Abstract
Pure barium ferrite and BaFe10.5−x Al1.5Cr x O19 (x = 0, 0.5, 1.0, 1.5, 2.0) nanoparticles were synthesized by auto-combustion sol–gel method. The effect of substituting Fe3+ ions by Al3+ and Cr3+ ions on the structural, chemical composition, morphology and magnetic properties of BaFe12O19 and BaFe10.5−x Al1.5Cr x O19 hexaferrites have been investigated using X-ray diffraction, Fourier transform infrared spectroscopy, field emission scanning electron microscopy and vibrating sample magnetometer (VSM), respectively. The results confirmed that the hexagonal phase has been formed in all of nanoparticles and the value of lattice parameter (c) decreased, while the value of lattice parameter (a) remained nearly constant with the increase in Cr3+ ions contents in comparison to pure barium ferrite. The crystallite size and mean particle size of the nanoparticles lie in the ranges 33–42 and 35–70 nm, respectively. The VSM measurements show that with substituting Al and Cr cations in the hexagonal structure of barium ferrite, the saturation magnetization (M s ) and remanence magnetization (M r ) of the nanoparticles reduce, while coercive field (H c ) increased from 4.45 kOe for pure barium ferrite to 6.9 kOe for BaFe10.5Al1.5O19 (x = 0) due to the increase in the magnetocrystalline anisotropy and the decrease of the particle size.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The M-type hexagonal hard ferrites BaFe12O19 with magnetoplumbite structure have been discovered in the 1950s and they have many interest for low costs and their specific magnetic properties like high coercive field, their high magnetization, high Curie temperature, excellent chemical stability, mechanical strength and corrosion resistivity [1–3]. Due to their properties, these ferrites have many applications such as data storage and recording, permanent magnets, electrical and microwave devices, plastoferrites, RAM and microwave/EM wave absorption, magnetoelectric (ME) and multiferroic (MF) applications and chip inductors [1, 2].
As it is well known that the magnetic properties can be changed by substitution for the Fe3+ with divalent, trivalent and tetravalent ions in crystallographic sites which are three octahedral (2a, 4f2 and 12k), one trigonal bipyramidal (2b) and one tetrahedral (4f1) [4–6]. One of the most important of magnetic properties for achievement to above mentioned applications is coercive field (H c ). The coercive field can be remarkably changed with changing of magnetocrystalline anisotropy field and superexchange interaction [4, 5, 7–9]. When trivalent ions (like Al3+ and Cr3+) are entered to the lattice of BaFe12O19, the coercive field could be increased due to the substitution of these ions instead of Fe3+ ions by effect on magnetocrystalline anisotropy field and superexchange interaction. On the other hand, with substituting Al3+ and Cr3+ ions in crystal structure of barium ferrite, it can be reduced particles size and lead to increase of the coercive field [10–12].
In the current study, the BaFe10.5−x Al1.5Cr x O19 (x = 0, 0.5, 1.0, 1.5, 2.0) nanoparticle were synthesized via auto-combustion sol–gel method. Then, the structural characteristics and magnetic properties of substituted barium ferrite were investigated and discussed. The substituted hexagonal nanoparticles have been characterized using the X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR) and field emission scanning electron microscopy (FE-SEM). The vibrating sample magnetometer (VSM) were also used to investigate the effect of Al3+ and Cr3+ ions substitution contents on magnetic properties of barium ferrite.
Materials and Methods
Preparation of Nanoparticles
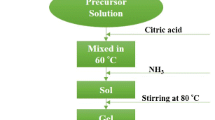
The raw materials used in the present work were purchased from Merck and were used as received. The pure barium hexaferrite and BaFe10.5−x Al1.5Cr x O19 nanoparticles with different composition (x = 0, 0.5, 1.0, 1.5, 2.0) were prepared via auto-combustion sol–gel method. A stoichiometric amounts of Ba(NO3)2, Fe(NO3)3·9H2O, Al(NO3)3·9H2O and Cr(NO3)3·9H2O were dissolved in deionized water and the mixture was vigorously stirred on a hotplate in 60 °C. Citric acid was weighed as a molar ratio of metal ions to citric acid of 1:5 and add into the solution. After dissolving the nitrate salts completely, the solution were allowed to cool down to room temperature and then ammoniac was added to adjust the pH value to ca. 7.0. These solution were slowly evaporated on a hotplate at 80 °C until a highly viscous residue dried gels were formed and then the viscous residue dried gels were heated at 250 °C to ignite with the formation of large amounts of gas, resulting in light weight voluminous powder. Before annealing, the resulting precursor powders was preheated to 450 °C for 1 h to decompose the organic precursor and volatile gases. Finally, the resulting precursor powders were calcined at 950 °C for 2 h. The procedure for the synthesis of pure and substituted barium hexaferrite has been illustrated in Fig. 1.
Materials Characterization
For structural analysis, X-ray diffraction measurements were performed. A Phillip X’pert diffractometer (Model MPD-XPERT) was used carry out the experiment. A CuKα beam having wavelength (λ) = 1.54 Å was used as the radiation source. The diffractometer was operated at 40 kV and 30 mA using a scanning step of 0.03 in 2θ and a dwell time of 1 s was used. The 2θ angle was varied from 10° to 100°. The FTIR spectra for all samples were characterized using a Bruker TENSOR 27 infrared spectrophotometer. The IR absorbance was measured in a range between 4000 and 400 cm−1. The morphology, microstructure and chemical composition of the substituted hexaferrite nanoparticles were examined using a field emission scanning electron microscope (FE-SEM; Model Mira3-XMU, TESCAN) and energy dispersive spectroscopy with 15 kV voltages, respectively. The magnetic properties of the ferrite nanoparticles such as saturation magnetization (M s ), remanence magnetization (M r ), and the coercive field (H c ) were calculated from the M–H loops, measured with a vibrating sample magnetometer (VSM; Model Kavir Magnet, Iran) in a maximum applied field of 15 kOe at room temperature.
Results and Discussion
Structural Characteristics
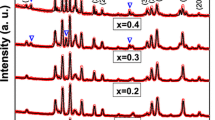
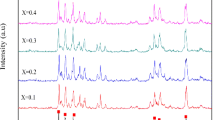
Figure 2 shows the X-ray diffraction patterns of pure barium ferrite and BaFe10.5−x Al1.5Cr x O19 nanoparticles with various Cr3+ ions contents calcined at 950 °C for 2 h. It can be seen that the positions and relative intensities of these nanoparticles peaks match well with the (110), (107), (114), (203), (205), (217) and (2011) planes and belong to the magnetoplumbite barium ferrite as revealed in the PDF. No. 01-078-0133. The powder XRD patterns of BaFe12O19 and BaFe10Al1.5Cr0.5O19 (x = 0.5) exhibited magnetoplumbite phase with undesired diffraction peak related to α-Fe2O3 phase (PDF. NO. 01-085-0599 and PDF. No. 01-084-0307, respectively). This is attributed to the incomplete reaction or blending of raw materials between Fe3+ and Ba2+ under synthesis conditions and due to the α-Fe2O3 is similar to FeTiO3 structure which belongs to trigonal crystal structure having orthorhombic hexahedron crystal cell, it is difficult to transform the α-Fe2O3 into magnetoplumbite phase [9]. In the other substituted nanoparticles, this phase was not observed, but with increasing substitution of Cr3+ ions contents from 1.0 to 2.0, the XRD patterns of substituted nanoparticles reflect extra peaks at 27.969°, 32.288° and 33.137° which is the typical Bragg peaks of BaFe2O4 intermediate phase as revealed in the PDF. No. 01-077-2337. The presence of BaFe2O4 intermediate phase reflects that both the Al3+ and Cr3+ ions contents substitution leading to lack of γ-Fe2O3 intermediate phase which can react with BaFe2O4 phase to form the BaFe12O19 phase, hence the amount of BaFe2O4 phase to remain unreacted [4]. The complete reaction proceeds as follow:
The lattice parameters of these nanoparticles (a and c) and unit cell volume (Vcell) have been calculated using the following equations [11].
where d(hkl) is the d-spacing value and (hkl) is the miller indices. Figure 3 (a and c) represents the lattice parameters and unit cell volume resulting from pure barium ferrite and substituted hexaferrite BaFe10.5−x Al1.5Cr x O19 nanoparticles. As it clear, the values of lattice parameter (c) and unit cell volume decreased with increasing the substitution contents in comparison to BaFe10.5Al1.5O19 (x = 0), while the value of lattice parameter (a) remained nearly constant with the increase in Cr3+ ions contents. On the contrary substitution of Al3+ that will change both the a and c, Cr3+ substitution in the M-type ferrite mainly affect the c lattice parameter [5, 13]. Also, it can be seen that except BaFe12O19 and BaFe8.5Al1.5Cr2O19 (x = 2), the contraction of crystal axis (c/a) ratio is nearly constant. These results could be due to smaller ionic radii of substituted Al3+ (0.535 Å) and Cr3+ (0.615 Å) ions in comparison to ionic radius of Fe3+ ions (0.645Å). Due to the solubility of Al3+ and Cr3+ ions in the M-type hexaferrite lattice, the cell dimensions could be reduced which is in agreement with the previous literature in this field [5, 11, 12].
The Scherrer’s formula was used to obtain the values of crystallite size as shown in Fig. 3b.
where λ is the X-ray wavelength, β is the full-width at half max and θ is the Bragg angle [11]. As it can be seen from Fig. 3b, with substitution of Cr3+ ions in barium hexaferrite lattice, the crystalline size of substituted barium ferrite reduce in comparison to pure barium ferrite. In fact, when the unit cell volume reduce due to substitute Al3+ and Cr3+ ions in the lattice of BaFe12O19, during the crystal growth process, the mass transportation through adjacent crystallite is reduced and cause to a decrease in the crystallite size [14].
The investigations of chemical structure changes of a precursor powder, pure barium ferrite and the substituted hexaferrite nanoparticles were carried out by FTIR, and the FTIR spectra at the range of wave number 400–4000 cm−1 are shown in Fig. 4. The main features of the spectra include bands corresponding to barium hexaferrite and OH group. The bands in the range 420–450 and 540–615 cm−1 are corresponding to stretching vibration of metal–oxygen bond indicating the formation of hexaferrite structure including octahedral and tetrahedral sites, respectively [9, 15, 16]. On the other hand, the wavenumber being inversely proportional to the atomic weight, hence with increasing the substitution of Al3+ and Cr3+ ions contents, the peaks shift to a higher wavenumber (from 582 to 615 cm−1) due to Fe3+ ions with a heavier atomic weight than Al3+ and Cr3+ ions and it confirms that the Al3+ and Cr3+ ions are entered to the lattice of BaFe12O19 [14]. The absorption bonds around 1623 cm−1 can be attributed to O–H bending band of the H2O molecules chemically adsorbed to the magnetic particle surface. The spectra exhibit an O–H stretching mode around 3200–3500 cm−1 which is represented by a broad band, where freely vibrating OH groups and hydrogen bonded OH groups are apparent [15, 16]. The peaks around 2850 and 2925 cm−1 corresponding to the presence of C-H stretching [10].
Figure 5 shows the FE-SEM micrographs of the pure barium ferrite and substituted hexaferrite BaFe10.5−x Al1.5Cr x O19 (x = 0–2) nanoparticles with various Cr3+ ions contents calcined at 950 °C for 2 h. As it can be seen, in all of the samples, the nanoparticles have been distributed homogeneously and closely packed throughout the surface of sample and the hexagonal structure is clear. As shown in Fig. 6, with substituting Cr3+ ions in the hexagonal structure of barium ferrite the mean particle size of BaFe10.5−x Al1.5Cr x O19 (x = 0–2) along c-axis are less than 55 nm and reduced in comparison to BaFe12O19. It can be attributed to the presence of Al3+ and Cr3+ ions on the grain boundaries which lead to hindering of growth in some of the crystallographic planes and encouraging of growth in other directions. On the other hand, with increasing substitution of Cr3+ ions, the morphology of nanoparticles are turning from nanospherical shape (equiaxed morphology) to nanodisk and nanorods (35–70 nm).
The elemental analysis were carried out by the energy-dispersive spectroscopy (EDS) to confirm the chemical composition of the substituted hexaferrite nanoparticles and the corresponding results are presented in Table 1. It is evident from the elemental analysis that the atomic percentage (at.%) of Ba, Fe, Al and Cr in the BaFe10.5−x Al1.5Cr x O19 nanoparticles basically agree with the designed composition and the stoichiometric Cr3+ ions substitution contents were increased while the Fe3+ ions contents decreased. Figure 7 shows the typical EDS spectra of the pure barium ferrite and substituted hexaferrite nanoparticles.
Magnetic Properties
The magnetic properties of the BaFe12O19 and substituted hexaferrite BaFe10.5−x Al1.5Cr x O19 nanoparticles with different Cr3+ ions contents were measured at a maximum applied of 15 kOe at room temperature. The typical hysteresis loops (M-H curves) of the nanoparticles are plotted in Fig. 8 and the magnetic parameters are presented in Table 2. The amount of substituting has a significantly influence on magnetic properties. As it can be observed in Fig. 9, in comparison to pure barium ferrite, the saturation magnetization (M s ) and remanence magnetization (M r ) is decreased, but the coercive field (H c ) did not have any specific trend with an increase in the amount of Cr3+ ions substitution in the composition. The decrease in M s and M r with chemical composition can be justified based on the different sites that occupied by substituted Al3+ and Cr3+ ions on the structure of barium hexagonal ferrite. The Al3+ (0 μ B ) and Cr3+ (3 μ B ) ions are mainly substituted Fe3+ (5 μ B ) ions in 12 k sites (upward spin) and lead to a reduction in M s and M r synthesized nanoparticles. On the other hand, small amounts of chromium substitution can be increased in saturation magnetization due to the number of substituted Cr3+ ions in 4f2 sites (downward spin) is more than 12 k and 2b sites (upward spin). It could be the reason for the higher saturation magnetization of small substituted Cr3+ nanoparticles (x = 0.5) in comparison to other substituted Cr3+nanoparticles. Also, the substitution of Fe3+ ions by Al3+ and Cr3+ ions lead to reduce superexchange interaction between Fe3+-O-Fe3+ and it may be caused to a non-collinear spin arrangement (spin canting) [5, 11, 12]. Moreover, the presence of impurity like α-Fe2O3 (in pure barium ferrite and BaFe10Al1.5Cr0.5O19) and BaFe2O4 (x > 0.5) have a significant influence on decrease in M s and M r . It is possible to reduce the particle size lower than single domain critical size, hence the nanoparticles show paramagnetic behavior with increasing the substitution of Cr3+ ions in the crystal structure of barium ferrite, as well as substituting Al3+ ions. Therefore the saturation magnetization and remanence magnetization are reduced.
It is observed from Fig. 9 and Table 2, the H c values of Cr3+ substituted barium hexaferrite did not have any specific trend and the BaFe10.5Al1.5O19 (x = 0) nanoparticle shows the highest coercivity field (6933 Oe) in comparison to pure barium ferrite and other substituted nanoparticles. The coercive field is strongly dependent on particle size and magnetocrystalline anisotropy. As concluded from FE-SEM data (Figs. 5, 6), the mean particle size of substituted barium ferrite (about 50 nm) was lower than critical diameter of the spherical M-type hexaferrite with single magnetic domain, hence all of substituted nanoparticle consist of single magnetic domains and they can act as a barrier to the movement of the domain walls, which leading to an increase in the H c . But the H c values have been decreased in BaFe10Al1.5Cr0.5O19 (x = 0.5) and BaFe9Al1.5Cr1.5O19 (x = 1.5) in comparison to pure barium ferrite. It can be attributed to simultaneous substitution of Al3+and Cr3+ ions in the structure of barium hexaferrite which leading to a decrease in particle size of nanoparticles lower than superparamagnetic area, so the hysteresis loops of nanoparticle with x = 0.5 and x = 1.5 have a mixture of ferrimagnetic and paramagnetic component of the sample [5, 17].
As it is well known, in according to H c = 2K 1 /M s (where H c is the coercive field, K 1 is anisotropic constant and M s is saturation magnetization), H c and M s are inversely proportional [4, 10]. However, as it can be observed from Table 2, the H c values not be increased with decreasing M s at Cr3+ substituted nanoparticles (except at x = 1). This result could be explained by reduction in magnetocrystalline anisotropy. Concerning the magnetic anisotropy, the Al3+ ions can occupied 2a, 4f1, 4f2 and 12 k sites, so it has no significant effect on magnetocrystalline anisotropy, while the M s values of substituted hexaferrite have been dramatically decreased [5]. On the other hand, there is strong competition between Cr cations and Al cations for substituting at these sites. Therefore, when one cation substituted at these sites (e.g. Al), another cation (e.g. Cr) may be substituted at the other sites (that have a significant effect on magnetocrystalline anisotropy), which lead to decrease in magnetocrystalline anisotropy and consequently decrease in H c .
Another factor that plays a role in the magnetocrystalline anisotropy changes, is 2b site in the crystal structure of M-type ferrites. The Al3+ ions (in small substitution, x = 1.5) are not replaced Fe3+ ions at trigonal bipyramidal 2b sites, thus it has a very little effect on the magnetiocrystalline anisotropy [5, 18, 19]. However, due to the presence of Cr3+ ions in addition to Al3+ ions in chemical composition, Cr3+ ions can occupy sites in the lattice that could already be occupied by Al3+ ions, thereby the Al3+ ions may be occupied 2b sites and lead to decrease in magnetiocrystalline anisotropy and coercive field. The ratio of c/a is also another factor that affected on coercive field. It can be seen from Fig. 3b that the ratio of c/a decrease with increasing substitution of Cr3+ ions due to the value of lattice parameter (c) decreased and the value of lattice parameter (a) remained nearly constant. This can reduce the H c values in the rotation magnetization process [13].
Conclusion
Pure barium ferrite and substituted Cr3+ nanoparticles of BaFe10.5−x Al1.5Cr x O19 (x = 0–2) have been successfully fabricated via auto-combustion sol–gel method. It was revealed from XRD patterns and FE-SEM micrographs that in all of nanoparticles the hexagonal phase has been formed and the value of lattice parameter (c) decreased, while the value of lattice parameter (a) remained nearly constant with the increase in Cr3+ ions contents. The crystallite size and mean particle size of the nanoparticles lie in the ranges 33–42 and 35–70 nm, respectively. The formation of hexaferrite structure including octahedral and tetrahedral sites was confirmed by FTIR. The saturation magnetization (M s ) has been dramatically decreased from 71 to 3.85 emu/g due to the reduction of the superexchange interaction between Fe3+-O-Fe3+ and the non-collinear spin arrangement magnetic moment, while coercive field (H c ) increased from 4.45 kOe for pure barium ferrite to 6.9 kOe for BaFe10.5Al1.5O19 (x = 0). The enhancement in coercivity field may be explained on the basis of the increase in the magnetocrystalline anisotropy and the decrease of the particle size. In the others substitution of Cr3+ ions, the H c values decreased in comparison to BaFe10.5Al1.5O19.
References
R. C. Pullar (2012). Prog. Mater. Sci. 57, 1191.
M. V. Kuznetsov, L. F. Barquín, Q. A. Pankhurst, and I. P. Parkin (1999). J. Phys. D 32, 2590.
A. Kaewrawang, A. Ghasemi, X. Liu, and A. Morisako (2009). J. Magn. Magn. Mater. 321, 1939.
C. Li, B. Huang, and J. Wang (2013). J. Mater. Sci. 48, 1702.
H. Luo, B. K. Rai, S. R. Mishra, V. V. Nguyen, and J. P. Liu (2012). J. Magn. Magn. Mater. 324, 2602.
A. Hojjati Najafabadi, R. Mozaffarinia, and A. Ghasemi (2015). J. Supercond. Nov. Magn. 28, 2821.
B. K. Rai, S. R. Mishra, V. V. Nguyen, and J. P. Liu (2013). J. Alloys Compd. 550, 198.
J. Luo (2012). Mater. Lett. 80, 162.
A. Thakur, R. R. Singh, and P. B. Barman (2013). Mater. Chem. Phys. 141, 562.
M. Asghari, A. Ghasemi, E. Paimozd, and A. Morisako (2013). Mater. Chem. Phys. 143, 161.
I. Ali, M. U. Islam, M. S. Awan, and M. Ahmad (2013). J. Alloys Compd. 547, 118.
S. Ounnunkad, P. Winotai, and J. Magn (2006). Magn. Mater. 301, 292.
Q. Fang, H. Cheng, K. Huang, J. Wang, R. Li, and Y. Jiao (2005). J. Magn. Magn. Mater. 294, 281.
M. Liu, X. Shen, F. Song, J. Xiang, and X. Meng (2011). J. Solid State Chem. 184, 871.
F. Khademi, A. Poorbafrani, P. Kameli, and H. Salamati (2012). J. Supercond. Nov. Magn. 25, 525.
A. Thakur, R. R. Singh, and P. B. Barman (2013). J. Magn. Magn. Mater. 326, 35.
A. Roberts, Y. Cui, and K. L. Verosub (1995). J. Geophys. Res. 100, 17909.
Y. Xu, G. L. Yang, D. P. Chu, and H. R. Zhai (1990). J. Physica Status Solidi B 157, 685.
G. Albanese, M. Carbucicchio, and A. Deriu (1973). J. II Nuovo Cimento B 15, 17.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hojjati Najafabadi, A., Ghasemi, A. & Mozaffarinia, R. Synthesis and Evaluation of Microstructural and Magnetic Properties of Cr3+ Substitution Barium Hexaferrite Nanoparticles (BaFe10.5−x Al1.5Cr x O19). J Clust Sci 27, 965–978 (2016). https://doi.org/10.1007/s10876-015-0963-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-015-0963-x