 3d electrons of the Ni atom will contribute to its magnetic property. In principle, both spin and orbital magnetic moments have contribution to the total magnetic moment in any system. The orbital magnetic moment can be produced by orbital motion of each electron while the spin magnetic moment can be produced by electron spin. For ferromagnetic transition metal clusters, the magnetic moment mainly comes from the spin motion of the electron, while the orbital magnetic moment has almost no contribution to the total magnetic moment, which is called “orbital quenching effect”. This conclusion has also been proven by recent experiments [25, 28]. Therefore, for the transition metals in the 3d group, the magnetic moment which is caused by the unpaired electron orbital motion is relatively small. The atomic total magnetic moment depends mainly on the electronic spin magnetic moment. As shown in Fig. 4a, the total magnetic moments obtained for NinGea clusters from our calculation have the same trend as pure Nin clusters, which increase monotonically as a function of cluster size except Ni28Gea. The calculated total magnetic moments decrease with the addition of Ge atom, but the Ge atom in all these clusters has negligible magnetic moments, which is in good agreement with previous results [19]. This is mainly due to a strong hybridization between the 3d8 of Ni with the 4s24p2 of Ge atom results in the magnetic moment of Ni being quenched with leftover part to hold its spin moment in the NinGe ground state cluster. The present investigation follows the same reported [25] and is one of the strongest evidence of the quenching of spin magnetic moment of the Ni atom. However the total magnetic moment of Ni28Gea is higher than Ni29. In order to analyze the reasons for this phenomenon, we have plotted the total density of states (TDOS) for Ni28 and Ni28Gea clusters in Fig. 5a, b and the projected density of states (PDOS) for Ni28Gea in Fig. 5c, d. It can be seen from Fig. 5a, b, the structure of Ni28Gea can be considered to be composed of two interpenetrating double icosahedron and double icositetrahedron (with three parallel hexagonal rings), which exhibits high (C2v) symmetry. This high symmetry makes the electronic state have a greater degree of degeneracy, resulting in high DOS. In addition, there is also charge transfer between the d orbit and s, p orbits of Ni (Fig. 5c), which leads to a reduction in the d-band width of Ni28Gea cluster and increasing the DOS, especially near the Fermi level. Finally exchange splitting induces a relative movement of the spin-up and spin-down band, which leads to different electron occupations in the spin-up and spin-down states, thus increasing the magnetic moment of Ni28Gea cluster. Next we have also plotted the total magnetic moment for the cationic and anionic clusters as shown in Fig. 4b, c, respectively. Similar to the corresponding neutral clusters, the total magnetic moment of Ni +/−n is larger than that of NinGe+/− except Ni +/−28 (Ni28Ge+/−). And the total magnetic moments of Ni19Ge+/− and Ni20Ge− are very close to Ni +/−19 and Ni −20 . The results obtained by the present study help us choose magnetic materials.
Charge Transfer
The charge transfer between the Ge atom and Ni clusters for neutral and ionic NinGea (n = 19–29) clusters are displayed in Fig. 6. Compared to the pure Nin clusters, the adsorption of Ge atom makes the electron cloud between the atoms lean toward the side of Nin clusters, and the electrons are transferred from the Ge atom to Nin clusters. In general, the electronegative of Ge(2.01) is more than Ni(1.91). But Mulliken population analysis showed that, in this system, charge is always transferred from Ge to Ni, so Ge acts as an electron donor in NinGe clusters. This mainly has two side reasons. On one hand, the whole Ni clusters have stronger electron withdrawing ability; on the other hand, the Ni atom has a large number of charges than Ge atom, so the transfer of electrons from Ge atom to Ni clusters. We find the amount of the charge transfer is less than 0.5e as a function of cluster size for NinGea (n = 19–22, 27 and 29) clusters, and the cationic clusters is larger than that for neutral and anionic clusters. And the local maxima value has appeared at Ni25Gea cluster.
Ionization Potential and Electron Affinity
The adiabatic ionization potential (AIP) is an important character in understanding the electronic properties of clusters, and it can also account for the metallicity of the clusters. The calculated AIP for Nin and NinGea (n = 19–29) clusters are shown in Fig. 7a. It can be seen from the plot that the AIP exhibits an oscillating behavior as the cluster size increases for Nin and NinGea clusters. And the AIP is larger for NinGea than that for Nin clusters except Ni21 (Ni21Gea). And the AIP is very close to each other for n = 22, 24–26. As is well known, when the AIP gets smaller, the cluster will be more close to a metallic system; hence the metallicity is weakened with the addition of Ge atom. And the strongest metallicity is Ni28Gea cluster, which can be applied in the field of alloy materials.
The adiabatic electron affinity (AEA) for Nin and NinGea (n = 19–29) clusters have been plotted in Fig. 7b as a function of cluster size. The clusters with greater AEA are more reactive. Similar to the trend of AIP, the AEA also exhibits an oscillating behavior as the cluster size increases for Nin and NinGea clusters. The local maxima and minima of the AEA are Ni24 and Ni19 for Nin clusters, Ni29Gea and Ni22Gea for NinGea clusters, respectively. And Ni20Gea, Ni23Gea, Ni27Gea and Ni29Gea clusters have the stronger ability to capture electrons.
Summary
The properties of the NinGe (n = 19–29) clusters have been studied using the DFT-PBE calculations. In this work, we focused on analyzing a series of properties of the lowest-energy Ni-Ge alloy clusters including binding energy, embedding energy, magnetic property, charge transfer, ionization potential and electron affinity. The binding energies of NinGe clusters are higher than the corresponding Nin clusters, and the curve of the binding energies is increasing monotonically as a function of cluster size. From the magnetic moment analyses, it is found that the addition of the Ge atom can decrease the magnetic moments of Nin clusters except Ni28 and Ni +/−28 , but the Ge atom in all these clusters has negligible magnetic moments. On the other hand, the charge is transferred from Ge atom to Ni clusters. And the local maxima value has appeared at Ni25Ge cluster. Finally both the calculated ionization potential and electron affinity exhibit an oscillating behavior as the cluster size increases.
References
C. J. Feng and L. L. Cai (2014). Comput. Theor. Chem. 1042, 57.
K. Dhaka, R. Trivedi, and D. Bandyopadhyay (2013). J. Mol. Model. 19, 1473.
Z. W. Ma and B. X. Li (2015). Comput. Theor. Chem. 1068, 88.
T. Mohri (2015). J. Mater. Sci. 50, 7705.
J. Teeriniemi, J. Huisman, P. Taskinen, and K. Laasonen (2015). J. Alloys Compd. 652, 371.
J. X. Zhu, P. Cheng, N. Wang, and S. P. Huang (2015). Comput. Theor. Chem. 1071, 9.
N. S. Venkataramanan, R. Sahara, H. Mizuseki, and Y. Kawazoe (2010). J. Phys. Chem. A 114, 5049.
J. A. Mary, A. Manikandan, L. J. Kennedy, M. Bououdina, R. Sundaram, and J. J. Vijaya (2014). Trans. Nonferrous Met. Soc. 24, 1467.
B. R. Wang, H. Y. Han, and Z. Xie (2014). J. Mol. Struct. 1062, 174.
C. M. Tang, M. Y. Liu, W. H. Zhu, and K. M. Deng (2011). Comput. Theor. Chem. 969, 56.
N. Kapila, V. K. Jindal, and H. Sharma (2011). Physica B 406, 4612.
A. Chikhaoui, K. Haddab, S. Bouarab, and A. Vega (2011). J. Phys. Chem. A 115, 13997.
X. J. Deng, X. Y. Kong, X. L. Xu, H. G. Xu, and W. J. Zheng (2016). Chin. J. Chem. Phys. 29, 123.
J. M. Goicoechea and J. E. McGrady (2015). Dalton Trans. 44, 6755.
Z. El-Bayyari (2005). J. Mol. Struct. (Theochem) 716, 165.
Z. Xie, Q. M. Ma, Y. Liu, and Y. C. Li (2005). Phys. Lett. A 342, 459.
W. Song, W. C. Lu, Q. J. Zang, C. Z. Wang, and K. M. Ho (2012). Int. J. Quantum Chem. 112, 1717.
W. Song, W. C. Lu, C. Z. Wang, and K. M. Ho (2011). Comput. Theor. Chem. 978, 41.
G. Kresse and J. Hafner (1993). Phys. Rev. B 47, 558.
G. Kresse and J. Furthmuller (1996). Phys. Rev. B 54, 11169.
M. B. Abreu, A. C. Reber, and S. N. Khanna (2014). J. Phys. Chem. Lett. 5, 3492.
D. Bandyopadhyay and P. Sen (2010). J. Phys. Chem. A 114, 1835.
S. N. Khanna, B. Rao, and P. Jena (2002). Phys. Rev. Lett. 89, 016803.
D. Bandyopadhyay, P. Kaur, and P. Sen (2010). J. Phys. Chem. A 114, 12986.
R. Trivedi, K. Dhaka, and D. Bandyopadhyay (2014). RSC Adv. 4, 64825.
D. Bandyopadhyay (2008). J. Appl. Phys. 104, 084308.
M. Kumar, N. Bhattacharyya, and D. Bandyopadhyay (2012). J. Mol. Model. 18, 405.
J. T. Lau, A. Föhlisch, M. Martins, R. Nietubyc, M. Reif, and W. Wurth (2002). New J. Phys. 4, 98.
Acknowledgements
This work is supported by the Natural Science Foundation of He’nan Department of Education (Grant Nos.: 15B150010; 18B430012 and 15A140032). This work is also supported by Xinxiang University Doctor Initial Research Program (Grant Nos.: 1366020018 and 1366020039) and Science and Technology Innovation Fund of Xinxiang University (Grant Nos.: 15ZP01 and 15ZB25). The computational resource is partly supported by the Performance Computing Center of Jilin University, China.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Song, W., Fu, Z., Liu, Th. et al. First-Principles Study of the Structures and Electronic Properties for NinGe (n = 19–29) Clusters. J Clust Sci 30, 131–139 (2019). https://doi.org/10.1007/s10876-018-1462-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-018-1462-7