Abstract
Background
Despite immunoglobulin replacement (IgRT) therapy, some patients with primary antibody deficiency (PAD) continue to develop respiratory infections. Recurrent and severe respiratory infections, particularly pneumonia, can lead to significant morbidity and mortality. Therefore, we sought to determine the risk factors of developing pneumonia in PAD patients, already receiving IgRT.
Methods
We evaluated clinical and laboratory features of PAD patients enrolled in the US Immune Deficiency Network (USIDNET) registry by April 2017. Patients were included if they met the following criteria: (1) PAD diagnosis (common variable immunodeficiency (CVID), agammaglobulinemia, hypogammaglobinemia, and specific antibody deficiency (SAD) and (2) available data on infections before and after IgRT. Patients were excluded if they were not receiving IgRT, or if no pre/post infections data were available. Descriptive and multivariable logistic regression analyses were used to identify factors associated with pneumonia post-IgRT.
Results
A total of 1232 patients met the inclusion criteria. Following IgRT, 218 patients (17.7%) were reported to have at least one pneumonia episode. Using multivariate logistic regression analysis, we found a statistically significant increased risk of pneumonia in patients with asthma (OR: 2.55, 95% CI (1.69–3.85), p < 0.001) bronchiectasis (OR: 3.94, 95% CI (2.29–6.80), p < 0.001), interstitial lung disease (ILD) (OR: 3.28, 95%CI (1.43–7.56), p < 0.005), splenomegaly (OR: 2.02, 95%CI (1.08–3.76), p < 0.027), allergies (OR: 2.44, 95% CI [1.44–4.13], p = 0.001), and patients who were not on immunosuppressives (OR: 1.61; 95%CI [1.06–2.46]; p = 0.027). For every 50 unit increase in IgA, the odds of reporting pneumonia post IgRT decreased (OR: 0.86, 95% CI [0.73–1.02], p = 0.062).
Infectious organisms were reported in 35 of 218 patients who reported pneumonia after IgRT. Haemophilus influenzae was the most frequently reported (n = 11, 31.43%), followed by Streptococcus pneumoniae (n = 7, 20.00%).
Conclusion
Our findings suggest PAD patients with chronic and structural lung disease, splenomegaly, and allergies were associated with persistent pneumonia. However, our study is limited by the cross-sectional nature of the USIDNET database and limited longitudinal data. Further studies are warranted to identify susceptible causes and explore targeted solutions for prevention and associated morbidity and mortality.
Clinical Implications
Patients with primary antibody deficiency with structural lung disease, allergies, and splenomegaly are associated with persistent pneumonia post-IgRT.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Primary antibody deficiency disorders (PADs) are humoral immune deficits characterized by defective immunoglobulin production and function and recurrent respiratory and gastrointestinal tract infections that significantly contribute to morbidity and mortality [1,2,3]. Recurrent and severe respiratory infections have been associated with poor quality of life in PAD [4, 5] and could lead to chronic, irreversible lung disease (e.g., bronchiectasis) [6,7,8,9], even in those with subclinical infections [10].
Treatment with immunoglobulin replacement therapy (IgRT) via intravenous (IV) or subcutaneous (SC) routes is used to reduce the incidence of infections [11]. However, many patients with PAD continue to have persistent respiratory infections [12,13,14]. There is a wide variation in the use of prophylactic antibiotics for patients with PAD, with some studies showing minimal efficacy for those patients already receiving IgRT [15, 16].
Given the challenge and importance of reducing the risk of recurrent respiratory infection, particularly pneumonia, in patients with PAD, we aimed in this study to identify the pathogens responsible for pneumonia in patients with PAD after initiation of IgRT and to explore factors that may predict risks of developing pneumonia after the initiation of IgRT.
Methods
Data Sources
Data for analyses were acquired in a de-identified format from the US Immunodeficiency Network (USIDNET) registry, a research consortium established to advance scientific research on inborn errors of immunity. The study was approved by the USIDNET Steering Committee and the Baylor College of Medicine Institutional Review Board (IRB). The registry is populated by participating clinician-investigators at thirty-nine academic institutions in the USA and Canada. Data in the registry include demographic, clinical, and laboratory information abstracted and entered by the investigators and medical records submitted by participants, which are confirmed and entered by the investigator. In April 2017, we queried the USIDNET patient registry for demographic, clinical, and laboratory data, including immunoglobulin levels, infectious pathogens, antibiotic therapy, and immunoglobulin therapies.
Patients were included if they had a confirmed PAD diagnosis of common variable immune disorder (CVID), agammaglobulinemia, specific antibody deficiency (SAD), hyper-IgM syndrome, excluding CD40L patients (HIGM), and hypogammaglobulinemia (unknown cause and no listed genetic defect). In addition, we excluded patients if they were not receiving IgRT or if pre/post infections data were unavailable.
Statistical Analysis
Categorical variables were summarized using frequencies and percentages, and continuous measures were summarized using mean ± standard deviation (SD), or median with interquartile ranges (IQRs).
Univariate comparisons were made using the t-test, chi-square test, Fisher’s exact test, and Wilcoxon’s rank-sum tests. Variables with a p < 0.25 were considered for inclusion in an exploratory multivariate logistic regression model.
A backward stepwise selection method was used to build the multivariable regression model. First, all variables were entered into a preliminary model and were reviewed. Then, the highest p-value was eliminated, and the model was re-run. This was repeated at every step until all p-values were significant (p < 0.1) [17].
Odds ratios (ORs) and their corresponding 95% confidence intervals (CIs) were calculated for each predictor to examine their contribution to the likelihood of having persistent respiratory infection after initiating IgRT. All data were analyzed using (Stata/IC, Version 12.11).
Results
Demographics and Clinical Characteristics of the Baseline Cohort
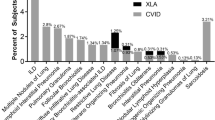
At the time of our query (April 2017), the USIDNET database included 2230 patients with PAD. Out of those, n = 1232 patients with a confirmed diagnosis of PAD were treated with IgRT and had available information on infections post-IgRT. The mean age at PI diagnosis was 15.7 years (0–84). Most patients (82.87%) identified as Caucasian, and 35% were female. The most-reported diagnosis was CVID (70.62%). Complete patients’ characteristics are summarized in Table 1s. Lung disease (34.01%) was the most commonly reported comorbidity, including asthma (22.32%) and bronchiectasis (7.79%), followed by hematological autoimmune disease and gastrointestinal disease (24.43% and 20.05%, respectively). Out of the 1232 included in the study, 159 patients were reported to be receiving prophylactic antibiotics. Patients’ comorbidities and immune phenotypes are summarized in Table 2s and Table 3s, respectively.
Post-treatment Pneumonia and Causative Pathogens
Pneumonia was reported in 218 patients after initiating IgRT (Table 1). Males reported a higher post-IgRT pneumonia frequency compared to females (60.09% vs. 39.91%). Females had a later diagnosis in this cohort with a median age of 28 years compared to median age of 7 years for males. A PAD diagnosis of CVID (70.64.1%) was most reported, followed by hypogammaglobinemia (24.77%) (Table 1). Lung disease was the most common comorbidity (57.80%) (Table 2). Thirty-five out of 218 patients who reported pneumonia after IgRT also reported the organism of infection that caused their pneumonia. Haemophilus influenzae was the most frequently reported (n = 11, 31.43%) followed by Streptococcus pneumoniae (n = 7, 20.00%). Thirty-five patients reported receiving prophylactic antibiotics post-IgRT. A list of reported prophylactic antibiotics is summarized in Table 4s.
Immunoglobulin Levels
We then evaluated the impact of immunoglobulin levels on post-IgRT infection. Of note, Ig levels were provided one-time value. There was no available data on when those values were reported in relation to IgRT. Patients’ immunological data are summarized in Table 3.
Multivariate Analysis for Pneumonia after IgRT Treatment in PAD Patients
To determine which variables to consider for building the multivariate logistic regression model for predicting pneumonia after beginning IgRT treatment, a univariate analysis was run, and variables with a p < 0.25 were considered for the multivariate logistic regression model.
Variables with a p-value of less than 0.25 included IgA (mg/dl—50-unit increments), absolute lymphocyte count (100-unit increments), age 18–26 compared to all other ages, COPD, autoimmune, granulomas, adenopathy, liver disease, EOE, IgRT type (SCIG or IVIG only), prophylactic antibiotics, and PID diagnosis (CVID vs. all others and XLA vs. all others).
The backward stepwise selection method was used to build the multivariate model. First, all variables were entered into a preliminary model, and the p-values were reviewed. Then, the highest p-value was eliminated, and the model was re-run. This continued at each step of the model until all p-values were considered significant (p < 0.10).
During the process of backward stepwise regression, the following variables were eliminated from the final model due to a p-value of larger than 0.10: absolute lymphocyte count (100-unit increments), age 18–26 compared to all other ages, COPD, autoimmune, granulomas, adenopathy, liver disease, EOE, IgRT type (SCIG or IVIG only), prophylactic antibiotics, and PID diagnosis (CVID vs. all others and XLA vs. all others).
After performing backward stepwise regression, the final multivariate model included IgA (mg/dl—50-unit increments), bronchiectasis, asthma, hematological, allergies, interstitial lung disease, splenomegaly, and taking immunosuppressives after beginning IgRT treatment.
Below are the predicted outcomes for each variable, summarized in Table 4:
-
Bronchiectasis
-
Compared to patients who did not report bronchiectasis, patients who did report bronchiectasis had an increase in the odds of reporting pneumonia after IGRT (OR: 3.94, 95% CI [2.29–6.79], p < 0.001) while holding all other variables constant.
-
-
ILD
-
Compared to patients who did not report ILD, patients who did report ILD had an increase in the odds of reporting pneumonia after IGRT (OR: 3.28, 95% CI [1.43–7.56], p = 0.005), while holding all other variables constant.
-
-
Asthma
-
Compared to patients who did not report asthma, patients who did report asthma had an increase in the odds of reporting pneumonia after IGRT (OR: 2.55, 95% CI [1.69–3.85], p < 0.001), while holding all other variables constant.
-
-
Allergies
-
Compared to patients who did not report allergies, patients who did report allergies had an increase in the odds of reporting pneumonia after IGRT (OR: 2.44, 95% CI [1.44–4.13], p = 0.001), while holding all other variables constant.
-
-
Splenomegaly
-
Compared to patients who did not report splenomegaly, patients who did report splenomegaly had an increase in the odds of reporting pneumonia after IGRT (OR: 2.02, 95% CI [1.08–3.76], p = 0.027), while holding all other variables constant.
-
-
Immunosuppressives
-
Compared to patients who were not on immunosuppressives, the patients who were immunosuppressives had a decrease in the odds of reporting pneumonia after IGRT (OR: 0.62, 95%CI [0.41–0.95]; p = 0.027), while holding all other variables constant.
-
The following 2 variables were significant in building the multivariate model to generate the best fit, but did not reach statistical significance as individual variables in the multivariate analysis:
-
Hematological
-
Compared to patients who did not report hematological comorbidities, patients who did report hematological comorbidities had an increase in the odds of reporting pneumonia after IGRT (OR: 1.44, 95% CI [0.94–2.20], p = 0.090), while holding all other variables constant.
-
-
IgA (mg/dL)
-
For every 50 unit increase in IgA, the odds of reporting pneumonia post-IgRT decreased significantly (OR: 0.86, 95% CI [0.73–1.02], p = 0.062).
-
Discussion
This exploratory analysis aimed to identify factors associated with pneumonia in patients with PAD following treatment with IgRT. Persistent respiratory symptoms and complications such as pneumonia pose a significant cause of morbidity and mortality among pediatric and adult patients with inborn errors of immunity [18]. However, its predictors are not well understood. As such, this study provides additional clarity to this challenging problem.
Consistent with previous literature, we have confirmed that Streptococcus pneumoniae and Haemophilus influenzae type b (hib) are the most common pathogens identified in patients with PAD with respiratory infections [19,20,21], even after IgRT. Although most commercially available IgRT products contain antibodies to Streptococcus pneumoniae and hib, the levels of those specific antibodies vary significantly between products [22, 23], and preparations are not standardized for specific antibody content for the pathogens that most commonly cause infections in patients with inborn errors of immunity [24]. Additionally, the inability to directly transport IgG to mucosal membranes has been postulated to be a risk factor for persistent infections in patients with PAD [16]. Equally important, respiratory viral infections could lead to persistent and severe respiratory infections despite IgRT [25].
From a host perspective, we have identified several significant predictors of pneumonia in patients with PAD after IgRT therapy. Consistent with previous studies, we noted that asthma, bronchiectasis, and interstitial lung disease significantly increased the risk of pneumonia in a patient with PAD despite receiving immunoglobulin replacement therapy [26,27,28,29]. Higher IgA serum levels were, in contrast, might be protective.
Furthermore, we show that having a diagnosis of CVID, or hypogammaglobinemia, increased the incidence of pneumonia while on IgGRT. CVID accounted for most patients, and most had chronic lung disease. In a multicenter study by Quinti et al., in patients with CVID, the presence of bronchiectasis was associated with persistent respiratory infections despite IgRT in those with IgA levels < 7 mg/dL and those with persistently IgG trough levels < 400 mg/dL) [14], while in XLA, bronchiectasis was the only predictor of increased risk of pneumonia following IgRT in the same center [14]. Together, these data raise the need for controlled studies to identify the best approach to prevent persistent infections in patients receiving therapeutic doses of IgRT.
We also found that patients with splenomegaly and hematological complications had higher odds of reporting pneumonia following IgRT. In many cases in patients with CVID, and other antibody deficiency disorders, the non-infectious complications present with multisystem involvement, including lung disease, liver disease, GI disease, and splenomegaly. Those patients are prone to persistent innate and adaptive immune activation, despite IgRT [30, 31].
We hypothesize that chronic immune activation can lead to immune exhaustion, which increases the risk of infections. Therefore, being on immune suppression might be beneficial and even perhaps protective from further infections in these patients.
We found that allergies were associated with increased odds of reporting pneumonia in PAD patients following IgRT. However, previous studies showed that many patients report symptoms of rhinitis and wheezing, despite having low to undetectable IgE levels [32]. Therefore, we hypothesize that allergy symptoms might be due to alternative diagnoses such as upper respiratory infections in those patients.
The main strengths of this study include the use of USIDNET registry data, its large size, and prospective data collection from thirty-nine institutions, and the broad representation of PAD increases our ability to generalize results. Our study was the largest of its kind to evaluate predictors of respiratory infections in patients with PAD. While registries allow the collection of rare diseases, there are limitations in that reporting cases is voluntary, and data might be entered once, or updated as per providers’ discretion. Hence, the frequency of infections/year could not be ascertained, as the USIDNET database does not allow for longitudinal study design. In addition, as this was a secondary analysis, due to the nature of the USIDnet database that relies on self-reporting by physicians, we do not have access to patient charts, dosing of IgRT or its timing, and laboratory data such as IgG trough levels, and dosing of IgRT cannot be ascertained. Despite these limitations, our study provides insights on predictors for persistent infections in patients with PAD from both patient and pathogen-related factors.
Conclusion
IgRT significantly reduces but does not eliminate respiratory infections in patients with PAD. In addition, chronic lung disease, splenomegaly, and allergies were associated with an increased risk of post-IgRT pneumonia. At the same time, higher serum IgA levels and immunosuppressive use decreased post-IgRT pneumonia risk.
Our study is limited by the cross-sectional nature of the USIDNET database and limited longitudinal data, particularly the lack of dosing data. Prospective studies exploring targeted solutions for prevention and associated morbidity and mortality are warranted.
Data Availability
The datasets generated during and analyzed during the current study are available from the corresponding author on reasonable request.
Code Availability
N/A.
Abbreviations
- AID:
-
Deficiency activation-induced cytidine deaminase deficiency
- aOR:
-
Adjusted odds ratio
- BTK:
-
Bruton’s tyrosine kinase
- CD40L:
-
CD40 ligand
- CVID:
-
Common variable immunodeficiency
- CI:
-
Confidence interval
- COPD:
-
Chronic obstructive pulmonary disease
- GLILD :
-
Granuloma, interstitial lung disease
- HIGM:
-
Hyper-IgM syndrome
- IG:
-
Immunoglobulin
- IgRT:
-
Immunoglobulin replacement therapy
- IM:
-
Intramuscular
- IQR:
-
Interquartile range
- IRB:
-
Institutional Review Board
- IV :
-
Intravenous
- IVIG:
-
Intravenous immunoglobulin
- OR:
-
Odds ratio
- PAD:
-
Primary antibody deficiency
- SAD:
-
Specific antibody deficiency
- SD:
-
Standard deviation
- SC :
-
Subcutaneous
- SCIG:
-
Subcutaneous immunoglobulin
- TACI Deficiency:
-
Transmembrane activator and CAML interactor deficiency
- USIDNET:
-
United States Immunodeficiency Network
- XLA:
-
X-linked agammaglobulinemia
References
Wood P. Primary antibody deficiencies: recognition, clinical diagnosis and referral of patients. Clin Med (Lond). 2009;9:595–9.
Hampson F, Chandra A, Screaton N, Condliffe A, Kumararatne D, Exley A, et al. Respiratory disease in common variable immunodeficiency and other primary immunodeficiency disorders. Clin Radiol. 2012;67:587–95.
Cunningham-Rundles C, Bodian C. Common variable immunodeficiency: clinical and immunological features of 248 patients. Clin Immunol. 1999;92:34–48.
Jiang F, Torgerson TR, Ayars AG. Health-related quality of life in patients with primary immunodeficiency disease. Allergy Asthma Clin Immunol. 2015;11:27.
Routes J, Costa-Carvalho BT, Grimbacher B, Paris K, Ochs HD, Filipovich A, et al. Health-Related quality of life and health resource utilization in patients with primary immunodeficiency disease prior to and following 12 months of immunoglobulin G treatment. J Clin Immunol. 2016;36:450–61.
Good RA, Mazzitello WF. Chest disease in patients with agammaglobulinemia. Dis Chest. 1956;29:9–35.
Pasteur MC, Bilton D, Hill AT, British Thoracic Society Bronchiectasis non CFGG. British Thoracic Society guideline for non-CF bronchiectasis. Thorax 2010; 65 Suppl 1:i1–58.
Sweinberg SK, Wodell RA, Grodofsky MP, Greene JM, Conley ME. Retrospective analysis of the incidence of pulmonary disease in hypogammaglobulinemia. J Allergy Clin Immunol. 1991;88:96–104.
Tarzi MD, Grigoriadou S, Carr SB, Kuitert LM, Longhurst HJ. Clinical immunology review series: an approach to the management of pulmonary disease in primary antibody deficiency. Clin Exp Immunol. 2009;155:147–55.
Kainulainen L, Varpula M, Liippo K, Svedstrom E, Nikoskelainen J, Ruuskanen O. Pulmonary abnormalities in patients with primary hypogammaglobulinemia. J Allergy Clin Immunol. 1999;104:1031–6.
Joud H, Nguyen AL, Constantine G, Kutac C, Syed MN, Orange JS, et al. Prophylactic antibiotics versus immunoglobulin replacement in specific antibody deficiency. J Clin Immunol. 2020;40(1):158–64. https://doi.org/10.1007/s10875-019-00716-2.
Busse PJ, Razvi S, Cunningham-Rundles C. Efficacy of intravenous immunoglobulin in the prevention of pneumonia in patients with common variable immunodeficiency. J Allergy Clin Immunol. 2002;109:1001–4.
Aghamohammadi A, Allahverdi A, Abolhassani H, Moazzami K, Alizadeh H, Gharagozlou M, et al. Comparison of pulmonary diseases in common variable immunodeficiency and X-linked agammaglobulinaemia. Respirology. 2010;15:289–95.
Quinti I, Soresina A, Guerra A, Rondelli R, Spadaro G, Agostini C, et al. Effectiveness of immunoglobulin replacement therapy on clinical outcome in patients with primary antibody deficiencies: results from a multicenter prospective cohort study. J Clin Immunol. 2011;31:315–22.
Lucas M, Lee M, Lortan J, Lopez-Granados E, Misbah S, Chapel H. Infection outcomes in patients with common variable immunodeficiency disorders: relationship to immunoglobulin therapy over 22 years. J Allergy Clin Immunol. 2010;125(1354–60): e4.
Kuruvilla M, de la Morena MT. Antibiotic prophylaxis in primary immune deficiency disorders. J Allergy Clin Immunol Pract. 2013;1:573–82.
Riffenburgh RH. Chapter 6 - statistical testing, risks, and odds in medical decisions. In: Riffenburgh RH, editor. Statistics in Medicine. 2nd ed. Burlington: Academic Press; 2006. p. 93–114.
Jesenak M, Banovcin P, Jesenakova B, Babusikova E. Pulmonary manifestations of primary immunodeficiency disorders in children. Front Pediatr. 2014;2:77.
Rezaei N, Aghamohammadi A, Siadat SD, Nejati M, Ahmadi H, Moin M, et al. Serum bactericidal antibody response to serogroup C polysaccharide meningococcal vaccination in children with primary antibody deficiencies. Vaccine. 2007;25:5308–14.
Aguilar C, Malphettes M, Donadieu J, Chandesris O, Coignard-Biehler H, Catherinot E, et al. Prevention of infections during primary immunodeficiency. Clin Infect Dis. 2014;59:1462–70.
Hermaszewski RA, Webster AD. Primary hypogammaglobulinaemia: a survey of clinical manifestations and complications. Q J Med. 1993;86:31–42.
Mikolajczyk MG, Concepcion NF, Wang T, Frazier D, Golding B, Frasch CE, et al. Characterization of antibodies to capsular polysaccharide antigens of Haemophilus influenzae type b and Streptococcus pneumoniae in human immune globulin intravenous preparations. Clin Diagn Lab Immunol. 2004;11:1158–64.
Lee S, Kim HW, Kim KH. Functional antibodies to Haemophilus influenzae type B, Neisseria meningitidis, and Streptococcus pneumoniae contained in intravenous immunoglobulin products. Transfusion. 2017;57:157–65.
Bonilla FA, Khan DA, Ballas ZK, Chinen J, Frank MM, Hsu JT, et al. Practice parameter for the diagnosis and management of primary immunodeficiency. J Allergy Clin Immunol 2015; 136:1186–205.e1–78.
Kainulainen L, Vuorinen T, Rantakokko-Jalava K, Osterback R, Ruuskanen O. Recurrent and persistent respiratory tract viral infections in patients with primary hypogammaglobulinemia. J Allergy Clin Immunol. 2010;126:120–6.
Lucas M, Lee M, Lortan J, Lopez-Granados E, Misbah S, Chapel H. Infection outcomes in patients with common variable immunodeficiency disorders: relationship to immunoglobulin therapy over 22 years. J Allergy Clin Immunol. 2010;125:1354-60.e4.
Brent J, Guzman D, Bangs C, Grimbacher B, Fayolle C, Huissoon A, et al. Clinical and laboratory correlates of lung disease and cancer in adults with idiopathic hypogammaglobulinaemia. Clin Exp Immunol. 2016;184:73–82.
Urm SH, Yun HD, Fenta YA, Yoo KH, Abraham RS, Hagan J, et al. Asthma and risk of selective IgA deficiency or common variable immunodeficiency: a population-based case-control study. Mayo Clin Proc. 2013;88:813–21.
Juhn YJ, Kita H, Yawn BP, Boyce TG, Yoo KH, McGree ME, et al. Increased risk of serious pneumococcal disease in patients with asthma. J Allergy Clin Immunol. 2008;122:719–23.
Wong GK, Huissoon AP. T-cell abnormalities in common variable immunodeficiency: the hidden defect. J Clin Pathol. 2016;69:672–6.
Paquin-Proulx D, Sandberg JK. Persistent immune activation in CVID and the role of IVIg in its suppression. Front Immunol 2014; 5:637-.
Lawrence MG, Palacios-Kibler TV, Workman LJ, Schuyler AJ, Steinke JW, Payne SC, et al. Low serum IgE is a sensitive and specific marker for common variable immunodeficiency (CVID). J Clin Immunol. 2018;38:225–33.
Funding
This research project was funded by the US immune deficiency network research grant.
Author information
Authors and Affiliations
Contributions
CK and JH performed material preparation, data collection, and analysis. MS and JH wrote the first draft of the manuscript. FK, JM, and RM contributed critical reviews and comments on data analysis, manuscript endpoints, and overall message. CCR, KS, and RF contributed significantly to the USIDNET database (> 10% of patients included). All the authors commented on previous versions of the manuscript. All the authors contributed to the study’s conception and design. Finally, all the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
The study was approved by the USIDNET Steering Committee and the Baylor College of Medicine Institutional Review Board (IRB).
Consent to Participate
N/A.
Consent for Publication
N/A.
Competing Interests
Hajjar received grants from Immune Deficiency Foundation, the US immunodeficiency network, the Chao-physician Scientist award, the Texas Medical Center Digestive Diseases Center, and the Jeffrey Modell Foundation. J. Hajjar received an honorarium/advisory from Horizon, Pharming, Baxalta, CSL Behring, the National guard, and Al-Faisal University Hospital outside the submitted work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Syed, M.N., Kutac, C., Miller, J.M. et al. Risk Factors of Pneumonia in Primary Antibody Deficiency Patients Receiving Immunoglobulin Therapy: Data from the US Immunodeficiency Network (USIDNET). J Clin Immunol 42, 1545–1552 (2022). https://doi.org/10.1007/s10875-022-01317-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-022-01317-2