Abstract
The present paper accounts for the synthesis, crystal structure, differential scanning calorimetry and vibrational spectroscopy of a new compound (1,3,5-triazinidium-2,4,6-triamine) hexabromidodicuprate (II) grown at room temperature by slow evaporation of aqueous solution. From X-ray diffraction data collected at room temperature, it is concluded that it crystallizes in the monoclinic system (P21/c space group). The anion and the cation are linked by N–H···Br hydrogen bonds. Furthermore, the room temperature IR and Raman spectra of the title compound were recorded and analyzed. The differential scanning calorimetric has also been investigated. Hirshfeld surfaces were used to confirm the existence of inter-molecular interactions in the compound.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
An inorganic–organic hybrids material is a fertile topic of research merging the advantages of the organic components (straightforward synthetic approach, easily tailored molecular structure and functional properties) to those of an inorganic network (chemical, thermal and mechanical stabilities). The synthesis of molecular solids based on inorganic complex anions and organic cations have attracted much attention not only due to particular physical properties such as magnetism, ferroelectricity, nonlinear optical activity and conductivity but also for their capability of exploring the new fields of condensed physics and materials science [1–6].
Melamine (1,3,5-triazine-2,4,6-triamine) has wide applications in industry. Melamine resin is used in automobile paints [7]. Crystals of melaminium salts exhibit interesting properties. The second harmonic generation has been observed recently in case of tetrakis(2,4,6-triamino-1,3,5-triazin-1-ium)bis(selenate)trihydrate [8] and 2,4,6-triamino-1,3,5-triazin-1,3-ium tartrate monohydrate [9], while low temperature phase transitions were registered for melaminiumdiperchlorate hydrate and melaminium butyrate [10]. Lot of works was performed to explain the behavior of melamine molecule in the solid state [11–17].
Magneto-structural studies in dimeric A2Cu2X6 compounds have been researched extensively in the past [18–22]. These compounds typically form chains or stacks of the [Cu2X6]2− dimers based on the formation of one or two pairs of semi-coordinate bonds between adjacent dimers.
In this paper we are interested to study the synthesis, crystal structure, vibrational, differential scanning calorimetry (DSC) and Hirshfeld surfaces of the novel compound (1,3,5-triazinidium-2,4,6-triamine) hexabromidodicuprate (II).
Materials and Methods
Synthesis of [H2mela]Cu2Br6
The title compound was synthesized through the reaction of 0.25 g of melamine (1.98 mmol); 0.885 g of CuBr2 (3.96 mmol) in a few drops of concentrated HBr (38 %).The reaction mixture is stirred until homogenized. After some days, purple crystals of [H2mela]Cu2Br6 suitable for X-ray analysis were obtained from this aqueous solution by slow evaporation at room temperature.
X-ray Crystallography
A suitable single crystal of the title compound was chosen for the structure determination and refinement. It was selected under a polarizing microscope and was mounted on a glass fiber. The data collected at room temperature using a Bruker APEX II Kappa CCD diffractometer with graphite-monochromated MoKα radiation [23]. The total number of measured reflections was 6807 among which 3559 were independent and 2213 had intensity I > 2r (I).
Cuprate and bromine atoms were located using direct methods with the SHELXS-97 program [24], and non-H atoms were refined by full-matrix least-square method with anisotropic temperature factor using SHELXL-97 software [25]. The positions of all H-atoms, N1, N2, N4, N5 and N6 of organic entities, were fixed geometrically with suitable instructions of SHELXL-97 program. Crystal data and details on data collection and refinement are summarized in Table 1. The atomic coordinates and the displacement parameters are reported in Tables S1 and S2. DIAMOND-2 [26] package was used for molecular graphics.
Spectroscopic Measurements
Infrared spectroscopy is effectively used not only to identify the functional groups but also to determine the molecular structure of the synthesized compound. In order to qualitatively analyze the presence of functional groups in [H2mela]Cu2Br6, FT-IR spectrum was recorded by means of a JASCOFT-IR-420 spectrometer using a crystal sample in the range of 400–4000 cm−1. As for the Raman spectra, they were performed at room temperature using a Dilor XY set-up. The excitation line was 488 nm. The laser beam was focused on the sample through a × 50 Microscope Objective and the laser spot dimensions were around 10 μm2. Concerning the laser power, it was kept less than 5 mW so as to avoid the sample heating.
Differential Scanning Calorimetry
The sample was studied by differential scanning calorimetry (DSC) was recorded using a DSC 822 METTLER TOLEDO instrument for temperatures ranging from 20 to 350 °C at a constant rate of 10 K min−1 under a nitrogen atmosphere.
Results and Discussion
Structure Description
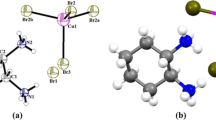
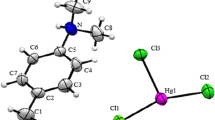
[H2mela]Cu2Br6 crystallizes in the monoclinic space group P21/c and its asymmetric unit contains one 1,3,5-triazinidium-2,4,6-triamine [H2mela]2+ cation and one [Cu2Br6]2− anions as shown in Fig. 1. The structural arrangement of [H2mela]Cu2Br6 can be described as an alternation of inorganic Cu2Br6 dimers and H-bonded dimers of [H2mela2+]2 (Fig. 2).
The Cu coordination geometries are best described as square-based pyramidal. The inorganic anion, illustrated in Fig. 3 consists of two crystallographically independent Cu atoms: Cu1 and Cu2. Both Cu atoms are in the square pyramidal sites, however, the material coordination environment of each Cu is distinct. The Cu1 and Cu2 atoms were surrounded by Br2, Br3, Br4, Br5, Br6 and Br1, Br2, Br3, Br4, Br6 respectively. The Cu1-Br and Cu2-Br bond lengths vary from 2.3962 (15) to 2.8683 (14) Å and from 2.3884 (15) to 2.9337 (14) Å, respectively. The Br-Cu1-Br and Br-Cu2-Br angles fall in the range 84.38 (5)–172.04 (6) and 84.53 (5)–174.82 (6)°. Selected bond lengths and angles are given in Table S3. The compound displayed a Cu–Cu distance of 3.659 Å, is comparable to corresponding bond lengths reported previously [27].
The ring C–N mean bond distances are in the range between 1.301 (1) and 1.376 (10) while the side chain C–N mean bond distances are in the range between 1.301 (13) and 1.317 (10) Å. The protonation of N1 an N2 leads the increase in C1–N1, C2–N1, C2–N2 and C3–N2 bond lengths that of the other ring C–N bonds. The protonation of melamine affect the side chain C1–N4, C2–N5 and C3–N6 bonds. The observed internal C1–N1–C2 (122.2°) and C2–N2–C3 (120.43°) angle at protanated N-atom is significantly greater than other two ring angles C1–N3–C3 (117.6°). It is worthwhile mentioning, that in recently determined structure of melaminium phthalate [28], melaminiumchloride hemihydrate [29] and bis(melaminium) sulphatedehydrate [30],the melaminium residues exhibit similar pattern of the internal C–N–C and N–C–N angles. Two H2mela2+ cations, each with two ring N sites protonated, form the [H2mela2+]2 dimer through the two intradimer N–H···N H-bonds in a R 22 (8) pattern [31–33] (Fig. 2), with N···N distances of 3.043 (10) Ǻ and N–H···N angles 179°. As for the crystal packing, the N–H···Br hydrogen bonds, whose geometrical parameters are reported in Table 2, play an important role in establishing the structure (see Fig. 4). The N···Br distances and the N–H···Br angles are in the ranges 3.310 (8)–3.620 (6) Å and 130–163, respectively.
Spectroscopic Study
Infrared Spectroscopy
To gain information on the crystal structure, we have carried out a vibrational study using infrared absorption. The infrared spectrum of the title compound [H2mela]Cu2Br6 recorded at room temperature is shown in Fig. 5. Based on some studies carried out for previous works and reported on similar compounds containing the same cations [34, 35], we propose an attempt of assignment of the observed bands.
The asymmetric and symmetric NH2 stretching is observed in the range 3380 and 3256 cm−1.
The bands at 2765 cm−1 is assigned to stretching vibrations of N–H band. The absorption at 1700 cm−1 is attributed to the deformation mode of (N–H). The bands at 1665 cm−1 in IR is assigned to -NH2 deformation mode. Side chain C–N stretching bands are assigned at 1509 cm−1. The band observed at 1403 cm−1 is assigned to C–N stretching vibration. The –NH2 rocking bands are observed at 1172 and 1118 cm−1. The C–N–C out of plane bending bands is assigned at 746 cm−1. The N–C–N out of plane band is assigned at 685 cm−1. In-plane bending C–N–C bands are assigned at 642 cm−1. In-plane bending N–C–N band is assigned at 523 cm−1. The interpretation of infrared spectra (Fig. 5) involves the correlation of absorption bands in the spectrum of [H2mela]Cu2Br6 compound with the spectrums of organic and hallogeno-organic absorption frequencies for types of bonds; can be attributed to the chemical environment change of (N–H) groups in the [H2mela]Cu2B6 compound. The major vibration bands (cm−1) and assignments of three compounds were listed in Table 3.
Raman Spectroscopy
The Raman spectrum of the title compound is illustrated in Fig. 6. In comparison with the literature for numerous bromocuprate (II) compounds containing [Cu2Br6] [36], the Raman spectrum of [H2mela]Cu2Br6 shows that the (asymmetric and symmetric) Cu–Br stretching vibrations should appear between 390 and 156 cm−1. The bands located at 136, 78 and 65 cm−1 are assigned to the bending vibrations of the [Cu2Br6]2− anion [δ (Br-Cu-Br)].
Differential Scanning Calorimetry Measurements
The thermal analysis results are reported in Fig. 7. The curve reveals a more intense endothermic peak at T = 265 °C. The corresponding reaction enthalpies are −1.4208 J g−1. The peak corresponded to the melting of the compound [H2mela]Cu2Br6.
Hirshfeld Surface Analysis
The Hirshfeld surfaces and the fingerprint analysis have been performed to study the nature of interactions and their quantitative contributions towards the crystal packing [37–43]. The value of d norm is negative or positive when intermolecular contacts are shorter or longer than rvdW [van der Waals (vdW) radii], respectively. The d norm values are mapped onto the Hirshfeld surface by using a red-blue-white colour scheme: where red regions correspond to closer contacts and negative d norm value; the blue regions correspond to longer contacts and positive d norm value; and the white regions correspond to the distance of contacts is exactly the vdW separation and with a d norm value of zero. The normalized contact distance (d norm) is based on both d e and d i.
The Hirshfeld surfaces mapped with d norm for the complexes are illustrated in Fig. 8. The Br···H and H···Br contacts combined, represented as points in the regions of bottom right (de < di, Br···H) and top left (de > di, H···Br) of the related plots in Fig. 9, comprise 46.3 % of the surface. So, the bromine and hydrogen atoms are often mutual partners in the crystal contacts and they are electrostatically favorable due to the partial positive charge, δ + of H atoms. The H···H contacts have important interactions due to the abundance of hydrogen on the molecular surface (7 %). The decomposition of the fingerprint plot shows that Cu···Br/Br···Cu contacts comprise 13.1 % of the total Hirshfeld surface area. The proportions of N···Br/Br···N and Br···Br interactions are 11.1 and 7.9 %.
Conclusion
Organic–inorganic single crystal of [H2mela]Cu2Br6 was grown by the slow evaporation method. The crystal structure of [H2mela]Cu2Br6 was reported for the first time. This compound crystallized in the monoclinic system with P21/c space group. The atomic arrangement consists of [Cu2Br6]2− anions and 1,3,5-triazinidium-2,4,6-triamine cation connected by N–H···Br hydrogen bonds. The organic cations are interconnected by N–H···N hydrogen bonds. The vibrational properties of this structure were studied by infrared and Raman spectroscopies. The assignment of the vibrational bands was performed by comparison with the vibration modes frequencies of homologous compounds. The DSC thermal analysis was performed to establish the thermal stability of the crystal. The Hirshfeld surface analysis reveals the percentage of intermolecular contacts of the title compound.
Appendix A Supplementary material
CCDC 1456537 contains the supplementary crystallographic data for [H2mela]Cu2Br6. These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html, or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: (+44) 1223 336 033; or email: deposit@ccdc.cam.ac.uk.
References
H. Zhang, X. M. Wang, K. C. Zhang, and B. K. Teo (1999). Coord. Chem. Rev. 183, 157.
T. Akutagawa and T. Nakamura (2002). Coord. Chem. Rev. 226, 3.
N. Robertson and L. Cronin (2002). Coord. Chem. Rev. 227, 93.
K. Wang and E. I. Stiefel (2001). Science 291, 106.
A. Shapiro, C. P. Landee, M. M. Turnbull, J. Jornet, M. Deumal, J. J. Novoa, M. A. Robb, and W. Lewis (2007). J. Am. Chem. Soc. 129, 952.
L. Liu and M. M. Turnbull (2007). Inorg. Chem. 46, 11254.
J. Zieba-Palus (1999). J. Mol. Struct. 511, 327.
M. K. Marchewka, J. Janczak, S. Debrus, J. Baran, and H. Ratajczak (2003). Solid State Sci. 5, 643.
M. K. Marchewka, J. Baran, A. Pietraszko, A. Haznar, S. Debrus, and H. Ratajczak (2003). Solid State Sci. 5, 509.
M. K. Marchewka and H. Ratajczak (2002). Bull. Pol. Acad. Sci. Chem. 3, 335.
R. J. Meier, A. Tiller, and S. A. M. Vanhommerig (1995). J. Phys. Chem. 99, 5457.
W. J. Jones and W. J. Orville-Thomas (1959). Trans. Faraday Soc. 55, 203.
P. J. Larkin, M. P. Makowski, N. B. Colthup, and L. A. Food (1998). Vib. Spectrosc. 17, 53.
R. J. Meier, J. R. Maple, M. J. Hwang, and A. T. Hagler (1995). J. Phys. Chem. 99, 5445.
J. R. Schneider and B. Schrader (1975). J. Mol. Struct. 29, 1.
P. J. Larkin, M. P. Makowski, and N. B. Colthoup (1999). Spectrochim. Acta 55A, 1011.
M. P. Fernandez-Liencres, A. Navarro, J. J. Lopez-Gonzalez, M. Fernandez-Gomez, J. Tomkinson, and G. J. Kearley (2001). Chem. Phys. 266, 1.
R. D. Willett (1986). Inorg. Chem. 25, 1918.
R. D. Willett, T. Grigereit, K. E. Halvorson, and B. Scott (1987). Proc. Indian Acad. Sci. 98, 147.
B. Scott and R. D. Willett (1987). J. Appl. Phys. 61, 3289.
B. Scott and R. D. Willett (1988). Inorg. Chim. Acta 141, 193.
T. Grigereit, J. E. Drumheller, B. Scott, G. Pon, and R. D. Willett (1992). J. Magn. Magn. Mater. 1, 104.
Bruker, APEX2 and SAINT. Bruker AXS Inc, Wisconsin, USA, Madison, 2009.
G. M. Sheldrick (2008). SHELXS-97. Acta cryst. 64, 112.
G. M. Sheldrick (2008). SHELXL-97. Acta cryst. 64, 112.
K. Brandenburg, DIAMOND 2.0, Visual Crystal Structure Information System, Crystal Impact Gbr, Bonn, Germany, 2007.
A. M. B. Salah, H. Naïli, M. Arczy_nski, and M. Fitta (2016). J. Organomet. Chem. 805, 42.
J. Janczak and G. J. Perpetuo (2001). Acta Cryst. C57, 123.
J. Janczak and G. J. Perpetuo (2001). Acta Crystallogr. C 57, 1120.
J. Janczak and G. J. Perpetuo (2001). Acta Crystallogr. C 57, 1431.
J. Bernstein, R. E. Davis, L. Shimoni, and N.-L. Chang (1995). Angew. Chem. Int. Ed. 34, 1555.
T. Steiner (2002). Angew. Chem. Int. Ed. 41, 48.
G. R. Desiraju and T. Steiner The Weak Hydrogen Bond In Structural Chemistry and Biology (Oxford University Press, New York, 1999).
V. Arjunan, M. Kalaivani, M. K. Marchewka, and S. Mohan (2013). Spectrochim. Acta Part A 107, 90.
V. Sangeetha, M. Govindarajan, N. Kanagathara, M. K. Marchewka, S. Gunasekaran, and G. Anbalagan (2014). Spectrochim. Acta Part A 125, 252.
Abderrazek Oueslati, Alain Buloub, Florent Calvayrac, Karim Adi, Mohamed Gargouri, and Faouzi Hlel (2013). Vib. Spectrosc. 64, 10.
S. K. Seth (2014). Inorg. Chem. Commun. 43, 60.
M. Mitra, P. Manna, A. Bauza, P. Ballester, S. K. Seth, S. Ray Choudhury, A. Frontera, and S. Mukhopadhyay (2014). J. Phys. Chem. B 118, 14713.
S. K. Seth, D. Sarkar, A. Roy, and T. Kar (2011). CrystEngComm. 13, 6728.
S. K. Seth, D. Sarkar, A. D. Jana, and T. Kar (2011). Cryst. Growth Des. 11, 4837.
M. Mitra, S. K. Seth, S. R. Choudhury, P. Manna, A. Das, M. Helliwell, A. Bauza, A. Frontera, and S. Mukhopadhyay (2013). Eur. J. Inorg. Chem. 2013, 4679.
S. K. Seth (2014). J. Mol. Struct. 1070, 65.
P. Manna, S. Ray Choudhury, M. Mitra, S. K. Seth, M. Helliwell, A. Bauza, A. Frontera, and S. Mukhopadhyay (2014). J. Solid State Chem. 220, 149.
Acknowledgments
The authors thank the members of units of common services, at the University of Sfax for their assistance in the measurements for X-ray diffraction. The authors are also thankful to Prof Hamadi KHEMAKHEM, for his co-operating in the Raman spectroscopy measurement.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mesbeh, R., Hamdi, B. & Zouari, R. Elaboration, Structural, Vibrational, DSC Investigations and Hirshfeld Surface Analysis of New Organic–Inorganic Hybrid Compound: [H2mela]Cu2Br6 . J Clust Sci 27, 1751–1762 (2016). https://doi.org/10.1007/s10876-016-1041-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-016-1041-8