Abstract
Chronic granulomatous disease (CGD) is a group of inherited disorder of phagocytes, resulting from mutations in the components of the NADPH oxidase complex. Reduced or absent oxygen radical synthesis seen in these patients leads to impaired killing of intracellular bacteria and fungi. CGD clinically presents with recurrent and life-threatening infections as well as granulomatous inflammatory responses. p47phox encoded by the NCF1 gene is the most common autosomal recessive form of CGD which is often clinically milder. Here, we are presenting the data on clinical and immunological findings in 21 Indian patients with Del GT mutation in the NCF1 gene. Diagnosis of these patients was based on detailed clinical evaluation, measurement of respiratory burst activity by nitro blue tetrazolium and dihydrorhodamine-1,2,3 assay, expression of p47phox by flow cytometry, and molecular confirmation by GeneScan method. Seventeen male and four female patients with median age of onset of 1 year ranging from 1.5 months to 6 years were included in the study. Sixty-two percent (13 out of 21) of patients belonged to a consanguineous marriage with only one family having a history of a previous sibling death. Significant variability in clinical presentation was observed in spite of identical genetic defect ranging from asymptomatic to very severe presentation leading to early death or requiring transplantation. However, none of these patients showed difference in immunological parameters to account for this variability. Thus, this study highlights the phenotypic heterogeneity seen in these patients with Del GT mutation in the NCF1 gene and its implication in management of these patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic granulomatous disease (CGD) is a primary immunodeficiency disorder marked by inability of phagocytes to produce microbicidal reactive oxygen species (ROS) essential for elimination of bacterial and fungal infections. Phagocytes convert molecular oxygen into potent ROS with the help of an enzyme called nicotinamide adenine dinucleotide phosphate-oxidase (NADPH oxidase). NADPH oxidase consists of spatially separated six heterosubunits, namely, gp91phox, p22phox, p47phox, p67phox, p40phox, and GTPase Rac, that associate in a stimulus-dependent manner to form active enzymes [1]. Two subunits gp91phox and p22phox are integral membrane proteins that together form heterodimeric flavocytochrome b558 at the catalytic core. Regulatory subunits p47phox, p67phox, and p40phox reside in the cytoplasm under unstimulated conditions. Upon activation, p47phox gets phosphorylated and the entire assembly eventually translocates to the membrane in order to form an active enzyme complex with b558 [2].
Each component of this enzyme complex is encoded by its own gene: gp91phox by the CYBB gene (cytochrome b-245 beta subunit) located on chromosome X, p22phox by the CYBA gene (cytochrome b-245 alpha subunit) located on chromosome 16, p47phox by the NCF1 gene (neutrophil cytosolic factor 1) on chromosome 7, and p67phox and p40phox by NCF2 (neutrophil cytosolic factor 2) and NCF4 (neutrophil cytosolic factor 4) genes located on chromosomes 1 and 22, respectively [3]. Mutation in any one of these genes can lead to inappropriate activation of NADPH oxidase resulting in rare, life-threatening genetic disorders like CGD.
Prevalence of CGD is approximately 1 in 200,000 to 250,000 live births [4]; however, due to the lack of definitive diagnostic facilities, lack of awareness of the doctors to this disease, and the access of the patients to good clinics, the incidence rate is likely to be underestimated especially in India. [5]. Mutation in the CYBB gene is reported in approximately 65 to 70 % of all CGD patients, making it largely X-linked disorder (XL-CGD) in the western world [6, 7]. Autosomal recessive (AR-CGD) mutations in the NCF1 gene affecting the expression of p47phox are reported in approximately 30 % of all cases. Defects in genes encoding for p22phox and p67phox are relatively uncommon, and only one case is reported with p40phox defect till now [8–12]. Though the pattern of genetic abnormality is vastly affected by ethnicity, autosomal recessive inheritance of CGD is more common in regions with high rate of consanguinity [13].
Patients with NCF1 gene defects have absent or non-functional expression of p47phox protein. A 2-bp GT deletion at the beginning of exon 2 in the NCF1 gene is the most common mutation in the NCF1 gene accounting for more than 95 % cases of p47phox defect. This 2-bp deletion is also a signature characteristic of two pseudo-NCF1 genes (ΨNCF1) that flank the NCF1 gene [14]. Due to a random recombination between NCF1 and ΨNCF1, this 2-bp GT deletion results in the loss of a functional gene on one allele. An individual who receives a defective gene carrying an allele from both mother and father lacks expression of p47phox resulting in autosomal CGD [15]. Due to high degree of sequence homology between NCF1 and ΨNCF1, direct sequencing and annotation become difficult, but a rapid GeneScan analysis can not only identify the NCF1 to ΨNCF1 ratio based on a 2-bp difference but also helps in the identification of patients and carrier status [16]. Generally, the ratio in normal individuals is 2:4, but in some cases 1:1 can also be seen. In NCF1 defect carriers, the ratio is 1:5 [17, 18].
Clinical manifestations seen in CGD vary significantly depending on the underlying genetic defect [19]. On the whole, XL-CGD is more severe as compared to AR-CGD with defect in the NCF1 gene [13]. Clinical severity also depends on the type of mutation seen in these patients resulting in variable residual NADPH activity. p47phox defect is the most common form of genetic defect identified in a cohort of Indian CGD patients from our center. Here, we are presenting clinical and immunological characteristics seen in this group with a single common gene defect.
Materials Methods
Samples
A total of 21 patients with AR-CGD were diagnosed and molecularly confirmed with NCF1 defect during the study at National Institute of Immunohaematology, ICMR, Mumbai. After obtaining appropriate informed consent as per the guidelines of the institutional ethics committee board under Declaration of Helsinki, 3 ml peripheral blood was collected in EDTA, plain, and heparin vacutainers.
Laboratory Evaluation and Neutrophil Function Test
Neutrophil functioning analysis was done using nitro blue tetrazolium (NBT), dihydrorohodamine-123 (DHR), and phorbol myristate acetate (PMA) from Sigma-Aldrich (Sigma-Aldrich Co., St. Louis, Missouri, USA). A defect in the NADPH oxidase enzyme complex was detected by NBT dye reduction test where upon activation, blue-black deposits of formazan particles were seen in functional neutrophils after stimulating with PMA; similarly, oxidation of DHR was studied using flow cytometry as a tool [5]. Complete blood counts were evaluated using a Sysmex XS-800i (Sysmex Co., Cobe, Japan) five-part automated hematological analyzer. Lymphocyte subset analysis was done in a few patients using BD Multitest 6-color TBNK reagent, and cells were acquired on FACSAria-I using FACSDiva software (BD Biosciences, San Jose, CA, USA). Serum immunoglobulin levels were estimated by nephelometry.
Dihydrorhodamine Assay
DHR was dissolved in DMSO to prepare 29 mM working stock stored at −20 °C. Out of this stock, 0.75 μl of DHR is added to 100 μl of EDTA anticoagulated whole blood sample in each unstimulated and stimulated tubes. PMA was dissolved in DMSO to give a 1-μg/μl stock solution, and aliquots were stored at −20 °C. Stimulation was carried out by adding 2 μl of PMA working solution, which is a 1/10 dilution of the stock solution. It is then incubated at 37 °C for 15 min. BD FACS lysing solution (1×) was used for RBC lysis. For analysis, at least 10,000 neutrophils are acquired (Fig. 1).
Flowcytometric Evaluation of NADPH Oxidase Component Expression
Expression of the NADPH component was evaluated using monoclonal antibodies including 7D5 (MBL International Co., Woburn, MA, USA) against gp91phox and -p22phox, and against p47phox (clone 1, BD Biosciences, San Diego, CA, USA) and p67phox (clone D-6, Santa Cruz Biotechnology Inc, Santa Cruz, CA, USA) in a flow cytometry-based approach. Stain-lyse-wash protocol was used to study the transmembrane components gp91phox and p22phox. The expression was evaluated on neutrophils and B and T lymphocytes, which were gated on phycoerythrin-Cy5-conjugated anti-CD20 and allophycocyanin-conjugated anti-CD3, respectively (Becton Dickinson, San Diego, CA, USA). Cytoplasmic components were evaluated using the previously described permeabilization protocol [20]. A minimum of 10,000 neutrophils per tube were acquired on BD FACSAria-I using FACSDiva software, and data was further analyzed using FlowJo software (Tree Star Inc., Ashland, USA).
GeneScan Analysis
Genomic DNA was isolated using whole blood DNA extraction kit (Sigma-Aldrich Co., St. Louis, Missouri, USA). The NCF1 gene and two flanking ΨNCF1 genes were amplified using 6-FAM tag-labeled primers as per the sequences provided earlier [21]. GeneScan analysis was done using GeneMapper software (Life Technologies, Carlsbad, CA, USA) on an Applied Biosystems 3130xl analyzer. Peak height corresponding to the copy number of genes was calculated. Ratio of NCF1 to ΨNCF1 was determined in order to establish normal, patient, and carrier status [19].
Results
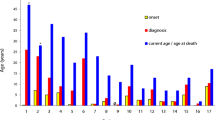
A total of 21 CGD patients belonging to 19 families were found to harbor the NCF1 gene defect. Sixty-two percent of these patients (13 out of 21) belonged to families with a consanguineous setting. Only one family had a history of death of patient’s twin sibling at the age of 2.5 years. Male predominance was seen in NCF1-defective patients as 17 out of 21 patients were male. Though the median age of onset of symptoms for these patients was found to be 1 year, the median age at diagnosis was about 2.6 years showing a considerable delay in diagnosis. Basic clinical parameters of CGD patients with NCF1 defect are given in Table 1.
Laboratory Investigation and Molecular Analysis
Superoxide burst activity of neutrophils was measured by NBT test ranging from 0 to 1 % (n = 21) and DHR assay ranging from 0 to 5 % (n = 17) positive, respectively. Lymphocyte subsets as evaluated in 17 patients showed normal count of B cells and with mild elevation of T and NK cells in almost half of the patients as given in Table 2. Leukocytosis was present in 52 % of the patients; majority of them showed neutrophilia. Similarly, serum immunoglobulin levels were elevated in 50 % of the patients. Anemia was also observed in 90 % of the patients due to chronic infections.
GeneScan analysis of 21 patients showed an NCF1/ΨNCF1 ratio of 0:6 indicating homozygous Del GT, while their parents had a ratio ranging from 1:5 to 1:7 showing a carrier status, except in two carrier parents with a ratio which is seen to be 2:4 (Table 3; Fig. 2). Normal controls showed a ratio of 2:4, except in two controls showing a ratio of >0.8. Expression of the p47phox antibody using flow cytometry was performed on 10 patients and found to be abnormal. Similarly, analysis in eight carrier parents showed reduced intensity of expression of cytoplasmic p47phox as compared to healthy controls (Fig. 3; Table 4).
(I) Flow cytometric evaluation of expression of the anti-p47phox antibody on neutrophils of a control, b patient, c mother, and d father, where the patient shows an absent expression of the p47phox antibody; parents show reduced expression as compared to healthy control. Unstained tube (sky blue), stained tube (dark blue). (II) GeneScan analysis of the NCF1 gene in a control, b patient, c mother, and d father, where the ratio of NCF1/pseudo-NCF1 genes is calculated by measuring peak height of the NCF1 gene at 207 bp and pseudogene at 205 bp. Patient b shows homozygous del GT mutation; parents show a carrier status with altered ratio of 1:6 and 1:7
Clinical Features
Pneumonia and tuberculosis were the most frequent clinical manifestation present in 76 and 48 % patients, respectively. Lymphadenitis, skin infections, and chronic diarrhea were among the other common presenting features as listed in Table 1. Interestingly, sternum osteomyelitis due to Aspergillus infection and lupus vulgaris was also seen in two cases. Apart from skin abscesses, no other deep abscesses were reported in these patients. Only one patient was documented to have chronic granulomatous infiltrations in lung requiring repeated immunosuppressive therapy.
Since most of the patients received empirical antimicrobial therapy, culture status could not be established. Of the confirmed culture reports, Aspergillus species and Staphylococcus aureus were isolated in four patients. Candida species and Chromobacterium violaceum were isolated in three patients as shown in Table 1.
All the patients were started with antibacterial and antifungal prophylaxis after the diagnosis. None of these patients were treated with IFNγ therapy. Out of 21 patients, 5 died due to severe infections and 2 underwent matched sibling donor transplantation for severe disease.
Discussion
CGD accounts for 12.5 % of primary immunodeficiency diseases in the ESID registry [22] and 8.5 % [23] in the US registry of primary immunodeficiency disorder. However, the incidence is reported to be higher where consanguinity is practiced; for example, in an Arab population, it is twice that in western parts of the world [8, 24]. Incidence in India is not known; however, CGD constitutes approximately 14 % among the diagnosed population of the primary immunodeficiency disorder cohort [25, 26].
A report from an Indian group suggests that the autosomal recessive (59 %) pattern of inheritance is more common than X-linked (41 %) CGD [5] and NCF1 gene defect accounts for 41 % (7 out of 17) of all the cases. Thus, identifying cases with NCF1 gene mutations from a cohort of CGD patients is important for management as well as for genetic counseling. If a mother’s neutrophils show a mosaic pattern on NBT and DHR assay, it clearly suggests XL-CGD, but if it shows normal NBT or DHR results, still the male child could have XL-CGD due to spontaneous mutation that requires molecular confirmation. The expression of p22phox, p47phox, and p67phox needs to be studied further for identifying the autosomal recessive genetic defect [27]. Western blot analysis is routinely practiced to infer the specific genotype of the patient, but it is very time consuming, laborious, and requires a large amount of blood sample. Most of the patients belong to the pediatric group where availability of a large amount of sample is often difficult. Direct molecular analysis is also time consuming and costly, and very few centers provide this facility in India. Flow cytometry offers a rapid and sensitive tool for studying the expression of NADPH oxidase component proteins p22phox, p47phox, and p67phox on neutrophils and B lymphocytes [20]. All the 10 patients tested by flow cytometry showed a consistent reduction in expression of p47phox compared to XL-CGD patients and healthy normal controls, thereby suggesting utility of this assay as a rapid screening tool at the time of diagnosis.
More than 95 % of the patients with NCF1 gene defect show presence of a single Del GT mutation at the beginning of exon 2 [28]. Molecular analysis by direct PCR sequencing is very difficult in the NCF1 gene due to presence of highly homologous pseudogenes. A rapid GeneScan method calculating the peak height of NCF1 and ΨNCF1 genes provides a reliable tool for identification of this particular mutation. Altered NCF1 to ΨNCF1 gene ratio is seen in carriers of this mutation [29]. A GeneScan ratio of 1:1 occurs in approximately 10 % of individuals from western countries, suggesting that they carry one copy of each gene. As per the study reported by Heyworth et al., there are three different haplotypes of NCF1/ΨNCF1 gene ratios observed in the general population due to reciprocal crossing over events during meiosis. These are 2:4, 1:1, and 4:2; this also explains one of the two carrier parents (pt 26, pt 62) showing a ratio of 2:4. In this case, testing their grandparents and other family members should then give a clue for the carrier haplotype in their family [29].
Defect in p47phox is the most common form of AR-CGD. Although clinical manifestations in different subtypes of CGD are found to be similar, differences in the pattern of severity and frequency of incidence are observed [30]. Male predominance is seen in this cohort of NCF1 patients with a male to female ratio of 4.25:1, perhaps due to gender bias in referring patients to hospitals. Ten patients are Hindu, nine Muslim, and two Christian, suggesting diversity in their ethnic background. Two patients were (pt. nos. 64, 65) diagnosed with CGD on family screening of the index case. They were asymptomatic till the age of 9 and 7 years of diagnosis, respectively. This conveys the significance of screening family members of affected patients. A similar observation reported in a series of an Egyptian cohort where siblings were diagnosed at 6 and 3.5 years of age after index case was diagnosed at 6 years of age [31].
Though lung is the most commonly affected organ among these patients, few patients also presented with infections in unusual sites such as brain, skin, bone, gastrointestinal tract, and urinary tract. There was a significant respiratory morbidity seen in these patients, with recurrent pneumonia and pulmonary tuberculosis being the major cause of death. This observation was supported by previous studies [5, 24]. In the present study, Aspergillus sp. was isolated in only 19 % cases as against 35 % reported previously [5]. Forty percent of patients received antitubercular therapy (ATT). Thus, morbidity due to mycobacterial tuberculosis infection is higher in the Indian cohort of patients with CGD which may be due to high endemicity of tuberculosis in India. This also highlights the need for investigating patients with recurrent tuberculosis for underlying CGD. All patients in this group had already received Bacillus Calmette-Guerin (BCG) immunization, as it is routinely administered at birth in India [5]. However, only three patients had left axillary and cervical lymphadenopathy following BCG vaccination; none of them had disseminated BCGiosis which is observed in XL-CGD patients. These three patients responded well to ATT.
Non-infectious inflammatory complications involving gastrointestinal tract, genitourinary tract, vasculitis, etc., is the major concern in patients with CGD. Inflammatory bowel disorder (IBD) is commonly seen in patients with CGD. Seven to 11 % of the patients with NCF1 defect showed IBD as a primary symptom of CGD [32, 33]. In our cohort of patients, 34 % showed history of chronic diarrhea at the time of diagnosis. Majority of the patients received antimicrobial therapy and showed response to the same. None of the patients were further evaluated for biopsy-proven IBD or colitis. These complications need to be kept in mind if the patient has persistent non-infectious diarrhea as they may require additional immunomodulatory or immunosuppressive therapy [33].
AR-CGD often has milder clinical presentation compared to XL-CGD [34]. Moreover, the clinical manifestations, age of onset, and condition of survival depend on the nature of the underlying genetic defect. However, our cohort of patients had a clinical heterogeneity despite having an identical genetic defect. Of the 21 patients, 52 % presented clinical manifestation within the first year of life. Twenty-four percent of patients (5 out of 21) expired due to severe infections. Two patients were transplanted at the age of 4.5 and 8 years, respectively, for severe clinical manifestations. Sixty-seven percent of patients had a relatively milder course and were significantly stable on antibacterial and antifungal prophylaxis. Interestingly, one of the family had three children who were affected, but only one child required frequent medications (pt. no. 57) whereas the other two (pt. nos. 64, 65) were relatively asymptomatic with normal growth and mild clinical manifestations in the form of URTI and could easily be treated with oral antibiotics. We tried to understand their clinical heterogeneity by looking at their other immunological parameters like lymphocyte subset analysis and immunoglobulin profile. However, no significant variation was observed between the patients with severe and milder phenotype.
It may be interesting to study the residual NADPH oxidase activity in these patients. Intact residual NADPH oxidase enzyme activity depends on the underlying genetic defect and the type of mutation which affects the severity of the disease, chances of survival, and clinical manifestation in the patient. It can be used as a tool for predicting the severity of the defect in CGD patients [13, 18]. Unfortunately, this was not done in the present study.
Despite of the advances in antimicrobial and antifungal prophylaxis and in the supportive management, CGD is still associated with significant morbidity and mortality. Average life span of CGD patients is 30 years with yearly mortality of 2 % [35]. Only hematopoietic stem cell transplantation (HSCT) currently offers the definitive therapy for CGD. The success of HSCT for CGD has improved significantly over the last few years even with matched unrelated donor (MUD), making it an important therapeutic option offered at diagnosis to all the patients especially in experienced centers [36, 37]. Two patients in our cohort with severe disease were successfully treated with matched sibling bone marrow transplantation, one for severe recurrent infections and one for chronic inflammatory complication. However, marked clinical heterogeneity seen in the present cohort clearly suggests that HSCT is not indicated in all the patients with CGD. Recurrent severe infections and chronic inflammatory or autoimmune complication requiring long-term steroid therapy are indicators of poor prognosis. Elevated alkaline phosphatase, a history of liver abscesses, and decline in platelet count reflecting portal hypertension are some other adverse prognostic indicators [36]. All these patients can be considered for early HSCT.
A study by Kuhns et al. has shown worse long-term survival with markedly reduced NADPH oxidase activity suggesting that these patients might be considered for early HSCT [18, 36]. The NADPH activity depends on the underlying genetic defect; however, it is not clear from the studies if differential residual NADPH oxidase activity is seen with identical genetic defect and it can be used as a predictive tool in patients with Del GT mutation. The effect of other genes and of epigenetic and environmental factors also plays an important role in having such wide heterogeneity [19].
In conclusion, early detection of NCF1 defect is important for timely management of CGD patients. Flow cytometry offers a rapid screening method for detection of p47phox defect from other forms of AR-CGD. Wide heterogeneity is seen in clinical manifestations of patients with Del GT mutation. Further studies are required to understand the underlying mechanisms for this phenotypic heterogeneity.
References
Diebold BA, Bokoch GM. Molecular basis for Rac2 regulation of phagocyte NADPH oxidase. Nat Immunol. 2001;2(3):211–5.
Knaus UG, Heyworth PG, Evans T, Curnutte JT, Bokoch GM. Regulation of phagocyte oxygen radical production by the GTP-binding protein Rac 2. Science. 1991;254(5037):1512–5.
Francke U, Hsieh CL, Foellmer BE, Lomax KJ, Malech HL, Leto TL. Genes for two autosomal recessive forms of chronic granulomatous disease assigned to 1q25 (NCF2) and 7q11.23 (NCF1). Am J Hum Genet. 1990;47(3):483–92. PubMed PMID: 2393022, PubMed Central PMCID: PMC1683885.
Winkelstein JA, Marino MC, Johnston Jr RB, Boyle J, Curnutte J, Gallin JI, et al. Chronic granulomatous disease. Report on a national registry of 368 patients. Medicine (Baltimore). 2000;79(3):155–69.
Rawat A, Singh S, Suri D, Gupta A, Saikia B, Minz RW, et al. Chronic granulomatous disease: two decades of experience from a tertiary care centre in North West India. J ClinImmunol. 2014;34(1):58–67.
Jones LB, Mcgrogan P, Flood TJ, Gennery AR, Morton L, Thrasher A, et al. Special article: chronic granulomatous disease in the United Kingdom and Ireland: a comprehensive national patient-based registry. Clin ExpImmunol. 2008;152(2):211–8.
Nunoi H, Ishibashi F. Statistical evaluation of chronic granulomatous disease in Japan and basic studies for gene therapy for CGD patients. Rinsho Byori. 1999;47(7):658–64.
Wolach B, Gavrieli R, de Boer M, Gottesman G, Ben-Ari J, Rottem M, et al. Chronic granulomatous disease in Israel: clinical, functional and molecular studies of 38 patients. Clin Immunol. 2008;129(1):103–14.
El Kares R, Barbouche MR, Elloumi-Zghal H, Bejaoui M, Chemli J, Mellouli F, et al. Genetic and mutational heterogeneity of autosomal recessive chronic granulomatous disease in Tunisia. J Hum Genet. 2006;51(10):887–95.
Agudelo-Flórez P, Prando-Andrade CC, López JA, Costa-Carvalho BT, Quezada A, Espinosa FJ, et al. Chronic granulomatous disease in Latin American patients: clinical spectrum and molecular genetics. Pediatr Blood Cancer. 2006;46(2):243–52.
Al-Zadjali S, Al-Tamemi S, Elnour I, Alkindi S, Lapoumeroulie C, Al-Maamari S, et al. Clinical and molecular findings of chronic granulomatous disease in Oman: family studies. Clin Genet. 2015;87(2):185–9.
Matute JD, Arias AA, Wright NA, Wrobel I, Waterhouse CC, Li XJ, et al. A new genetic subgroup of chronic granulomatous disease with autosomal recessive mutations in p40 phox and selective defects in neutrophil NADPH oxidase activity. Blood. 2009;114(15):3309–15.
Köker MY, Camcıoğlu Y, van Leeuwen K, Kılıç SŞ, Barlan I, Yılmaz M, et al. Clinical, functional, and genetic characterization of chronic granulomatous disease in 89 Turkish patients. J Allergy Clin Immunol. 2013;132(5):1156–63.
Roesler J, Curnutte JT, Rae J, Barrett D, Patino P, Chanock SJ, et al. Recombination events between the p47-phox gene and its highly homologous pseudogenes are the main cause of autosomal recessive chronic granulomatous disease. Blood. 2000;95(6):2150–6.
Noack D, Rae J, Cross AR, Ellis BA, Newburger PE, Curnutte JT, et al. Autosomal recessive chronic granulomatous disease caused by defects in NCF-1, the gene encoding the phagocyte p47-phox: mutations not arising in the NCF-1 pseudogenes. Blood. 2001;97(1):305–11.
Dekker J, de Boer M, Roos D. Gene-scan method for the recognition of carriers and patients with p47(phox)-deficient autosomal recessive chronic granulomatous disease. Exp Hematol. 2001;29(11):1319–25.
Roos D, Kuhns DB, Maddalena A, Bustamante J, Kannengiesser C, de Boer M, et al. Hematologically important mutations: the autosomal recessive forms of chronic granulomatous disease (second update). Blood Cells Mol Dis. 2010;44(4):291–9. doi:10.1016/j.bcmd.2010.01.009. PubMed PMID: 20167518, PubMed Central PMCID: PMC4568122, Epub 2010 Feb 18. Review.
Hayrapetyan A, Dencher PC, van Leeuwen K, de Boer M, Roos D. Different unequal cross-over events between NCF1 and its pseudogenes in autosomal p47(phox)-deficient chronic granulomatous disease. BiochimBiophysActa. 2013 Oct;1832(10):1662–72.
Kuhns DB, Alvord WG, Heller T, Feld JJ, Pike KM, Marciano BE, et al. Residual NADPH oxidase and survival in chronic granulomatous disease. N Engl J Med. 2010;363(27):2600–10.
Wada T, Muraoka M, Toma T, Imai T, Shigemura T, Agematsu K, et al. Rapid detection of intracellular p47phox and p67phox by flow cytometry; useful screening tests for chronic granulomatous disease. J Clin Immunol. 2013;33(4):857–64.
Roos D, de Boer M, Köker MY, Dekker J, Singh-Gupta V, Ahlin A, et al. Chronic granulomatous disease caused by mutations other than the common GT deletion in NCF1, the gene encoding the p47phox component of the phagocyte NADPH oxidase. Hum Mutat. 2006;27(12):1218–29.
Gathmann B, Grimbacher B, Beauté J, et al. ESID Registry Working Party. The European Internet-based patient and research database for primary immunodeficiencies: results 2006–2008. Clin Exp Immunol. 2009;157 suppl 1:3–11.
Joshi AY, Iyer VN, Hagan JB, St Sauver JL, Boyce TG. Incidence and temporal trends of primary immunodeficiency: a population-based cohort study. Mayo Clin Proc. 2009;84(1):16–22.
Carnide EG, Jacob CA, Castro AM, Pastorino AC. Clinical and laboratory aspects of chronic granulomatous disease in description of eighteen patients. Pediatr Allergy Immunol. 2005;16(1):5–9.
Madkaikar M, Mishra A, Desai M, Gupta M, Mhatre S, Ghosh K. Comprehensive report of primary immunodeficiency disorders from a tertiary care center in India. J Clin Immunol. 2013;33(3):507–12.
Verma S, Sharma PK, Sivanandan S, Rana N, Saini S, Lodha R, et al. Spectrum of primary immune deficiency at a tertiary care hospital. Indian J Pediatr. 2008;75(2):143–8.
Vowells SJ, Fleisher TA, Sekhsaria S, Alling DW, Maguire TE, Malech HL. Genotype-dependent variability in flow cytometric evaluation of reduced nicotinamide adenine dinucleotide phosphate oxidase function in patients with chronic granulomatous disease. J Pediatr. 1996;128:104–7.
Casimir CM, Bu-Ghanim HA, Rodaway ARF, Bentley DL, Rowe P, Segal AW. Autosomal recessive chronic granulomatous disease caused by deletion at a dinucleotide repeat. Proc Natl Acad Sci USA. 1991;88:2753–7.
Heyworth PG, Noack D, Cross AR. Identification of a novel NCF-1 (p47-phox) pseudogene not containing the signature GT deletion: significance for A47 degrees chronic granulomatous disease carrier detection. Blood. 2002;100(5):1845–51.
Marciano BE, Spalding C, Fitzgerald A, Mann D, Brown T, Osgood S, et al. Common severe infections in chronic granulomatous disease. Clin Infect Dis. 2015;60(8):1176–83. doi:10.1093/cid/ciu1154. Epub 2014 Dec 23. PubMed PMID: 25537876; PubMed Central PMCID: PMC4400412.
Meshaal S, El Hawary R, Abdelaziz D, Alkady R, Galal N, Boutros J, et al. Chronic granulomatous disease: review of a cohort of Egyptian patients. Allergol Immunopathol (Madr). 2015;43(3):279–85.
Marciano BE, Rosenzweig SD, Kleiner DE, Anderson VL, Darnell DN, Anaya-O’Brien S, et al. Gastrointestinal involvement in chronic granulomatous disease. Pediatrics. 2004;114:462–8. PubMed: 15286231.
Freudenberg F, Wintergerst U, Roesen-Wolff A, Albert MH, Prell C, Strahm B, et al. Therapeutic strategy in p47-phox deficient chronic granulomatous disease presenting as inflammatory bowel disease. J Allergy Clin Immunol. 2010;125(4):943–6. doi:10.1016/j.jaci.2010.01.035.
Roos D, de Boer M. Molecular diagnosis of chronic granulomatous disease. Clin Exp Immunol. 2014;175(2):139–49. doi:10.1111/cei.12202. PubMed PMID: 24016250, PubMed Central PMCID: PMC3892405, Review.
Goldblatt D. Recent advances in chronic granulomatous disease. J Infect. 2014;69 Suppl 1:S32–5. doi:10.1016/j.jinf.2014.07.013. Epub 2014 Sep 26. Review.
Kang EM, Marciano BE, Deravin S, Zarember KA, Holland SM, Malech HL. Chronic granulomatous disease: overview and hematopoietic stem cell transplantation. J Allergy Clin Immunol. 2011;127(6):1319–26. doi:10.1016/j.jaci.2011.03.028. PubMed PMID: 21497887, PubMed Central PMCID: PMC3133927, quiz 1327-8. Epub 2011 Apr 17. Review.
Seger RA. Hematopoietic stem cell transplantation for chronic granulomatous disease. Immunol Allergy Clin North Am. 2010;30(2):195–208. doi:10.1016/j.iac.2010.01.003. Review.
Acknowledgment
We would like to thank the Foundation of Primary Immunodeficiency (FPID) for providing antibodies for NADPH oxidase components. We also thank Prof. Martin de Boer, Sanquin Blood Supply Foundation, Amsterdam, The Netherlands, for his valuable suggestions to study the molecular mutations in this cohort.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Kulkarni, M., Desai, M., Gupta, M. et al. Clinical, Immunological, and Molecular Findings of Patients with p47phox Defect Chronic Granulomatous Disease (CGD) in Indian Families. J Clin Immunol 36, 774–784 (2016). https://doi.org/10.1007/s10875-016-0333-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-016-0333-y