Abstract
This paper studies the effects of Ag atomic segregation from the inner (100) or (111) planes on the melting of Ag–Pd clusters with different sizes by a molecular dynamics simulation. The results show that Ag segregation leads to the atomic energy decreases with increasing the temperature. Furthermore, the effect of the (100) segregation is larger than that of the (111) segregation. Meanwhile, the influence of segregation on the energy decreases with increasing the cluster size. The melting points of the clusters without segregation are the largest, followed by the clusters with a (111) and (100) segregation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alloy cluster exhibits unique properties due to the synergy effects of alloy elements and has attracted much attention [1]. Their large surface-volume ratio and states of electrons differing from bulk make bimetallic clusters have fascinating potential applications in the fields of heterogeneous catalysts, microelectronic, and optoelectronic devises [2]. However, the element with a low surface energy would segregate to the surface of clusters during the fabrication and application processes. When the temperature is higher than a given temperature, Pt atoms begin to partially alloy with the surface atoms although the most stable position is in the inner of the Pt–Ag core–shell cluster [3]. This would deteriorate the structure and leads to the failure of device. Therefore, it is essential to explore the segregation in alloy clusters.
Since the experiment is very sophisticated, it is almost impossible to study the atomic segregation only by experimental method. Computer simulation has become an effective method to study the processes related to alloy clusters. The current studies have proved that segregation phenomena existed in almost all the alloy clusters. For instance, Pd atoms penetrated into the Ag cluster and preferentially segregated at the subsurface layer of the cluster [4]. The difference of surface energies between Ag and Co has made Ag-Co cluster form the geometries of Janus-like and core–shell structure [5]. Surface nucleation was inhibited when Al atoms segregated to Ni–Al surface and formed a pure Al surface. This leaded to the formation of amorphous Ni–Al cluster [6]. Ag atomic segregation induced icosahedral Ag–Pd cluster to transform to decahedron [7]. Furthermore, atomic segregation induced the irregular variation of atomic energy, namely, the atomic energy increased, remained unchanged or decreased with increasing the temperature [8, 9]. In addition, it has been known that the structures and properties of clusters are closely related to their sizes [10, 11]. The frozen structures of alloy clusters were size and composition dependence [12]. Therefore, it is necessary to probe the size-dependent atomic segregation in alloy clusters. Moreover, the atomic nearest neighbors are different when the atoms distribute different planes, such as (100) and (111). This leads to that the energy barriers overcame by the segregated atoms are also different. This means that the energy variations are different when the atoms segregate to the surface from different planes. However, less attention has been paid to this.
In this study, in order to explore the effect of atomic segregation from different planes on the melting, Ag–Pd clusters with different sizes and also atomic distributions in the (100) or (111) planes were artificially constructed as the projects. Then, molecular dynamics simulation with a general embedded atom method was employed to study the effects of Ag atomic segregation from different planes on the size-dependent melting of Ag–Pd clusters. Finally, the effects of atomic segregation on the melting point, atomic energy, and atomic distribution were explored.
Simulation Details
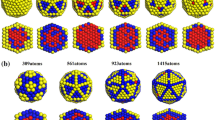
Molecular dynamics simulation with a general embedded atom method was used to study the melting. The accuracy and details were displayed in our previous studies [7, 13, 14]. Four kinds of cluster sizes were selected in this study. The atomic numbers in the clusters are 490, 826, 1288, and 1896, respectively. Since truncated octahedron are mainly composed by the (111) and (100) planes, the initial geometry of the clusters in this study is truncated octahedral like. Ag content in each cluster is about 30 % in atomic number. In order to study various segregations, four kinds of Ag atomic distributions were considered. (1) Ag atoms only distribute in the inner (111) (Fig. 1a) or (100) (Fig. 1b) planes. For these two cases, Ag atomic segregation only occurs in the (111) or (100) planes. (2) Ag atoms distribute randomly in various positions, namely, mixed clusters, as shown in Fig. 1c. In these clusters, Ag atoms will segregate to the surface from both the (111) and (100) planes. (3) Ag atoms only distribute in the surface layer, namely, Ag surface cluster (Fig. 1d). In this case, there would be no Ag atomic segregation. By comparing the melting among these clusters with different sizes and Ag atomic distributions, the effects of Ag atomic segregation from the (111) and (100) planes on the size-dependent melting of Ag–Pd clusters can be defined. Figure 1 shows the initial geometries of four kinds of (AgPd)1896 clusters. In this study, the clusters were heated up to 1400 from 500 K to ensure the cluster completely melted. The temperature step is 20 K and the relaxation time is 0.2 ns. The time step is 1 fs. The surface atoms are defined that the atom has a coordination number lower than 10.
Results and Discussion
Figure 2 shows the temperature–energy curves which were calculated to explore the energy variations during the melting processes. It can be seen that the melting curves are similar. The lowest atomic energy at 500 K is the cluster with Ag atoms in the surface and in turn the mixed, Ag in the (100) and (111) planes. This implies that the most stable position of Ag atoms is in the surface. When Ag atoms are in the inner of the cluster, the case of Ag in the (100) planes is more stable than that in the (111) planes. It also found that the atomic energy does not monotonously increase with the increase of the temperature except for the cases of Ag surface clusters with different sizes. After the clusters are melted, the atomic energies are identical. This indicates that the atomic distributions are same for the melted clusters. Additionally, the melting is different with the change of Ag atomic distributions. For the Ag surface clusters, since there is no segregation, the atomic energy increases with increasing the temperature. There is a sharp increase of energies which indicates the melting of the cluster. While for the clusters with Ag atoms in the (100) and (111) planes, the tendency of atomic energy variation with the temperature are similar, which are different from the melting of Ag surface clusters because the segregation occurs. For the mixed clusters, the effect of segregation on the energy variation is not obvious as the clusters with Ag in the planes because there are some Ag atoms on the surface for the mixed clusters. The atomic energy at 500 K reduces with the increase of cluster size. In addition, the energy reduction with the increase of the temperature is significant at the initial stage of segregation for (AgPd)490 cluster. However, the reduction amount due to atomic segregation decreases with the increase of cluster size. This means that the effect of atomic segregation on the energy is size-dependent.
In order to confirm whether atomic segregation affects the melting or not, the atomic segregation rates were calculated by using the segregated atomic number versus the total Ag atomic number. Figure 3 shows the segregation rates of Ag atoms from the inner (100) planes to the surface for the clusters with different sizes and corresponding temperature-energy curve for (AgPd)1896 cluster. By comparing the segregation rate of (AgPd)1896 and its temperature-dependent energies, it is found that the atomic segregation occurs at 600 K, while the atomic energy decrease with the increase of temperature occurs until 720 K. Meanwhile, the segregation rate is increased sharply at this temperature. This means that the melting is affected only when the segregated atoms reach a threshold. From 760 to 1060 K, the segregation rate linearly increases with increasing the temperature. As the energies are concerned, they almost remain constantly with the increase of the temperature. This means that the energy decrease due to the atomic segregation is similar with the energy increase due to the increase of temperature. At 1080 K, the clusters are melted, the segregation rate also increases sharply. After the cluster is melted, there is no atomic segregation, the segregation rate remains constantly. Therefore, the atomic energy increases with increasing the temperature. These results indicate that the energy variation is closely related to the atomic segregation. Then, the segregation rates of the clusters with different sizes were compared. It is found that the segregation rate at the same temperature is increased with the decrease of the cluster sizes. The larger the cluster sizes results in the smaller the segregation rates. After the clusters are melted, the segregation rates are almost 100 % for (AgPd)490. This means that all the inner Ag atoms segregate to the surface for the small cluster. While for the clusters with other sizes, the segregation rates decrease with the increase of the cluster sizes. This indicates that the segregation ability decreases with the increase of cluster size. This leads to the effect of atomic segregation on the melting decreases with the increase of cluster size.
Then, according to the sharp increase of the temperature-energy curves, the melting points of the clusters were measured, as shown in Fig. 4. It can be seen that melting points increase with increasing the cluster size. The melting points are different for the clusters with different Ag atomic distributions. That is to say, the melting points are affected by the Ag atomic segregation. Since the structures of the Ag surface clusters are the most stable, their melting points are the highest in various sizes. The clusters with Ag in the (100) planes have the lowest melting points. The melting points of clusters with Ag in the (111) planes are between them. For the mixed clusters, the melting point change is irregular compared to those of other clusters. There are two possible reasons for this. One is that the temperature step is 20 K, which is larger than the difference of the melting points. The other reason is that the Ag atoms in the surface and inner are not to the same 30 % strictly. In other words, the segregated atomic numbers may be different for various sizes, it influences the melting point.
In order to explore the reasons of the effect of Ag atomic segregation on the melting, the Pd clusters with 12 Ag atoms distributing in the (100) and (111) planes of different layers from the surface to inner were constructed. These clusters were relaxed for 0.2 ns at 300 K. The atomic energies at 300 K were obtained, as shown in Fig. 5. The atomic energy is the lowest when Ag atoms are in the surface (1st layer). When the atoms distributed in the 2nd layer, their energies increase obviously. Then, the energy decreases slightly with increasing the layer number. For both (AgPd)1288 and (AgPd)1896, the energy variation with the layers are similar. While for the Ag atoms in the (100) and (111) planes, the atomic energies are different. For the first layer, the atomic energy of Ag in the (100) plane is lower than that of Ag in the (111) planes. In the subsurface layer (2nd layer), their energies are almost same. While in the 3rd layer, the energy of Ag in the (100) planes is larger than that of Ag in the (111) planes. When Ag atoms are in the more inner of the clusters, their energies became same again. That is to say, when the atoms segregate from the more inner (100) and (111) planes, it is no influence on the melting.
Then, the energy variations of the clusters with the atoms segregating from the inner (100) or (111) planes to the surface were calculated by using the above method. When one Ag atom segregated from the inner to the surface, the energy changes are shown in Fig. 6. It can seen that the energy decrease ∆E changes slightly for both (100) and (111) segregations with the increase of the temperature. The ∆E of the (111) segregation is lower than that of the (100) segregation. In addition, the ∆E decreases with the increase of the cluster sizes. Also, the difference of ∆E between (100) and (111) segregation decreases. This is the reason why the effect of Ag atomic segregation becomes weak with the increase of the cluster size. Concerning the melting points, they decrease when there are Ag atomic segregations. The atomic segregation is companied by the energy decrease and also the melting point decrease. While for the cases of Ag in the (100) and (111) planes, the melting points of the clusters with Ag in the (100) planes are lower than that with Ag in the (111) planes. This is because the energy decrease of (100) segregation is larger than that of (111) segregation and also the melting points of the clusters with Ag in the (100) planes decreases more than that of the clusters with Ag in the (111) planes.
Conclusions
The effect of Ag atomic segregation from different planes of the clusters with different sizes on the melting was studied by molecular dynamics simulation. And it was found that Ag segregation from inner to the surface decreased the system energy of clusters. The energy decrease of Ag (100) segregation was larger than that of Ag (111) segregation. Furthermore, with the increase of clusters size, energy decreased due to atomic segregation. The difference of energy decrease between (100) and (111) segregation was also decreased. This leaded to that the atomic energy was not monotonously increase with the increase of the temperature. Furthermore, with increasing the cluster sizes, the effect of atomic segregation decreased. The energy decrease influenced the melting points of the clusters. Higher the energy decrease due to atomic segregation resulted in larger the melting point decrease. This provided a theoretical fundament for analyzing the structural evolution and failure of devices.
References
R. Ferrando, J. Jellinek, and R. L. Johnston (2008). Chem. Rev. 108, 845.
G. H. Wang Cluster Physics (Shanghai Science & Technology Press, Shanghai, 2003), pp. 1–9.
F. Calvo, E. Cottancin, and M. Broyer (2008). Phys. Rev. B 77, 121406(R).
H. Y. Kim, H. G. Kim, J. H. Ryu, and H. M. Lee (2007). Phys. Rev. B 75, 212105.
I. Parsina and F. Baletto (2010). J. Phys. Chem. C 114, 1504.
Y. G. Chushak and L. S. Bartell (2003). J. Phys. Chem. B 107, 3747.
G. J. Li, Q. Wang, Y. Z. Cao, K. Wang, J. J. Du, and J. C. He (2012). Comput. Mater. Contin. 30, 195.
G. J. Li, Q. Wang, T. Liu, K. Wang, C. Wu, and J. C. He (2012). J. Nanoparticle Res. 14, 1068.
X. Y. Xiao, Z. F. Cheng, and J. H. Xia (2012). Mod. Phys. Lett. B 26, 1250051.
S. L. Lai, J. Y. Guo, V. Petrova, G. Ramanath, and L. H. Allen (1996). Phys. Rev. Lett. 77, 99.
I. M. L. Billas, A. Chatelain, and W. A. D. Heer (1994). Science 265, 1682.
G. J. Li, Q. Wang, Y. Z. Cao, J. J. Du, and J. C. He (2012). Phys. Lett. A 376, 534.
G. J. Li, Q. Wang, D. G. Li, X. Lü, and J. C. He (2009). Mater. Chem. Phys. 114, 746.
G. J. Li, Q. Wang, D. G. Li, X. Lü, and J. C. He (2008). Phys. Lett. A 372, 6764.
Acknowledgment
This work was supported by the National Natural Science Foundation of China (Grant Nos. 51101034, 51271056, 51425401) and the Fundamental Research Funds for the Central Universities (Grant Nos. N140901001, N140902001, N130509002).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, K., Li, G., Wu, C. et al. Effects of Ag Atomic Segregation from Different Planes on the Size-Dependent Melting of Ag–Pd Clusters. J Clust Sci 27, 55–62 (2016). https://doi.org/10.1007/s10876-015-0898-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-015-0898-2