Abstract
Numerous reports are available for the use of plant extract in nanoparticles synthesis. The traditional methodologies adopted for analyzing nanosynthesis potential of plant materials is tedious and time-consuming. Rapid, selective, low-cost and easy protocols for on-site detection of nanoparticles synthesizing potential of plant extract have tremendous potential in nanotechnology. In the present study, a simple and selective nanoparticles synthesis screening approach is developed by coating metal salts (precursor for metal nanoparticles) on an inert paper strip and allowing them to react with test plants extract such as plant latex, whose nanosynthesis potential have to be screened. The visual color change of paper strip after reaction of metal salts with latex is the ultimate colorimetric endpoint indicating the formation of nanoparticles. Diverse nanoanalysis techniques qualitatively confirmed the nanoparticles formed on the paper strip. UV–visible spectroscopy exhibit characteristic absorption peaks for AuNPs and AgNPs at around 520 and 430 nm. Scanning electron microscopy confirms the size of 87 and 114 nm for AuNPs and AgNPs. The simplicity, selectivity and rapid response make this approach a promising layman-friendly, on-site useful detection tool. This screening platform can rapidly screen diverse plant species for their capacity to fabricate nanoparticle to select most potent species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rapid development in nanotechnology has increased the use of nanomaterials in several sectors such as agriculture, medicine, food, health, and energy [1,2,3]. Around 2300 nano-based products are available just within the European market with an estimated annual nanomaterial production up to thousands of tons/year [4]. Inorganic metal nanoparticles are an essential element of nanotechnology and are a topic of interest among researchers working in different disciplines [5]. Silver and gold nanoparticles gained extensive attention in both research and industrial platform, owing to their utility in diverse areas such as biocidal agent, textiles, food packaging, biosensors, nanocomposites, nanoelectronics, nanodiagnostics, cosmetics, and bioremediation [6,7,8,9]. Currently, large-scale synthesis of nanomaterials is achieved by chemical and physical methods. However, hazardous and toxic effects of these methods (and hence nanoparticles synthesized by these methods) are well established as a cause of high concern [10, 11]. Thus there is increased pressure on synthesizing nanomaterials especially by green chemistry approach instead of environmentally toxic chemicals [12]. Several International regulatory agencies have also suggested “safe by design” strategy to lower the harmful effects of nanomaterials and promote their sustainable use in different applications [13]. The apparent alternative and promising strategy for researchers to avoid the use of the harmful chemical in nanosynthesis is to utilize extensively available bioresources like plants and microorganisms as a mini-factory to produce nanoparticles. This ultimately gives rise to the concept of ‘green nanosynthesis’ that involves the use of natural sources (plant, bacteria, and algae) for nanoparticle synthesis. The natural resources may work as reducing (enzymes, ascorbic acid, flavonoids, phenolics, etc.) and capping agents (protein, starch, etc.) in the process of nanoparticles formation [8, 14].
According to the state of the world’s plants report-2016, there are about 391,000 plants are known to science [15]. Such massive plant biodiversity is already available and waiting for further exploration in nanotechnology. Plant metabolites have been well documented for their use in different disciplines like Ayurveda, food, pharmaceuticals, and medicine. Besides this, various plants families differ in their potential to reduce the bulk metal salts into nanosized particles. The phytosynthesis of nanomaterial is promises green chemistry approaches in nanotechnology [14]. Although plants are identified as an eco-friendly agent for nanomaterial synthesis, available methods for screening are not enough to determine most potent and promising plant species from vast diversity for their nanomaterial synthesizing potential. The traditional approach to analyze nanosynthesis potential of plant species involves several steps such as selection of plant followed by a collection of plant material and further processing (drying and extraction of phytochemicals) before use in nanosynthesis. Above discussed steps are time-consuming and limit the screening of a vast number of samples to check their potential. In this investigation, an instant method of screening of nanoparticle synthesizing potential of plants is developed by merely using the metal salts solution impregnated Whatman filter paper strip.
Paper, a highly abundant and low-cost material has become of potential interest due to attractive physical and chemical properties such as cellulose-based structure, highly controllable porosity and surface nature [16]. Recently, the development of paper-based analytical and clinical devices has emerged as a flexible, economical, handy and user-friendly. The well-known example is pregnancy test kit where a sample loaded on a pre-impregnated paper strip provides ‘yes/no’ type colorimetric detection [12]. It also implies the ideal format of colorimetric test kit for the developing world to be detected by naked eyes and without the requirement of any additional instrument setup [17].
In the present investigation, silver and gold metal salts impregnated on paper strip produce a yellow to ruby-red color respectively when undergoing the reduction in the presence of biological compounds (plant latex). The intensity of color change of strip mainly depends on size, shape and surface plasmon resonance phenomenon of metal nanoparticles [18]. Hence color change is the selective indicator key to develop the colorimetric assay for nanoparticles synthesis.
The combination of metal impregnated Whatman paper substrate, and colorimetric detection of nanoparticle formation in the presence of biological agents like plants extract, makes this a promising layman-friendly, field useful tool. The authors believe that this simple and functional screening platform will be of interest to analyze the nanoparticle synthesizing potential of massive plants diversity.
Materials and Methods
Whatman filter paper Number 10347509, silver nitrate and Gold chloride trihydrate was purchased from Sigma-Aldrich and S D Fine-Chemicals Limited, India.
Preparation of Test Paper Strips
In the present study, the inert solid matrix to perform nanosynthesis reaction is Whatman filter paper with medium porosity from 8 to 12 μm. The filter paper was soaked in acetone for 1 h followed by drying at room temperature and then cutting the paper into a size of 1.5 cm × 6.5 cm strips. Paper strips were then separately soaked in aqueous solutions of 0.1 M silver nitrate and 0.01 M gold chloride for 5 min (1.5 cm × 6.5 cm strip maximum absorb ~ 200 µl solution of each metal salts) and dried at room temperature. The metal salt solution impregnated paper strips were sealed in a plastic bag and stored in a dry place till further use.
Analysis of Samples
Plant latex (natural plant secretion) from selected species—Pergularia daemia (A) Euphorbia heterophylla (B) Jatropha curcas (C) and Jatropha gossypifolia (D) were used as a test sample to analyze nanosynthesis potential. The latex was extruded out from the plant after cutting near young leaves, and immediately the extruded latex (a small drop, ~ 10 µl) was spotted on a metal salt (silver nitrate and gold chloride) impregnated paper strip for analyzing nanoparticles synthesizing potential. After latex addition, the strips were incubated for 15 min and observed for color change. The strips without latex addition were kept as a control and a separate control strip without silver nitrate, or gold chloride was also observed for color change after latex addition.
Recovery of Nanoparticles Synthesized on a Paper Strip
The different colored spots from both gold and silver test strips were cut and suspended in sterile distilled water (5 ml) with shaking for 2–3 h on a shaker at 120 rpm. Later, the paper strip was removed, and the colored suspension was collected and used for further nano-characterization.
Characterization of Nanoparticles
UV–Visible Spectral Analysis
The colored suspension recovered from paper strips was analyzed for its characteristic UV Visible absorption spectrum for silver and gold nanoparticles. The absorption spectrum was monitored on UV–vis spectrophotometer (Shimadzu 1601 model, Tokyo, Japan) at the resolution of 1 nm from the wavelength range of 300–700 nm.
Scanning Electron Microscopy (SEM) and Energy-Dispersive Spectroscopy (EDS)
The morphology of nanoparticles was analyzed using SEM. The nanoparticles suspension obtained from silver and gold impregnated strips, as well as the whole colored paper strip, were used as a specimen for SEM and EDS characterization. The sample was mounted on specimen stubs with double-sided adhesive tape and coated with gold in a sputter coater (Bal-Tec SCD-050) and examined under SEM, Philips® XL 30 (Amsterdam, Netherlands) at 12–15 kV with a tilt angle of 45°. The EDS analysis was used to determine the presence of elemental silver and gold in the sample.
Fourier Transform Infra Red (FT-IR) Analysis
The control (Whatman filter paper strip, Whatman filter paper strip impregnated with silver nitrate, and gold chloride) and test samples (metal salt impregnated Whatman filter paper strip after reaction with plant latex) were analyzed by Shimadzu FTIR spectrophotometer (IR Prestige-21, Shimadzu, Japan) for functional groups identification. The samples were independently blended with KBr and spectra were observed in transmittance mode at 4000–400 cm−1 [19].
Results and Discussion
Formation of Silver and Gold Nanoparticles on a Paper Strip
In the present study, for the first time, a paper-based colorimetric screening test (paper-based sensor) is developed to rapidly analyze nanoparticle synthesizing potential of an eco-friendly nanosynthesizing agent such as plants. The silver and gold metal salt impregnated paper strips after spotted with plant latex produced a colored reaction and this colorimetric change is a specific indicator for nanoparticles formation.
Change in color of the metal salt solution after interactions with biological agents is a primary detection method to ascertain reduction of bulk metal and their conversion to nanoform. Addition of plant metabolites changes colorless solution of silver nitrate into yellow color, while in case of gold nanoparticles the yellow colored gold chloride solution transformed into ruby-red color [9]. However, an interesting fact is that the intensity of change in color, i.e., light to dark varies according to the size of formed nanoparticles.
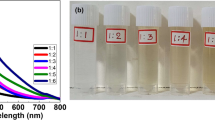
In the present study it was found that as soon as the drop of plant latex was spotted onto the metal impregnated paper strips, a rapid color change in the strip was developed. Formation of yellowish brown color on silver nitrate impregnated strip after spotting plant latex is an indication of fabrication of silver nanoparticles [14] (Fig. 1c). Similarly, gold chloride coated strips were changed into a ruby-red color after interaction with plant latex which indicated the formation of gold nanoparticles (Fig. 1a). Interestingly, it was observed that different color shades were produced on the paper strip by different plant latex under study which signifies that each plant latex can fabricate nanoparticles of different dimensions. This latex-specific variability in the nanoparticles synthesis is apparently because of different biochemical’s present in each of the plant latex (Table S1). In contrast, the controls strips without metal salt impregnation did not show any observable color change after spreading plant latex on its surface suggesting that the color change reaction specifically arises after interaction of metal salt and latex on the inert paper matrix (Fig. 1a, c).
Performance Analysis of Paper-Based Nanosynthesis with the Traditional Approach of Nanosynthesis
Comparative analysis of nanoparticles synthesizing potential of paper-based approach and the conventional approach of nanosynthesis by solution method was made in the present study. The traditional nanoparticle synthesis procedure involved mixing of plant latex with aqueous solutions of silver nitrate, and gold chloride, the resulted color change of solutions were checked for their absorption maxima by UV–vis spectrophotometry. The appearance of specific absorbance maxima and acquirement of color after nanoparticles formation is due to the resonant oscillations that are known as surface plasmon resonance (SPR). It was found that the paper strips and traditional method show nearly similar performance in synthesizing nanoparticles. As clearly outlined in Fig. 1, after addition of Pergularia daemia (A) latex on paper strip impregnated with gold chloride exhibit characteristic surface plasmon resonance (SPR) absorption of gold nanoparticles at 517 nm, while the addition of same plant latex in aqueous solution gold chloride showed SPR absorption at 521 nm. The slight differences in absorbance maxima of gold nanoparticles produced by above two methods suggest the formation of different size nanoparticles. Similar pattern of color and absorption maxima was obtained with remaining plant latex samples, i.e., gold nanoparticles fabricated on paper strip by latex of Euphorbia heterophylla (B) Jatropha curcas (C) and Jatropha gossypifolia (D) with SPR peaks at 525, 535 and 553 nm respectively, while solution method synthesized gold nanoparticles exhibit SPR absorption peak at 530, 535 and 545 nm. Similar absorption and the color trend were also observed in case of silver nanoparticles produced by the paper-based and traditional method of nanosynthesis (Fig. 1b, d). For the smaller monodisperse gold nanoparticles below 30 nm, the SPR phenomenon causes absorption of light in the blue-green portion of the spectrum, i.e., 450 nm and reflecting red light (~ 700 nm), giving bright red color to the solution. As the size of gold nanoparticles size tends to increase, the wavelength of SPR related absorption shifts to longer wavelengths (red shift). Red light is then absorbed, and blue light is reflected to yield a solution with a ruby-red color [20].
The observations made in present investigation suggest that the plant latex showing immediate color shades on silver and gold chloride coated paper strips are not only due to nanoparticle formation but also due to various size nanoparticles synthesizing potential (from 20 to 100 nm). Similar observations were reported earlier by several researchers [20,21,22].
Confirmation of Paper-Based Synthesis of Metal Nanoparticles
SEM, EDS and Particle Size Analysis
The development of silver nanoparticles and gold nanoparticles on the paper strip was further qualitatively confirmed by extracting nanoparticles formed on the paper strip in distilled water followed by SEM, EDS and particle size analysis. The extracted solution of nanoparticles, when viewed under an electron microscope, showed well-dispersed nanoparticles with the different morphology of grain shape and irregular tetragonal (Fig. S1 b). The entire paper strip showing color change was also analyzed in SEM; it showed aggregates of nanoparticles on its surface (Fig. S1 c), this could be due to rigid matrix structure of paper which allows limited mobility of the nanoparticles (as compared with solution method, providing enough space for dispersion of nanoparticles). Particle size analysis showed average size of 87 and 114.4 nm for gold nanoparticles and silver nanoparticles respectively (Fig. S1 a). The respective peaks of silver and gold were detected in EDS analysis indicating the presence of elemental silver and gold in the sample.
Whatman Filter Paper as Solid Support for Coating Silver and Gold Salt Solution
One of the essential priorities for enhancing the selectivity of nanosynthesis is to provide rigid and inert biomatrix facilitating the nanosynthesis reaction. Whatman filter paper was selected for this purpose. Comparison of FT-IR spectrum of Whatman filter paper strip alone and after absorption of silver nitrate (AgNO3) and gold chloride trihydrate (HAuCl4) was tabulated (Table S2). Several characteristic peaks pointed toward cellulose (a major component of paper) such as 2951, 1768, 1438, 1105 cm−1, these peaks were also observed after absorption of silver and gold salt solution on it, suggesting the property of Whatman filter paper to act as solid (inert) matrix facilitating nanosynthesis reaction platform.
Mechanism of Silver and Gold Nanoparticles Formation on Metal Salt Impregnated Paper Strip
Spotting of plant latex over silver nitrate impregnated paper strips resulted in a rapid color change of strips (from white to yellow) indicating silver nanoparticles formation over rigid cellulose paper matrix network due to the reduction of silver into nanosilver by biochemical’s present in latex. Characteristic FT-IR spectra exhibited shifting of peaks positions (Table S2, Fig. S2) and the emergence of new peaks suggested the role of different plant latex metabolites in the reduction of silver salt into nanosilver and stabilization of formed silver nanoparticles. The peak at 3483 cm−1 in paper-AgNO3 was shifted to 3470 cm−1 after silver nanoparticles formation pointing towards the role of OH group of protein and phenol present in latex in nanosynthesis. The emergence of a new peak at 3246 cm−1 suggests the presence of NH stretching due to the amide. The peak at 2951 cm−1 (C–H stretching) in paper-AgNO3 was shifted to 2955 cm−1 after silver nanoparticles synthesis. Silver nitrate alone is having a characteristic peak at 1396 cm−1 representing nitrate ion, but this peak was not observed in silver nanoparticles, this may be due to the removal of nitrate ion (Fig. S2).
Similar to that of silver nanoparticles, peak shifting in paper-gold chloride (HAuCl4) strip was observed after gold nanoparticles synthesis. The broad peak at 3520 cm−1 (O–H stretching vibration) was found to be nearly disappeared and was shifted to 3146 cm−1. New peaks at 3367, 3207, 3141 cm−1 pointed the role of N–H stretching vibration found in proteins (carboxylic acid, amines). Peak shifting from 1768 to 1759 cm−1 suggest O–H bending waves in phenols, proteins. The peak at 1641 cm−1 in gold chloride salt indicated Au3+ ion, and it was shifted to 1751 cm−1 in AuNPs showing the reduction of Au3+ to Au0 [23], while the peak at 1269 cm−1 denoted NH bending (Fig. S3).
FTIR spectroscopy of silver nanoparticles and gold nanoparticles formed on paper strip suggests a significant role of proteins, flavonoids, phenols of plants latex in nanosynthesis.
Conclusion
Owing to increasing pressure from regulatory agencies regarding the safety of nanomaterial, green chemistry perspective of nanosynthesis is the most potent answer to nanotoxicity issue. Currently, the available protocol to screen nanosynthesis potential of bio-agent (plant and microbes) is a tedious and time-consuming affair. In the present report, a rapid, one step paper-based indicator method to qualitatively analyze the potential of biological agents- plant latex for the synthesis of silver and gold nanoparticles is described. Paper strips are not only useful for checking nanoparticles synthesizing potential of the plants, but it also provides valuable primary knowledge about the possible size of the nanoparticles.
This tool is of particular interest for onsite detection of nano synthesis potential of vast biodiversity of plants before proceeding to precise nanofabrication and expensive characterization. This kind of paper strips could also be explored for the detection of nanomaterial synthesizing microorganisms. This technique could also apply to other commercially essential metals like chromium, titanium to develop rapid nanoparticle analyzing paper strips.
References
G. Benelli and C. M. Lukehart (2017). J. Clust. Sci. 28, 1–2.
K. Gopinath, S. Kumaraguru, K. Bhakyaraj, S. Mohan, K. S. Venkatesh, M. Esakkirajan, and G. Benelli (2016). Microb. Pathog. 101, 1–11.
G. D. Saratale, R. G. Saratale, G. Benelli, G. Kumar, A. Pugazhendhi, D. S. Kim, and H. S. Shin (2017). J. Clust. Sci. 28, 1709–1727.
L. M. Skjolding, S. N. Sørensen, N. B. Hartmann, R. Hjorth, S. F. Hansen, and A. A. Baun (2016). Angew. Chem. Chem. Int. Ed. 55, 15225–15239.
P. D. Shankar, S. Shobana, I. Karuppusamy, A. Pugazhendhi, V. S. Ramkumar, S. Arvindnarayan, and G. Kumar (2016). Enzym. Microb. Technol. 95, 28–44.
S. Vijayakumar, B. Vaseeharan, B. Malaikozhundan, N. Gopi, P. Ekambaram, R. Pachaiappan, and M. Suriyanarayanamoorthy (2017). Microb. Pathog. 102, 173–183.
P. Venkatachalam, T. Kayalvizhi, J. Udayabanu, G. Benelli, and N. Geetha (2017). J. Clust. Sci. 28, 607–619.
P. P. Gan and S. F. Y. Li (2012). Rev. Environ. Sci. Biotechnol. 11, 169–206.
H. P. Borase, C. D. Patil, R. B. Salunkhe, R. K. Suryawanshi, B. K. Salunke, and S. V. Patil (2014). Int. J. Cos. Sci. 36, 571–578.
P. C. Ray, H. Yu, and P. P. Fu (2009). J. Environ. Sci. Health Part C. 27, 1–35.
B. Park, P. Martin, C. Harris, R. Guest, A. Whittingham, P. Jenkinson, and J. Handley (2007). Part Fibre Toxicol. 4, 12.
P. Von Lode (2005). Clin. Biochem. 38, 591–606.
K. P. Madhwani (2013). Indian J. Occup. Environ. Med. 17, 87.
H. P. Borase, B. K. Salunke, R. B. Salunkhe, C. D. Patil, J. E. Hallsworth, B. S. Kim, and S. V. Patil (2014). Appl. Biochem. Biotechnol. 173, 1–29.
R. B. G. Kew The state of the world’s plants report–2016 (Royal Botanic Gardens, Kew, 2016), p. 2016.
D. D. Liana, B. Raguse, J. J. Gooding, and E. Chow (2012). Sensors. 12, 11505–11526.
W. Zhao, M. M. Ali, S. D. Aguirre, M. A. Brook, and Y. Li (2008). Anal. Chem. 80, 8431–8437.
R. Brause, H. Moeltgen, and K. Kleinermanns (2002). Appl. Phys. B Laser Opt. 75, 711–716.
S. Y. Oh, D. I. Yoo, Y. Shin, and G. Seo (2005). Carbon Res. 340, 417–428.
D. Paramelle, A. Sadovoy, S. Gorelik, P. Free, J. Hobley, and D. G. Fernig (2014). Analyst. 139, 4855–4861.
D. Huang, F. Liao, S. Molesa, D. Redinger, and V. Subramanian (2003). J. Electrochem. Soc. 150, G412–G417.
G. Peng, U. Tisch, O. Adams, M. Hakim, N. Shehada, Y. Y. Broza, S. Billan, R. Abdah-Bortnyak, A. Kuten, and H. Haick (2009). Nat. Nanotech. 4, 669–673.
M. Venkatachalam, K. Govindaraju, A. M. Sadiq, S. Tamilselvan, V. G. Kumar, and G. Singaravelu (2013). Spectrochim. Acta Part A Mole Biomol. Spectrosc. 116, 331–338.
Acknowledgements
Dr. Satish Patil is thankful to all mentors who encouraged for societal research with the low economic budget. SVP is also grateful to Prof. Ananda Mohan Chakrabarty, University of Illinois, Chicago and Prof. Ashok Mulchandani, University of California, Reverside, US for their constant inspiration and support. Authors are grateful to University Grants Commission (UGC) and Department of Science and Technology (DST) for making research facilities available under the UGC-SAP and DST-FIST programmes sanctioned to the School of Life Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declared that they have no competing interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Patil, S.V., Borase, H.P., Patil, C.D. et al. Fabrication of Paper Sensor for Rapid Screening of Nanomaterial Synthesizing Potential of Plants. J Clust Sci 29, 737–742 (2018). https://doi.org/10.1007/s10876-018-1396-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-018-1396-0