Abstract
Deep dermatophytosis has been described in HIV and immunosuppressed patients. Recently, CARD9 (caspase recruitment domain-containing protein 9) deficiency has been reported in individuals with deep dermatophytosis previously classified as “immunocompetent”. We report a 24-year-old Brazilian male patient with deep dermatophytosis born to an apparently non-consanguineous family. The symptoms started with oral candidiasis when he was 3 years old, persistent although treated. At 11 years old, well delimited, desquamative and pruriginous skin lesions appeared in the mandibular area; ketoconazole and itraconazole were introduced and maintained for 5 years. At 12 years of age, the lesions, which initially affected the face, started to spread to thoracic and back of the body (15 cm of diameter) and became ulcerative, secretive and painful. Terbinafine was introduced without any improvement. Trichophyton mentagrophytes was isolated from the skin lesions. A novel homozygous mutation in CARD9 (R101L) was identified in the patient, resulting in impaired neutrophil fungal killing. Both parents, one brother (with persistent superficial but not deep dermatophytosis) and one sister were heterozygous for this mutation, while another brother was found to be homozygous for the CARD9 wild-type allele. This is the first report of CARD9 deficiency in Latin America.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dermatophytes are ubiquitous filamentous fungi, usually responsible for benign superficial infections, such as tinea capitis, tinea corporis and/or onychomycosis [1]. In rare cases, dermatophyte infection can lead to deep dermatophytosis with the invasion of the dermis and hypodermis by dermatophytes, sometimes affecting lymph nodes, brain, digestive tract and bones [2]. Most cases of deep dermatophytosis have been reported among HIV patients or patients on immunosuppressive therapy [3]. However, a number of cases were reported with deep dermatophytosis but otherwise healthy, mainly from North Africa, often born to consanguineous families and/or multiplex families, suggesting a genetic origin for the disease [4]. In 2013, the main genetic etiology for deep dermatophytosis was identified in 17 patients from eight unrelated North African families, bearing homozygous loss-of-function mutations in CARD9 (caspase recruitment domain-containing protein 9). A homozygous premature stop codon mutation (Q289*) was identified in 15 patients from seven unrelated Algerian and Tunisian families while a homozygous missense mutation (R101C) was found in two Moroccan siblings [5]. More recently, an additional patient born to Egyptian parents with extensive skin and nail dermatophytosis was also identified with the CARD9 Q289* mutation [6]. CARD9 is an adaptor molecule, found mainly expressed in macrophages and myeloid dendritic cells in mice, that plays a central role in antifungal defense by receiving signals from several C-type lectin-like receptors and stimulating pro-inflammatory responses [7]. Indeed, CARD9 deficient cells showed a selective impairment of TNF-α and IL-6 production upon fungal agonist stimulation [5, 8, 9]. We herein report a Brazilian patient with deep dermatophytosis and CARD9 deficiency due to the homozygous R101L mutation.
Case Report
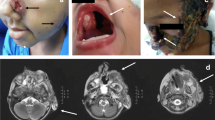
A 24-year-old Caucasian male, born to a non-consanguineous family of Italian origin, living in rural area, developed thrush at 3 years of age and erythematous cutaneous scaly lesions in mandibular area which have evolved to the whole body within the following years. At the same time, he lost his hair. Therapy has been introduced with nistatin for 6 years, followed by ketoconazole for 1 year (local and oral use) and the lesions were maintained under control at the beginning. At 11 years, well delimitated, scaly and pruritic skin lesions appeared which initially affected the face. Ketoconazole was thus reintroduced and maintained for 2 years with mild improvement at the beginning of the treatment. At 12 years, ulcerative, secretive and painful lesions installed in the face (lips) and spread to all the mandibular area. He was then referred to the Infectious Disease outpatient unit and itraconazole was introduced and maintained for 3 years, followed by terbinafin for the next 4 years. Transient improvement was observed after each therapeutic agent. In the following years, the lesions increased affecting his back (15 cm of diameter) and shoulders. Semi alopecia and onycodystrophy were observed (Fig. 1). Posaconazole was given for a period of 1 year (between 19 and 20 years old) and it was the best result obtained with antifungal therapy. In the last months, amphotericin B was administered with no response. Direct mycologic examination and biopsy identified hyaline septated hyphae positive for Trichophyton mentagrophytes after culture. Routine evaluation identified eosinophilia and high serum IgG and IgE levels. Immunophenotyping showed normal CD4+ and CD8+ T and CD19+ lymphocyte counts and low numbers of NK (CD16+/CD56+) cells. Lymphoproliferative T cell responses showed no T cell response to Candidin but normal to phytohaemaglutinin. Delayed-type hypersensitivity skin tests were negative for Candidin and of 8 mm for Trychophitin. Neutrophil killing of Candida or Staphylococcus was evaluated according to Saresella et al., 1997, modified [10] and found to be impaired in the patient’s sample for Candida only (Table 1). Neither his parents nor two of his siblings presented any symptom of fungal infection. However, one of his brothers had superficial dermatophytosis, usually treated with local antifungal cream; his lesions did not progress but were recurrent. We were unable to study viable cells in vitro or to perform flow cytometry for CARD9 protein from the patient and family members due to logistical difficulties. DNA samples from five family members were collected with their approval. AIRE was shown to be of wild-type sequence in the index case. CARD9 was amplified with specific primers. The patient was found to be homozygous for a novel c.302G>T variation in the exon 3 of the CARD9 gene (R101L) (CADD score at 9.927 with a cutoff of 6.612); his parents, his brother with superficial dermatophytosis and his sister were found to be heterozygous for this mutation (Fig. 2), and one brother was found to be homozygous wild-type. No homozygous or heterozygous individuals with that variation were reported in any of the various public databases (Human Gene Mutation Database, Ensembl, NHLBI GO Exome Sequencing Project [ESP], 1000 Genomes Project, and the Exome Aggregation Consortium [ExAC]) or in our in-house WES database (>2000 exomes), suggesting that this variant is extremely rare, possibly private to this kindred, and defines a novel AR deep dermatophytosis-causing allele. In addition, the Arginine at position 101 is highly conserved among species, and finally R101L is predicted to be deleterious by Sift (with a score of 0), and probably damaging by Polyphen 2 (with a score of 1.0) (Fig. 3).
Discussion
CARD9 is a key transducer of Dectin-1, Dectin-2 and Mincle signalling. CARD9 couples to BCL10 and regulates BCL10-MALT1-mediated NF-κB activation induced by various fungal ligands such as β-glucans, (such as curdlan, a selective Dectin-1 agonist) [11]. In humans, autosomal recessive CARD9 deficiency is associated with susceptibility to fungal infections, caused by Candida spp, dematiaceous fungi (Exophiala sp., Phialophora verrucosa) or Tricophyton spp, while these patients displayed normal immunity to common bacteria, intracellular bacteria, or viruses [4–6, 8, 9, 12–15]. The first clinical manifestation of the patient described here was oral candidiasis that started at 3 years of age and lasted until 11 years of age despite the use of several antifungal therapies. His symptoms worsened early in the adolescence, as previously reported for most CARD9 deficient patients with deep dermatophytosis [5]. Although our patient had dermatophytosis with profound and extended cutaneous involvement, he did not develop disseminated disease, as already reported for few patients [5, 6]. However, most patients with deep dermatophytosis and AR CARD9 deficiency developed invasive infections with bone involvement in one patient, brain involvement in another patient, and lymph node reaction in most patients [5]. Brain infections, mainly with Candida spp, but also Exophiala dermatitidis have been reported for several CARD9 deficient patients [5, 8, 9, 12, 13, 15].
The patient was found to be homozygous for the R101L CARD9 allele, even though the family did not report any consanguinity. However, the patient was born in a town with 15,000 inhabitants (Roncador, Parana State) suggesting cryptic consanguinity. Both parents were found heterozygous for the R101L CARD9 mutation and asymptomatic. This mutation has probably a high impact according to CADD, Polyphen 2 and Sift scores. Two of the patient’s siblings were also found heterozygous for the R101L CARD9 allele with one of them presenting persistent cutaneous dermatophytosis. Whether this clinical manifestation is incidental or caused by the heterozygous R101L mutation is unknown. To date, none of the heterozygous carriers reported displayed fungal infections [5, 8, 9, 12, 14, 15]. The R101L allele could affect CARD9 protein expression and/or interaction with its partners BCL-10/MALT1, as the mutation is located just after the CARD interacting domain. Unfortunately we were unable to retrieve blood in good condition to test these hypotheses. The patient displayed eosinophilia and high serum IgE levels, with normal T and B lymphocyte counts as previously reported [4–6, 12, 15], but with low numbers of CD16+/CD56+ NK cells. Furthermore, we found a negative delayed-type hypersensitivity skin test for Candidin but not Trychophitin. The patient presented a normal DHR response to PMA (phorbol mirystate acetate) suggestive of a normal NADPH oxidase activity. However, while Staphylococcus aureus killing by neutrophils was similar to a healthy non-related control tested in parallel, neutrophil Candida killing was impaired, as already reported [13]. This defect could contribute to the invasive nature of the fungal infection. Low levels of IL-6 and TNF-α by whole blood cells or monocyte-derived dendritic cells and low percentages of IL-17A-producing T cells were also reported in some CARD9 deficient patients, and may further contributing to the impaired defense against fungal microorganisms [4].
Conclusion
In summary, we describe a novel mutation related to CARD9 deficiency and deep dermatophytosis. This is the first CARD9 mutation reported in Latin American continent. Heterozygous individuals might be asymptomatic or present with mild infection. Neutrophil evaluation showed specific impaired fungal killing probably related to the severity of the disease.
Abbreviations
- CARD9:
-
caspase recruitment domain-containing protein 9
- CADD:
-
Combined Annotation Dependent Depletion
References
Seebacher C, Bouchara JP, Mignon B. Updates on the epidemiology of dermatophyte infections. Mycopathologia. 2008;166:335–52.
Hay RJ, Baran R. Deep Dermatophytosis: rare infections or common, but unrecognized, complications of lymphatic spread? Curr Opin Infect Dis. 2004;17:77–9.
da Silva BC, Paula CR, Auler ME, Ruiz Lda S, Dos Santos JI, Yoshioka MC, et al. Dermatophytosis and immunovirological status of HIV-infected and AIDS patients from Sao Paulo city. Brazil Mycoses. 2014;57(6):371–6.
Lanternier F, Cypowyj S, Picard C, Bustamante J, Lortholary O, Casanova JL. Puel A Primary immunodeficiencies underlying fungal infections. Curr Opin Pediatr. 2013;25:736–47.
Lanternier F, Pathan S, Vincent QB, Liu L, Cypowyj S, Prando C, et al. Deep dermatophytosis and inherited CARD9 deficiency. N Engl J Med. 2013;369:1704–14.
Jachiet M, Lanternier F, Rybojad M, Bagot M, Ibrahim L, Casanova JL, et al. Posaconazole treatment of extensive skin and nail dermatophytosis due to autosomal recessive deficiency of CARD9. JAMA Derm. 2015;151:192–4.
Hsu YMS, Zhang Y, You Y, Wang D, Li H, Duramad O, et al. Lin X The adaptor protein CARD9 is required for innate immune responses to intracellular pathogens. Nat Immunol. 2007;8(2):198–205.
Lanternier F, Barbati E, Meinzer U, Liu L, Pedergnana V, Migaud M, et al. Inherited CARD9 deficiency in 2 unrelated patients with invasive exophiala infection. J Infect Dis. 2015;211(8):1241–50.
Lanternier F, Mahdaviani SA, Barbati E, Chaussade H, Koumar Y, Levy R, et al. Inherited CARD9 deficiency in otherwise healthy children and adults with Candida species-induced meningoencephalitis, colitis, or both. J Allergy Clin Immunol. 2015. doi:10.1016/j.jaci.2014.12.1930 [Epub ahead of print].
Saresella SMKR, Speciale L, Taramelli D, Mendozzi E, Guerini F. A rapid evaluation of phagocytosis and killing of Candida albicans by CD13q leukocytes. J Immunol Methods. 1997;210:227–34.
Gross O, Gewies A, Finger K, Schafer M, Sparwasser T, Peschel C, et al. Card9 controls a non-TLR signaling pathway for innate anti-fungal immunity. Nature. 2006;442:651–6.
Glocker EO, Hennigs A, Nabavi M, Schäffer AA, Woellner C, Salzer U, et al. Grimbacher B a homozygous CARD9 mutation in a family with susceptibility to fungal infections. N Engl J Med. 2009;361(18):1727–35.
Drewniak A, Gazendam RP, Tool ATJ, van Houdt M, Jansen MH, van Hamme JL, et al. Invasive fungal infection and impaired neutrophil killing in human CARD9 deficiency. Blood. 2013;121(13):2385–92.
Wang X, Wang W, Lin Z, Wang X, Li T, Yu J, et al. CARD9 mutations linked to subcutaneous phaeohyphomycosis and TH17 cell deficiencies. J Allergy Clin Immunol. 2013;133(3):905–908e3.
Gavino C, Cotter A, Lichtenstein D, Lejtenyi D, Fortin C, Legault C, et al. Vinh DC CARD9 deficiency and spontaneous central nervous system candidiasis: complete clinical remission with GM-CSF therapy. Clin Infect Dis. 2014;59(1):81–4.
Acknowledgments
We are grateful to Mrs Ilda Sizue Kunii, BSc and Fernanda G Weiler, MD for checking AIRE gene for mutation. This work was supported by l’Agence Nationale de la Recherche (grant HGDIFD to A.P.), and a Translational Research grant from the Jeffrey Modell Foundation (to A.P.).
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Grumach, A.S., de Queiroz-Telles, F., Migaud, M. et al. A Homozygous CARD9 Mutation in a Brazilian Patient with Deep Dermatophytosis. J Clin Immunol 35, 486–490 (2015). https://doi.org/10.1007/s10875-015-0170-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-015-0170-4