Abstract
Non-methane volatile organic compounds (NMVOCs) play key roles in local and regional atmospheric chemistry as precursors for the production of ozone and secondary organic aerosols. Ambient air C2-C5 NMVOCs were measured at a tropical forest site in the central Western Ghats and urban site of Udaipur in India during the late monsoon period of 2016–17 and 2015, respectively. In the Western Ghats, air samples were collected from the protected Bhagwan Mahaveer Sanctuary. Ethene, propene, and isoprene were the dominant biogenic compounds with mean concentrations of 4.8 ± 2, 1.6 ± 0.66 and 1.05 ± 0.43 ppb, respectively. The concentrations of anthropogenic compounds such as propane and pentane were significantly lower than those of light alkenes. The contributions of ethene and propene among different NMVOCs were ~ 44 and 14%, respectively. However, the contributions of isoprene were highly variable of 3–22%. The tight correlation (r2 = 0.90) between the mixing ratios of ethene and propene and their ratio indicates their common formation and emission mechanisms. The molar emission ratio of ethene/propene (2.9 ± 0.17 ppb ppb−1) was comparable to those measured at other biogenic sites of Asia while higher than those reported for mid-latitude sites. The concentrations of light alkenes and isoprene at the Western Ghats were 4–5 times higher than those measured in an urban environment in the same season. The higher ozone formation potentials and Propylene-Equivalent concentrations of alkenes and isoprene than those of other NMVOCs indicate important implications of biogenic emissions on ozone photochemistry in the forest regions of India.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The global carbon cycle studies have been mainly focused on the cycling of carbon dioxide (CO2) between different land and oceanic reservoirs. In addition to the cycles of carbon in the forms of atmospheric CO2 and methane (CH4), it is important to consider the non-CO2 loss in the form of volatile organic compounds (VOCs). For example, the emission of isoprene and other biogenic-VOCs (BVOCs) from terrestrial vegetation is sensitive to changes in atmospheric CO2 and temperature (e.g., Malik et al. 2018; Singh et al. 2008). Therefore, non-CO2 loss of carbon should be accounted for the accurate regional carbon balance. More importantly, non-CO2 carbon loss in tropical forests is expected to be higher than those of other biomes (Unger 2014). The main factors responsible for the additional non-CO2 carbon loss in the tropics are high temperature and intense solar flux. The complex biosynthetic pathways of many VOCs produced in plants are linked to CO2 exchange between the vegetation and the atmosphere (Fasbender et al. 2018).
VOCs describe a wide range of chemical compounds emitted from various anthropogenic (Ambade et al. 2020a, 2020b; Kumar et al. 2020; Sahu et al. 2020b; Sahu et al. 2016) and natural sources, such as vegetation, marine algae and microbiological decomposition (Kuzma et al. 1995; Zemankova and Brechler 2010). VOCs are ubiquitous in the atmosphere and play an important role in regional and global atmospheric chemistry and climate change (Velasco et al. 2008). The estimated emission rates of ~1150 TgC yr−1 of VOCs from biogenic sources are about 7–8 times greater than those of non-biogenic VOCs (Stewart et al. 2003; Myhre et al. 2013). Monoterpenes (C10H16) and isoprene (2-methyl-1,3-butadiene, C5H8) are two major BVOCs. On a global scale, BVOCs are composed of 44% isoprene, 11% monoterpenes and 22.5% other reactive VOCs (Guenther et al. 2012; Guenther et al. 1995). Thus far, most of the studies have focused on emission and atmospheric processes of isoprene and monoterpenes among the group of BVOCs. In particular, isoprene emissions from forest regions have been extensively studied in the past decades (Sharkey and Yeh 2001 and references therein). In addition to their key roles in the global atmospheric chemistry, BVOCs are important chemical messengers in regulating the growth and reproduction of plants, resisting environmental stress, and preventing the harm of animals and insects (Lun et al. 2020).
The light alkenes, also defined as the C2-C4 alkenes, which include ethene (C2H4), propene (C3H6), 1-butene, trans-2-butene, and cis-2-butene (C4H8) are important BVOCs. Globally, light alkenes are emitted from both anthropogenic and biogenic sources (Sahu 2012). Light alkenes have local and regional anthropogenic emissions due to the use of fossil fuels (Goldstein et al. 1996). Among the biogenic sources, the formation and emission mechanisms of light alkene are not well established (Rhew et al. 2017). Nonetheless, emissions could be induced when a plant experiences any forms of stress, either internal or external (Holopainen and Gershenzon 2010). The emissions of light alkenes have been reported from vegetation for various forest environments (Goldstein et al. 1996; Rhew et al. 2017). Considerable variability of biogenic emissions can be due to various environmental factors, particularly ambient temperature and intensity of the photosynthetically active radiation (PAR) (Kesselmeier and Staudt 1999; Tripathi and Sahu 2020). The light-independent fraction of emission flux (FTLIDF) of temperature responses can be parameterized according to the following equation (Schade and Goldstein 2001).
Where β is an empirical coefficient, T is the ambient temperature (°C), and Fref is the observed emission flux at reference temperature (Tref) of 30 °C.
Ethene is a major plant hormone and used as a ripening agent for agricultural products. It is an important phytohormone and its emission flux from vegetation has been used as an indicator of stress (Wellburn and Wellburn 1996). Emission of BVOCs can be induced at any time from leaves of plant species associate with both abiotic (breakdown of organic matter) and biotic (enzymatically) stresses (Dicke and Baldwin 2010; Loreto et al. 2006; Loreto and Schnitzler 2010). Unlike alkanes, light alkenes are also emitted significantly from the surface seawater biological activity (Bonsang and Lambert 1985; Liss and Johnson 2014; Ratte et al. 1993; Tripathi et al. 2020a, 2020b). The emissions of light alkenes are about 10% of isoprene from biogenic sources (Guenther et al. 2006; Poisson et al. 2000). Emissions of ethene from vegetation are estimated to be ~11.8 TgC yr−1, accounting for about one-third of the global budget of 34.5 TgC yr−1 (Poisson et al. 2000). Recent studies on BVOC emissions suggest that plant residue decomposition can also emit a large number of light non-methane hydrocarbons (NMHCs) from natural soils (Hayward et al. 2001; Hellén et al. 2006). However, in contrast to living plants and vegetation, the studies on BVOC emissions from leaf litter and degrading plant matter are very limited. Isidorov and Jdanova (2002) have reported the emissions of ~70 different VOC compounds from decomposing leaf litter of deciduous trees.
The literature on the emission rates of BVOCs from a specific plant or tree species in the tropical regions of South Asia is not readily available mainly due to the lack of measurement-based studies characterizing the emissions of BVOCs. Varshney and Singh (2003) have reported the emission fluxes of isoprene and monoterpenes for some tropical plants of India. So far, there are no reports on the ambient air measurement of light alkenes in the forested regions of the Indian subcontinent. The lack of observational studies at regional scales is one of the major limitations hampering scientific progress for assessing biogenic sources and related atmospheric processes (Chaliyakunnel et al. 2019). The tropical forests with large biodiversity have attracted many researchers working in different tropics (Hoorn et al. 2010). The Western Ghats (WG) in tropical central and southern India is one of the globally recognized biodiversity hotspots (Myers et al. 2000). The present study focuses on the results of atmospheric measurements of biogenic NMVOCs at the densely forested central region of the WG during the late monsoon of the years 2016 and 2017. We have also compared the present NMVOCs data with the measurements at an urban site in India during the late monsoon of the year 2015. Our main objective was to investigate the composition of different NMVOCs and characterize the biogenic sources of light alkenes.
2 Material and methods
2.1 Study region and vegetation
The WG is a long mountain range (~1600 km) along the west coast of peninsular India between 8° to 21°N through the states of Maharashtra, Goa, Karnataka, Tamil Nadu and Kerala (Joshi and Karanth 2013). The WG, also known as the Sahyadri (Benevolent Mountains), is home to four tropical and subtropical moist broadleaf forests, namely the northwest WG moist deciduous forests, north WG montane rain forests, south WG moist deciduous forests and southwest WG montane rain forests. The moderate to dense forests cover ~30% of the total geographical area (3702 km2) of Goa, while ~69% of the area is covered by forest and tree (https://www.forest.goa.gov.in/fca/). There is significant diversity in the forests due to the variation in altitude, soil characters, slope, etc. The percentages of the tropical wet evergreen, tropical semi-evergreen, and tropical moist deciduous forests are about 25%, 19%, and 25%, respectively. To the best of our knowledge, despite the great biogenic significance of the WG, the measurements based studies of BVOCs are not reported to understand the emissions and associated atmospheric processes. As shown in Fig. 1, the site at Mahadev Temple, Tambdi Surla (15.4390° N, 74.2526° E; 890 m amsl) located in the Bhagwan Mahaveer Sanctuary (BMS) is mainly influenced by biogenic emissions with negligible activities of anthropogenic emissions as most of the areas are protected. The area of protected BMS and Mollem National Park is about 207 km2 at an average elevation of 890 m.
The left panel shows Goa state (province) and Udaipur (UDR, red triangle) urban site marked in the map of India and the right panel shows the location of Bhagwan Mahaveer Sanctuary (BMS, red square). The maps are retrieved from the Bhuvan earth visualization portal of the National Remote Sensing Centre (NRSC), Indian Space Research Organisation (https://bhuvan.nrsc.gov.in/)
The vegetation cover of the wildlife sanctuary is quite diverse with a mixture of moist deciduous, semi-evergreen and evergreen forests. Evergreen species like Actinodaphne angustifolia, Knema attenuata, Polyalthia fragrans, etc., are common trees in this ecosystem. Semi-evergreen species like Calamus thwaitesii are found at lower elevation areas while species like Maesa indica, Gnidiaglauca, Macaranga peltata, Canthium dicoccum, etc., are found at elevations above 200 m. Between the altitudes of 80 to 200 m, a wide range of moist deciduous forest species like Careyaarborea, Haldinacordifolia, Careyaarborea, etc., are common. The dominant families in these domains are Rubiaceae, Bignoniaceae, etc. Besides these forest cover, there are plateau and grassland which give the national park its rich diversity (Datar and Lakshminarasimhan 2013).
2.2 Sampling and analysis of VOCs
The air samples were collected in 800 to 1000 mL of flasks using an oil-free ‘Metal Bellow’ compressor (High speed Appliances, India, mini diaphragm type, Model No. IS 4722). We collected 60 samples at the BMS biogenic site and 23 samples at Udaipur (UDR, 24.58°N, 73.68°E; 598 m amsl) urban site with replicas for 10% of the samples. The samples at BMS were collected during daytime (9:00 h to 18:00 h), while during both day and night at UDR. These air samples were analyzed using a gas chromatography-flame ionization detector (GC-FID) (7890A-G3440A, Agilent, Palo Alto, CA, USA) coupled with thermal desorption (TD) system (UNITY2, Air Server 3 channel module, Markes International, UK) at Physical Research Laboratory (PRL), Ahmedabad, India. In the first step, air samples were pre-concentrated on the cold trap (U-T17O3P-2S: Ozone precursors, Markes International GmbH, Germany) at a low temperature of −15 °C and then desorbed rapidly by heating at 275 °C. The desorbed samples were then transferred into the GC column via a heated (100 °C) transfer line (capillary). An aluminum oxide PLOT (Al2O3/Na2SO4) column (50 m × 0.53 mm) was used to separate different C2-C5 NMVOCs. An ultra-high pure Helium (He) gas (Research Grade 99.9999%, Linde, USA) was used as a carrier gas. The zero-air generated by Parker HPZA-3500-220 and hydrogen gas using Parker H2PD-300-220 were used as fuel gases for the FID. The nitrogen gas (99.9999%, Parker UHPN2–1100-220) was used as a purge and make-up gas in the TD and the FID, respectively. The flow rates of zero air and hydrogen gas were set at 350 and 35 mL min−1, respectively. The GC oven temperature was programmed for the desired separation as follows: initially heated at 97 °C (0.5 min hold), increased to 110 °C at the rate 2 °C min−1 (6 min hold), and finally increased to 190 °C at the rate 5.2 °C min−1 (10 min hold).
The signals of different NMVOCs were calibrated using dynamic dilutions of a standard mixture containing ~1.0 (±5%) ppm of each compound (LE732C 4/15, Linde, USA). A gas calibration unit (GCU-advanced v2.0, Ionicon Analytik GmbH, Austria) was used to set the known concentrations required for the multi-point calibration. The flow rate of calibration standard was controlled using a mass flow controller (MFC-standard) to set variable amounts of standard gas into the flow of dilution gas (zero-air) set at 500 sccm (standard cubic centimeters per minute) using another mass flow controller (MFC-dilution). In the GCU system, the zero-air gas is produced by allowing ambient air via a heated (350 °C) VOC-scrubber catalyst. It is desirable to get a very stable zero-air supply to determine the background signal, sensitivity and limit of detection (LOD). Therefore, we configured ultra-purity Parker HPZA-3500-220 zero-air generator in tandem with GCU to generate stable and VOC-free zero-air.
For each VOC, the limit of detection (LOD) was determined by the following equation (Rovelli et al. 2019).
Where σblank is the standard deviation of blank (zero-air). The sensitivity was determined using the multi-point calibration fit between the set volume mixing ratio (ppb) and normalized peak area, which showed excellent linear relations with correlation coefficients (r2) > 0.98 at p < 0.05.
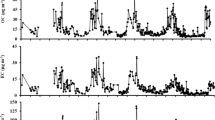
The precision was estimated as the relative standard deviation (RSD) at different set mixing ratios of ~2, 4, 8 ppb with 5 replicates for each value. The overall analytical precisions of different NMVOCs were in the range of 2–15%. Accuracies of all NMVOCs were estimated to be better than 10% including the uncertainties of calibration standard (~5%) and flow rates (~2%) of both the standard gas and zero-air. The representative chromatograms of the analysis of VOCs in air samples collected during the sunny, cloudy sky-conditions and for a blank sample (zero-air) are shown in Fig. 2. The peaks of light alkenes in the samples for the sunny sky conditions were greater than those in the cloudy sky-conditions. Further details of the TD-GC-FID system and key operational parameters are provided in our recent papers (Dave et al. 2020; Sahu et al. 2020a; Tripathi et al. 2020a, b).
Typical chromatograms of VOCs analyzed by TD-GC-FID for air samples collected during the Sunny, Cloudy sky conditions, and blank sample (zero-air). The amplitude and area under the peaks of alkenes in the air samples collected during the Sunny sky conditions were greater than those for the Cloudy sky conditions. However, the signals of anthropogenic compounds do not show much differences between the samples collected during different sky conditions
During the sampling period, the ambient air temperature, relative humidity (RH) and solar radiation flux varied in ranges of 27–32 31 °C, 64–84% and 90–870 W m−2, respectively. The calm winds (<1.0 m s−1) mostly from the south/southwest (S/SW) directions prevailed during the study period. Average wind fields using the National Centers for Environmental Prediction (NCEP) Reanalysis 1 project data and 7-day back trajectories using the Hybrid Single Particle Lagrangian Integrated Trajectory (HYSPLIT) model (Rolph et al. 2017; Stein et al. 2015) for the sampling periods are shown in Fig. 3.
Average wind fields during September 13 and 14 of the year 2017 using the National Centers for Environmental Prediction (NCEP) Reanalysis 1 project data (https://www.esrl.noaa.gov/psd/data/gridded/data.ncep.reanalysis.html). The 7-day back trajectories (dark pink) calculated for every 2 h interval during the sampling days are also plotted. The back trajectory data were obtained from the Air Resources Laboratory’s (ARL’s) Hybrid Single Particle Lagrangian Integrated Trajectory (HYSPLIT) model
3 Results and discussion
3.1 Concentrations of VOCs and their ratios
The time series concentrations of different VOCs in different samples collected at the BMS site are plotted in Fig. 4. On clear sky conditions, ethane, ethene, propene, and isoprene were the dominant compounds with the concentration ranges of 0.63–2.7, 2.4–10.2, 0.8–3.6, and 0.27–2.01 ppb, respectively. The concentrations of propane and other compounds were significantly lower than those of ethane and light alkenes. However, for rainy conditions on September 14 (15:45–16:30 h), the concentrations of all VOCs except n-pentane were within the ranges of their levels measured during the clear sky conditions. The concentrations of n-pentane (1.1–2.3 ppb) for rain-influenced samples were significantly higher compared to 0.04–0.18 ppb measured under normal or clear-sky conditions. In the afternoon hours (14:00–14:30 h) of September 13, unusually lower concentrations of isoprene (<1.0 ppb) were measured under cloudy conditions. At the BMS site, the average concentrations of ethene, propene, and isoprene were 4.8 ± 2, 1.6 ± 0.7 and 1.05 ± 0.4 ppb, respectively in the year 2017. The higher concentration of ethane (1.1 ± 0.4 ppb) could be due to the contributions from biogenic sources and transport from other sources due to its higher lifetime in the atmosphere. The concentrations of ethene, propene and isoprene in the samples taken during the rainy conditions were about 3.0, 1.1 and 1.3 ppb, respectively. The rainy conditions seem to inhibit the biogenic emissions of light alkenes emitted from trees and plants. The emission rates of BVOCs from vegetation show strong dependence on ambient temperature and sunlight intensity. Therefore, the decrease of solar insolation can inhibit biogenic emissions from plants during cloudy or rainy weather conditions. The reaction rate constants and statistics (range, mean, standard deviation) of the concentration and percentage contribution of different VOCs sites are presented in Table 1. The concentrations of aromatic VOCs (benzene and toluene) were also measured at the BMS site but are not presented in this study. The concentration of both benzene and toluene were very low of ~100 and 210 ppt, respectively. Relatively much lower concentrations of these anthropogenic compounds support the discussion that the BMS region is least influenced by anthropogenic activity. The analysis of 7 day back trajectory and wind field plots also confirms that the BMS site was mainly influenced by local biogenic emission sources and transport of oceanic air masses from the Arabian Sea during the study period. Tripathi et al. (2020b) have reported high levels of light alkenes in the marine boundary layer of the Arabian Sea during the inter-monsoon spring. Hence, oceanic emission of ethene and propene could also contribute to the higher levels of alkenes. We have compared the levels of some common VOCs measured at the UDR urban site located in Rajasthan province of India. The annual data for 2015 at UDR is reported in Yadav et al. (2019), but we have used the data measured in September for the comparison. The average concentrations of ethene (2.3 ± 0.7 ppb), propene (0.73 ± 0.4 ppb), and isoprene (0.72 ± 0.2 ppb) at the UDR site were lower than those measured at the BMS site indicating their dominant emissions from biogenic sources. While the concentrations of other compounds at UDR were significantly higher than their levels at BMS indicating their anthropogenic emissions. Although the emission from biogenic sources is a major contributor of isoprene in the global atmosphere (e.g., Guenther et al. 1995; Jobson et al. 1994) the recent studies have reported that the anthropogenic emissions, mainly from the vehicular exhaust in urban regions could be significant (e.g., Borbon et al. 2001; Yadav et al. 2019). Studies in urban areas have reported relatively strong anthropogenic emissions in winter (e.g., Sahu et al. 2017). However, both biogenic and anthropogenic sources could be significant during the summer (Borbon et al. 2001; Nichol and Wong 2011; Wang et al. 2013). Therefore, the concentrations of isoprene measured at the UDR site are mainly attributed to anthropogenic emissions. This is consistent with the fact that the biogenic contributions are small at Udaipur which has a semi-arid climate condition with a total annual rainfall of ~580 mm (recorded mainly during the south-west monsoon period).
The ratios of different pairs of VOCs have been used to broadly characterize and differentiate the contributions of biogenic and anthropogenic sources (Sahu et al. 2017; Tang et al. 2009). However, with certain uncertainties, the ratios measured in the atmosphere can also account for the variations caused by the boundary layer dynamics and meteorological processes, but not necessarily the loss due to oxidations mainly by OH radicals. The time series of ethene/propene, ethene/ethane, propene/ethane, and ethane/propane ratios are also plotted in Fig. 4. Given negligible biogenic emissions, propane concentration has been used as a reference for the long-range transport from anthropogenic sources. Except for ethene/propene, the ratios of other pairs of VOCs at the BMS site show higher values than their ratios measured at the UDR. In addition to different levels, the VOCs ratios at the BMS site were relatively more stable and showed small variability than those at the UDR site. At biogenic site (BMS), the ethene/propene, ethene/ethane, propene/ethane and ethane/propane ratios were 3.1 ± 0.4, 4.2 ± 0.7, 1.4 ± 0.3, and 2.1 ± 0.6 ppb ppb−1, respectively. In contrast, these ratios at the UDR site were 5.2 ± 4.5, 1.2 ± 0.5, 0.3 ± 0.2, and 1.3 ± 0.6 ppb ppb−1, respectively. In particular, the significantly higher ratios of ethene/ethane and propene/ethane characterize the prevalence of biogenic sources at the BMS site. The result of compassion is consistent as biogenic emissions of ethane are much smaller than those of light alkenes (Guenther et al. 2006). The ethene/propene ratios of ~3 ppb ppb−1 measured in this study are slightly higher than the values of ~1.5–2.0 ppb ppb−1 reported at several other forested sites of Harvard forest (42o N, 72o W) and Rocky Mountains (39.1o N, 105.1o W), USA in the mid-latitude region (e.g., Goldstein et al. 1996; Lamanna and Goldstein 1999; Rhew et al. 2017).
3.2 Diurnal dependence
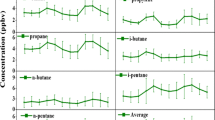
The diurnal dependence of ethene, propene, isoprene, and ethane of the concentrations and their ratios at the BMS site are compared with measurements at UDR (Fig. 5). The concentrations of light alkenes and isoprene at the BMS site were 4–5 times higher than their values measured at the UDR site. On the other hand, the daytime concentrations of ethane at the BMS site were slightly lower than those measured at UDR for the same period of the day. In comparison, the nighttime concentrations of ethane at the UDR site were much higher than those measured during the daytime. Similarly, the ratios of ethene, propene and isoprene concentrations to that of ethane concentration show major enhancements, particularly in the afternoon hours. However, the ethene/propene ratio at the BMS site does not show much variation with the local time.
In contrast to the measurements at the BMS, the ratios of ethene/ethane, propene/ethane, isoprene/ethane at the UDR site show decline in the afternoon hours. Overall, the diurnal patterns of the light alkenes and isoprene concentrations show distinct variations at the BMS and UDR sites. The distinct diurnal patterns also elucidate the major contributions of biogenic sources in the WG while that of anthropogenic sources in Udaipur. One of the important features at the BMS site is the sharp enhancement of isoprene concentration and isoprene/ethane ratio compared to those light alkenes and their ratios with ethane in the afternoon hours (12–15 h). Both light alkenes and isoprene are predominantly emitted from the biogenic sources but could be through different mechanisms. However, the dependence of BVOCs emissions from the WG on weather conditions is an important topic to investigate. For example, isoprene emissions are known to be very sensitive to the intensity of solar radiation and mainly emitted from the living vegetation (Malik et al. 2018; Singh et al. 2008). While emissions of light alkenes are known to be very sensitive to ambient temperature and emitted from both living vegetation and degradations of leaf litter and plant matter (Isidorov and Jdanova 2002). For the living vegetation, Rhew et al. (2017) have reported the light-independent fraction (LIDF) and light-dependent fraction (LDF) of the temperature emission response for light alkenes and isoprene. In addition to local time dependence, the hour-to-hour variations of VOCs were also influenced by the weather conditions. Typically, the concentrations of BVOC measured under cloudy conditions were lower than their levels during the clear-sky (sunny) conditions (Fig. 4).
3.3 Species composition and percentage contribution
The time series variation of the percentage contributions of different VOCs to all compounds at the BMS site in Goa is shown in Fig. 6. The data measured at the BMS site under clear-sky conditions have been used to discuss the composition and emission characteristics of BVOCs emitted from biogenic sources. On clear sky conditions, light alkenes make major contributions to ambient concentrations with 30–54% (mean 44 ± 5%) by ethene and 12–21% (14 ± 2%) by propene at the BMS site. The percentage contributions of ethane and isoprene were also significant which varied in the ranges of 8–16% and 3–22%, respectively. The contributions of propane were in the range of 3–10% (5 ± 1%). The samples collected under rain/drizzle conditions during 17:45–18: 30 h on 14 September 2017 show reductions in the contributions of almost all VOCs except n-pentane. The percentage contributions of ethene and propene were ~ 33 and 11%, respectively for rainy conditions. In 2016, the contributions of ethene, propene, and isoprene at the BMS site were about 52, 6 and 4%, respectively.
The percentage contributions of eight common VOCs (ethane, ethene, propane, propene, 1-butene, n-pentane, 1-pentene, and isoprene) measured at both BMS and UDR sites during the same season (late monsoon) are compared in Fig. 7. Unlike the BMS site, the contributions of some VOCs (e.g., ethane) at the UDR exhibit diurnal dependence. The abundances of ethene, propene, isoprene, and ethane account for about 48.6, 15.9, 11.7 and 11.2% of VOCs at the BMS site, while these were 21.4, 6.5, 6.6 and 19.2% at UDR, respectively. Other species like propane, 1-butene, and 1-pentene also make significant contributions at UDR as these VOCs are predominantly emitted from anthropogenic sources. The average percentage contributions of light alkenes (sum of ethene and propene) to the total measured VOCs were much higher of ~65% at BMS compared to 28% at UDR (Fig. 8). In summary, the abundances of major VOCs show distinct contributions at biogenic and anthropogenic sites.
3.4 Correlation between biogenic VOCs
The correlations between the concentrations of various VOCs can provide information about the major sources if the measurements are conducted near the emissions (e.g., Hough 1991; Rudolph 1995). Evidence of late monsoon biogenic emissions of ethene, propene, ethane, and some other VOCs is apparent in the comparison of scatter plots of ambient concentrations measured at biogenic (BMS) and urban (UDR) sites (Fig. 9). At the BMS site, the ethene, propene, and ethane concentrations were tightly correlated (r2 = 0.81–0.92). However, the concentrations of the light alkenes with the propane show poor correlations (r2 < 0.25) indicating their negligible contributions from anthropogenic sources (Fig. 10). The tight correlation between ethene and propene suggests that they are emitted from a common formation mechanism (Rhew et al. 2017). However, the light alkenes are highly reactive and hence have short-lifetimes in the atmosphere. As presented in Table 1, the reaction rate constant of ethene (kOH = 8.5 × 10−12 cm3 molecule−1 s−1) is ~3 times lower than that for propene (2.6 × 10−11 cm3 molecule−1 s−1) (Atkinson et al. 1997; Atkinson and Arey 2003). Therefore, the relation between ethene and propene can be influenced by photochemical aging once emitted into the atmosphere. The estimated atmospheric lifetimes of ethene and propene are ~1.5 days and 5.2 h, respectively (Sahu et al. 2011). However, stronger correlations between ethene, propene and ethane indicate simultaneous emissions of these species from local sources. The influence of aged air is negligible because the lifetimes of these VOCs are different. If the transport of aged air masses is dominant, then the correlations between these species may not be stronger. For the correlated pairs, the median molar emission ratios (ERs) were ethene/propene (2.9), ethene/ethane (4.6) and propene/ethane (1.5). In this study, the concentration ratios of propene/ethene, butene/ethene and butene/ethene were in the ranges of 0.28–0.59, 0.01–0.06, and 0.04–0.17, respectively. Rhew et al. (2017) have reported the median molar concentration ratios of ethene/propene of (1.82) at the Manitou Experimental Forest Observatory (MEFO), south-southwest of Denver, Colorado, USA. Tang et al. (2009) have reported the comparison of several VOCs including ethene and propene measured at various rural or remote sites in Asia. Based on the data presented in Tang et al. (2009), the ethene/propene ratios of 3.0–5.5 ppb ppb−1 were determined for predominantly biogenic sites. Goldstein et al. (1996) have reported biogenic emissions of ethene, propene and 1-butene with emission ratios of 4:2:1. Zimmerman et al. (1988) reported significant emissions of ethene and propene from the Amazon forest. The estimates of ethene/propene ratios of 0.9–2.1 ppb ppb−1 were published for the oceanic emissions.
Correlations between the concentrations of ethene, propene, and ethane measured at BMS (biogenic) and UDR (urban) sites. The slopes of ethene/propene measured at tropical site of Jianfeng Mountain (Tang et al. 2009) and mid-latitude sites of Harvard forest, USA (Goldstein et al. 1996), Rocky Mountains, USA (Rhew et al. 2017) and forested Central-Eastern Venezuela and other sites (Donoso et al. 1996 and references therein) are also plotted
As plotted in Fig. 9(a), the ethene/propene ratios in the present study fall within the range of values reported for distinct biogenic sites of the world. In comparison, ethene/ethane and propene/ethane ratios at the BMS site are significantly higher than those measured at other biogenic sites (Fig. 9a, b). At the BMS site, the median molar concentration ratios of ethene/ethane, propene/ethane, ethene/propane and propene/propane were about 4.6, 1.5, 9.5 and 3.2 ppb ppb−1, respectively. While at the UDR site, these were 1.2, 0.45, 1.4 and 0.44 ppb−1, respectively. The distinct values of these ratios measured at BMS and UDR suggested the influence and contributions of very different emission processes. In summary, the significantly higher molar concentration ratios of alkene/alkane at the BMS site characterize the biogenic emissions.
3.5 Ozone formation potential and propylene equivalent concentration of NMVOCs
Ozone is a greenhouse gas in the troposphere and can contribute to global climate change. The photochemical formation of ozone (O3) from NMVOCs is initiated by OH radical reactions and varies significantly for each NMVOC. The ozone formation potential (OFP) has been used to determine the contribution of different VOCs in the formation of O3 following their respective mechanism (e.g., Tan et al. 2012). The OFP defined by the following equation has also been used to assess the importance of different NMVOCs in the formation of ozone (Carter 2009; Carter 1994).
Where OFPi is the ozone formation potential of a given VOCi. While [VOCi] and MIRi are the concentration and maximum incremental reactivity (MIR) of VOCi, respectively. The values of MIR were taken from Carter (1994) for the estimation of OFP. The MIR and OFP value of different VOCs measured at both BMS and UDR are listed in Table 2. At the BMS site, the OFPs of ethene (40 μg m−3), propene (25 μg m−3) and isoprene (25 μg m−3) are significantly higher than the values (<10 μg m−3) calculated for other NMVOCs. On the other hand, at the UDR site, 1-butene (33 μg m−3), 1-Pentene (22 μg m−3) and isoprene (18 μg m−3) were the major contributors to the OFP. The comparison highlights the predominance of biogenic and anthropogenic NMVOCs at BMS and UDR sites, respectively.
Another scale is known as the Propylene-Equivalent (Propy–Equiv) concentration (Chameides et al. 1992), and for a given VOC (i) it can be defined as here.
The Propy–Equiv (i) provides a measure of the concentration of species (i) on OH reactivity based scale normalized with the reactivity of propylene (C3H6). Ci is expressed in ‘ppbC’ obtained by multiplying the volume mixing ratio (ppb) with the number of carbon atoms in a given NMVOC compound. As listed in Table 2, the Propy–Equiv concentrations of isoprene (19.2 ppbC), propene (4.7 ppbC), and ethene (3.1 ppbC) are significantly higher than the values (<2.0 ppbC) calculated for other NMVOCs at the BMC site. On the other hand, at the UDR site, isoprene (18 ppbC), 1-butene (7.7 ppbC), and 1-Pentene (7.5 ppbC) were the major contributors to the Propy–Equiv concentrations. For both the scales, the comparison of data highlights the predominance of biogenic and anthropogenic NMVOCs at the BMS and UDR sites, respectively. However, the formation of O3 in the lower atmosphere is influenced by other factors which include the reaction rate of NMVOCs, level of NOx, solar flux intensity and weather conditions (Tan et al. 2012). Therefore, in reality, both OFP and Propy–Equiv based methods have their limitations as the efficiency of O3 production also depends on the level of NOx and the intensity of sunlight. The sensitivity of photochemistry for the production of O3 is limited by the levels of both NMVOCs or NOx and is usually estimated by the ratio of NMVOCs/NOx (Chameides et al. 1992). Therefore, to reduce the production rate of O3, the reductions of NMVOCs emissions are only effective under the VOC-sensitive regime and reductions of NOx will only be effective under the NOx-sensitive regime (NRC 1992). Typically, under high NOx regimes, the rate of O3 production is determined by the levels of peroxy radicals formed during the oxidations of NMVOCs. At lower NOx values, it is the level of NOx that limits the formation of O3 (Carter 1994). Thus far, the impact of biogenic emission in the formation of ozone is not yet established over forested regions of India due to the lack of comprehensive VOC measurements. One of our future research programs is to make a comprehensive measurement of NMVOCs along with O3 and NOx at biogenic sites of India in different seasons.
4 Conclusions
The ambient air concentrations of C2-C5 NMVOCs were measured at forest sites in the central Western Ghats and an urban site of India during the late monsoon period. There is significant diversity in the forest vegetation of the Western Ghats covered by the tropical wet evergreen, tropical semi-evergreen, and tropical moist deciduous forests. The concentrations of different NMVOCs were measured at protected Bhagwan Mahaveer Sanctuary and Mollem national park. Ethane, ethene, propene, and isoprene were the dominant compounds while the contributions of propane and other compounds were relatively small. The concentrations of biogenic NMVOCs were elevated in the afternoon, but slightly lower values were measured in the morning and evening hours. The ethene/propene, ethene/ethane, propene/ethane, and ethane/propane ratios at the BMS site were significantly different from those measured at a typical urban site of Udaipur. In particular, the higher ratios of the light alkenes to ethane characterize the prevalence of biogenic emission and negligible influences of anthropogenic activities at BMS. The diurnal patterns of light alkenes and isoprene show much higher daytime values than those measured at an urban site. Another important feature is the sharp enhancements of isoprene concentration and isoprene/ethane ratio compared to light alkenes and their ratios with ethane in the afternoon hours. Among the different NMVOCs, light alkenes make major contributions of 30–54% by ethene and 12–21% by propene. The contributions of isoprene were highly variable with a mean of ~10%. The molar emission ratios of ethene/propene (2.9 ppb ppb−1) fall in the range of values reported for several biogenic sites of Asia while higher than those reported for the mid-latitude sites. The tropical forests are the major sources of biogenic NMVOCs in the global atmosphere. Therefore, the emission flux measurements of biogenic NMVOCs are necessary to parameterize and evaluate their emissions in the regional and global models. The ozone formation potential (OFP) and propylene equivalent concentration (PEC) of light alkenes and isoprene at BMS were significantly higher than those estimated for other NMVOCs. The comparison of data for both the scales of OFP and PEC highlights the predominance of biogenic NMVOCs. India is a tropical country with large and diverse forest coverage tends to emit abundant amounts of biogenic NMVOCs, which need to be quantified by in situ measurement-based validations. Our future work will be focused on the measurements of eddy emission fluxes of various BVOCS such as isoprene, monoterpenes and oxygenated compounds using our state-of-art PTR-TOF-MS 8000 instrument in the Western Ghats for the different seasons. Comprehensive measurements of VOCs and other reactive trace gases are required to understand the photochemistry leading to O3 and SOA formation in forest regions of South Asia.
Data availability
Data used in this present are available in figshare repository (https://figshare.com/s/f9e34ad1363906e96905).
References
Ambade, B., Sankar, T.K., Kumar, A., Sethi, S.S.: Characterization of PAHs and n-alkanes in atmospheric aerosol of Jamshedpur City, India. J. Hazardous Toxic Radioactive Waste. 24, 04020003 (2020a). https://doi.org/10.1061/(ASCE)HZ.2153-5515.0000490
Ambade, B., Sethi, S.S., Kumar, A., Sankar, T.K., Kurwadkar, S.: Health risk assessment, composition, and distribution of polycyclic aromatic hydrocarbons (PAHs) in drinking water of Southern Jharkhand. East India. Arch Environ Contam Toxicol. 80, 120–133 (2020). https://doi.org/10.1007/s00244-020-00779-y
Atkinson, R., Arey, J.: Atmospheric degradation of volatile organic compounds. Chem. Rev. 103, 4605–4638 (2003). https://doi.org/10.1021/cr0206420
Atkinson, R., Baulch, D., Cox, R., Hampson Jr., R., Kerr, J., Rossi, M., Troe, J.: Evaluated kinetic and photochemical data for atmospheric chemistry: supplement VI. IUPAC subcommittee on gas kinetic data evaluation for atmospheric chemistry. J. Phys. Chem. Ref. Data Monogr. 26, 1329–1499 (1997). https://doi.org/10.1063/1.556048
Bonsang, B., Lambert, G.: Nonmethane hydrocarbons in an oceanic atmosphere. J. Atmos. Chem. 2, 257–271 (1985). https://doi.org/10.1007/BF00051076
Borbon, A., Fontaine, H., Veillerot, M., Locoge, N., Galloo, J., Guillermo, R.: An investigation into the traffic-related fraction of isoprene at an urban location. Atmos. Environ. 35, 3749–3760 (2001)
Carter, W.P.: Development of ozone reactivity scales for volatile organic compounds. Air Waste. 44, 881–899 (1994)
Carter, W.P.: Updated maximum incremental reactivity scale and hydrocarbon bin reactivities for regulatory applications. California Air Resources Board Contract. 2009, 339 (2009)
Chaliyakunnel, S., Millet, D.B., Chen, X.: Constraining emissions of volatile organic compounds over the Indian subcontinent using space-based formaldehyde measurements. J. Geophys. Res.: Atmos. 124(19), 10525–10545 (2019). https://doi.org/10.1029/2019JD031262
Chameides, W., Fehsenfeld, F., Rodgers, M., Cardelino, C., Martinez, J., Parrish, D., Lonneman, W., Lawson, D., Rasmussen, R., Zimmerman, P.: Ozone precursor relationships in the ambient atmosphere. J. Geophys. Res. Atmos. 97, 6037–6055 (1992)
Datar, M.N., Lakshminarasimhan, P.: Check list of wild angiosperms of Bhagwan Mahavir (Molem) National Park, Goa, India [with erratum]. Check List. 9, 186–207 (2013)
Dave, P.N., Sahu, L.K., Tripathi, N., Bajaj, S., Yadav, R., Patel, K.: Emissions of non-methane volatile organic compounds from a landfill site in a major city of India: impact on local air quality. Heliyon. 6, e04537 (2020)
Dicke, M., Baldwin, I.T.: The evolutionary context for herbivore-induced plant volatiles: beyond the ‘cry for help’. Trends Plant Sci. 15, 167–175 (2010)
Donoso, L., Romero, R., Rondón, A., Fernandez, E., Oyola, P., Sanhueza, E.: Natural and anthropogenic C 2 to C 6 hydrocarbons in the central-eastern Venezuelan atmosphere during the rainy season. J. Atmos. Chem. 25, 201–214 (1996)
Fasbender, L., Yáñez-Serrano, A.M., Kreuzwieser, J., Dubbert, D., Werner, C.: Real-time carbon allocation into biogenic volatile organic compounds (BVOCs) and respiratory carbon dioxide (CO2) traced by PTR-TOF-MS, 13CO2 laser spectroscopy and 13C-pyruvate labelling. PLoS One. 13, e0204398 (2018)
Goldstein, A., Fan, S., Goulden, M., Munger, J., Wofsy, S.: Emissions of ethene, propene, and 1-butene by a midlatitude forest. J. Geophys. Res. Atmos. 101, 9149–9157 (1996)
Guenther, A., Hewitt, C.N., Erickson, D., Fall, R., Geron, C., Graedel, T., Harley, P., Klinger, L., Lerdau, M., McKay, W.: A global model of natural volatile organic compound emissions. J. Geophys. Res. Atmos. 100, 8873–8892 (1995). https://doi.org/10.1029/94JD02950
Guenther, A., Jiang, X., Heald, C., Sakulyanontvittaya, T., Duhl, T., Emmons, L., Wang, X.: The model of emissions of gases and aerosols from nature version 2.1 (MEGAN2. 1): an extended and updated framework for modeling biogenic emissions. (2012). https://doi.org/10.5194/gmd-5-1471-2012
Guenther, A., Karl, T., Harley, P., Wiedinmyer, C., Palmer, P.I., Geron, C.: Estimates of global terrestrial isoprene emissions using MEGAN (model of emissions of gases and aerosols from nature). Atmos. Chem. Phys. 6, 3181–3210 (2006)
Hayward, S., Muncey, R., James, A., Halsall, C.J., Hewitt, C.N.: Monoterpene emissions from soil in a Sitka spruce forest. Atmos. Environ. 35, 4081–4087 (2001)
Hellén, H., Hakola, H., Pystynen, K.-H., Rinne, J., Haapanala, S.: C 2-C 10 hydrocarbon emissions from a boreal wetland and forest floor. Biogeosciences. 3, 167–174 (2006)
Holopainen, J.K., Gershenzon, J.: Multiple stress factors and the emission of plant VOCs. Trends Plant Sci. 15, 176–184 (2010)
Hoorn, C., Wesselingh, F., Ter Steege, H., Bermudez, M., Mora, A., Sevink, J., Sanmartín, I., Sanchez-Meseguer, A., Anderson, C., Figueiredo, J.: Amazonia through time: Andean uplift, climate change, landscape evolution, and biodiversity. Science. 330, 927–931 (2010). https://doi.org/10.1126/science.1194585
Hough, A.M.: Development of a two-dimensional global tropospheric model: model chemistry. J. Geophys. Res. Atmos. 96, 7325–7362 (1991)
Isidorov, V., Jdanova, M.: Volatile organic compounds from leaves litter. Chemosphere. 48, 975–979 (2002)
Jobson, B., Wu, Z., Niki, H., Barrie, L.: Seasonal trends of isoprene, C2–C5 alkanes, and acetylene at a remote boreal site in Canada. J. Geophys. Res. Atmos. 99, 1589–1599 (1994)
Joshi, J., Karanth, P.: Did southern Western Ghats of peninsular India serve as refugia for its endemic biota during the cretaceous volcanism? Ecol. Evol. 3, 3275–3282 (2013)
Kesselmeier, J., Staudt, M.: Biogenic volatile organic compounds (VOC): an overview on emission, physiology and ecology. J. Atmos. Chem. 33, 23–88 (1999). https://doi.org/10.1023/A:1006127516791
Kumar, A., Ambade, B., Sankar, T.K., Sethi, S.S., Kurwadkar, S.: Source identification and health risk assessment of atmospheric PM2.5-bound polycyclic aromatic hydrocarbons in Jamshedpur, India. Sustainable cities and society 52, 101801. (2020). https://doi.org/10.1016/j.scs.2019.101801
Kuzma, J., Nemecek-Marshall, M., Pollock, W.H., Fall, R.: Bacteria produce the volatile hydrocarbon isoprene. Curr. Microbiol. 30, 97–103 (1995)
Lamanna, M.S., Goldstein, A.H.: In situ measurements of C2-C10 volatile organic compounds above a Sierra Nevada ponderosa pine plantation. J. Geophys. Res. Atmos. 104, 21247–21262 (1999)
Liss, P.S., Johnson, M.T.: Ocean-Atmosphere Interactions of Gases and Particles. Springer (2014)
Loreto, F., Barta, C., Brilli, F., Nogues, I.: On the induction of volatile organic compound emissions by plants as consequence of wounding or fluctuations of light and temperature. Plant Cell Environ. 29, 1820–1828 (2006)
Loreto, F., Schnitzler, J.-P.: Abiotic stresses and induced BVOCs. Trends Plant Sci. 15, 154–166 (2010)
Lun, X., Lin, Y., Chai, F., Fan, C., Liu, J.: Reviews of emission of biogenic volatile organic compounds (BVOCs) in Asia. J. Environ. Sci. 95, 266–277 (2020). https://doi.org/10.1016/j.jes.2020.04.043
Malik, T.G., Gajbhiye, T., Pandey, S.K.: Plant specific emission pattern of biogenic volatile organic compounds (BVOCs) from common plant species of Central India. Environ. Monit. Assess. 190, 631 (2018)
Myers, N., Mittermeier, R.A., Mittermeier, C.G., Da Fonseca, G.A., Kent, J.: Biodiversity hotspots for conservation priorities. Nature. 403, 853–858 (2000)
Myhre, G., Shindell, D., Bréon, F.-M., Collins, W., Fuglestvedt, J., Huang, J., Koch, D., Lamarque, J.-F., Lee, D., Mendoza, B.: Anthropogenic and natural radiative forcing. Climate Change. 423, 658–740 (2013). https://doi.org/10.1017/CBO9781107415324.018
Nichol, J., Wong, M.S.: Estimation of ambient BVOC emissions using remote sensing techniques. Atmos. Environ. 45, 2937–2943 (2011)
NRC (National Research Council): Rethinking the ozone problem in urban and regional air pollution. National Academies Press, Washington, District of Columbia (1992)
Poisson, N., Kanakidou, M., Crutzen, P.J.: Impact of non-methane hydrocarbons on tropospheric chemistry and the oxidizing power of the global troposphere: 3-dimensional modelling results. J. Atmos. Chem. 36, 157–230 (2000)
Ratte, M., Plass-Dülmer, C., Koppmann, R., Rudolph, J., Denga, J.: Production mechanism of C2-C4 hydrocarbons in seawater: field measurements and experiments. Global Biogeochem. Cycles. 7, 369–378 (1993). https://doi.org/10.1029/93GB00054
Rhew, R.C., Deventer, M.J., Turnipseed, A.A., Warneke, C., Ortega, J., Shen, S., Martinez, L., Koss, A.R., Lerner, B.M., Gilman, J.B.: Ethene, propene, butene and isoprene emissions from a ponderosa pine forest measured by relaxed eddy accumulation. Atmos. Chem. Phys. 17, 13417–13438 (2017)
Rolph, G., Stein, A., Stunder, B.: Real-time environmental applications and display sYstem: READY. Environ Model Softw. 95, 210–228 (2017). https://doi.org/10.1016/j.envsoft.2017.06.025
Rovelli, S., Cattaneo, A., Fazio, A., Spinazzè, A., Borghi, F., Campagnolo, D., Dossi, C., Cavallo, D.M.: VOCs measurements in residential buildings: quantification via thermal desorption and assessment of indoor concentrations in a case-study. Atmosphere. 10, 57 (2019)
Rudolph, J.: The tropospheric distribution and budget of ethane. J. Geophys. Res. Atmos. 100, 11369–11381 (1995)
Sahu, L.: Volatile organic compounds and their measurements in the troposphere. Current Science. 1645–1649. (2012) http://www.jstor.org/stable/24084821
Sahu, L., Lal, S., Venkataramani, S.: Seasonality in the latitudinal distributions of NMHCs over bay of Bengal. Atmos. Environ. 45, 2356–2366 (2011)
Sahu, L., Pal, D., Yadav, R., Munkhtur, J.: Aromatic VOCs at major road junctions of a Metropolis in India: measurements using TD-GC-FID and PTR-TOF-MS instruments. Aerosol Air Qual. Res. 16, 2405–2420 (2016). https://doi.org/10.4209/aaqr.2015.11.0643
Sahu, L., Tripathi, N., Yadav, R.: Contribution of biogenic and photochemical sources to ambient VOCs during winter to summer transition at a semi-arid urban site in India. Environ. Pollut. 229, 595–606 (2017)
Sahu, L., Tripathi, N., Yadav, R.: Observations of trace gases in Earth’s lower atmosphere: instrumentation and platform. Curr. Sci. 118, 1893 (2020a)
Sahu, L., Yadav, R., Tripathi, N.: Aromatic compounds in a semi-urban site of western India: seasonal variability and emission ratios. Atmos. Res. 246, 105114 (2020b)
Schade, G.W., Goldstein, A.H.: Fluxes of oxygenated volatile organic compounds from a ponderosa pine plantation. J. Geophys. Res. Atmos. 106, 3111–3123 (2001)
Sharkey, T.D., Yeh, S.: Isoprene emission from plants. Annu. Rev. Plant Biol. 52, 407–436 (2001)
Singh, U.K., Kumar, M., Chauhan, R., Jha, P., Ramanathan, A., Subramanian, V.: Assessment of the impact of landfill on groundwater quality: a case study of the Pirana site in western India. Environ. Monit. Assess. 141, 309–321 (2008). https://doi.org/10.1007/s10661-007-9897-6
Stein, A., Draxler, R.R., Rolph, G.D., Stunder, B.J., Cohen, M., Ngan, F.: NOAA’s HYSPLIT atmospheric transport and dispersion modeling system. Bull. Am. Meteorol. Soc. 96, 2059–2077 (2015)
Stewart, H.E., Hewitt, C., Bunce, R., Steinbrecher, R., Smiatek, G., Schoenemeyer, T.: A highly spatially and temporally resolved inventory for biogenic isoprene and monoterpene emissions: Model description and application to Great Britain. J. Geophys. Res.: Atmos. 108(D20), 4644 (2003). https://doi.org/10.1029/2002JD002694
Tan, J.-H., Guo, S.-J., Ma, Y.-L., Yang, F.-M., He, K.-B., Yu, Y.-C., Wang, J.-W., Shi, Z.-B., Chen, G.-C.: Non-methane hydrocarbons and their ozone formation potentials in Foshan, China. Aerosol Air Qual. Res. 12, 387–398 (2012). https://doi.org/10.4209/aaqr.2011.08.0127ccccc
Tang, J., Chan, L., Chang, C., Liu, S., Li, Y.: Characteristics and sources of non-methane hydrocarbons in background atmospheres of eastern, southwestern, and southern China. J. Geophys. Res.: Atmos. 114, (2009). https://doi.org/10.1029/2008JD010333
Tripathi, N., Sahu, L., Singh, A., Yadav, R., Karati, K.K.: High levels of isoprene in the marine boundary layer of the Arabian Sea during spring inter-monsoon: role of phytoplankton blooms. ACS Earth Space Chem. 4, 583–590 (2020a)
Tripathi, N., Sahu, L., Singh, A., Yadav, R., Patel, A., Patel, K., Meenu, P.: Elevated levels of biogenic nonmethane hydrocarbons in the marine boundary layer of the Arabian sea during the intermonsoon. JJ Geophys Res: Atmos. 125, e2020JD032869 (2020b)
Tripathi, N., Sahu, L.K.: Emissions and atmospheric concentrations of α-pinene at an urban site of India: role of changes in meteorology. Chemosphere. 127071, 127071 (2020). https://doi.org/10.1016/j.chemosphere.2020.127071
Unger, N.: On the role of plant volatiles in anthropogenic global climate change. Geophys. Res. Lett. 41, 8563–8569 (2014). https://doi.org/10.1002/2014GL061616
Varshney, C., Singh, A.P.: Isoprene emission from indian trees. J. Geophys. Res.: Atmos. 108, (2003). https://doi.org/10.1029/2003JD003866
Velasco, E., Márquez, C., Bueno, E., Bernabé, R., Sánchez, A., Fentanes, O., Wöhrnschimmel, H., Cárdenas, B., Kamilla, A., Wakamatsu, S.: Vertical distribution of ozone and VOCs in the low boundary layer of Mexico City. Atmos. Chem. Phys. 8, 3061–3079 (2008)
Wang, J.-L., Chew, C., Chang, C.-Y., Liao, W.-C., Lung, S.-C.C., Chen, W.-N., Lee, P.-J., Lin, P.-H., Chang, C.-C.: Biogenic isoprene in subtropical urban settings and implications for air quality. Atmos. Environ. 79, 369–379 (2013)
Wellburn, F., Wellburn, A.: Variable patterns of antioxidant protection but similar ethene emission differences in several ozone-sensitive and ozone-tolerant plant selections. Plant Cell Environ. 19, 754–760 (1996)
Yadav, R., Sahu, L., Tripathi, N., Pal, D., Beig, G., Jaaffrey, S.: Investigation of emission characteristics of NMVOCs over urban site of western India. Environ. Pollut. 252, 245–255 (2019). https://doi.org/10.1016/j.envpol.2019.05.089
Zemankova, K., Brechler, J.: Emissions of biogenic VOC from forest ecosystems in Central Europe: estimation and comparison with anthropogenic emission inventory. Environ. Pollut. 158, 462–469 (2010)
Zimmerman, P., Greenberg, J., Westberg, C.: Measurements of atmospheric hydrocarbons and biogenic emission fluxes in the Amazon boundary layer. J. Geophys. Res. Atmos. 93, 1407–1416 (1988)
Acknowledgments
The authors would like to thank Dr. Sanjeev Kumar, Dr. Niharika Sharma from PRL and Mr. K. Suresh from CSIR-NIO for their supports during the field campaign. The authors gratefully acknowledge the NOAA Air Resources Laboratory (ARL) for the provision of the HYSPLIT transport and dispersion model and/or READY website (https://www.ready.noaa.gov), and the National Centers for Environmental Prediction (NCEP) Reanalysis 1 project data (https://www.esrl.noaa.gov/psd/data/gridded/data.ncep.reanalysis.html used in this publication.
Author information
Authors and Affiliations
Contributions
The manuscript is written by Nidhi Tripathi. Dr. Lokesh Sahu has designed the experiment and suggested the corrections in the manuscript.
Corresponding author
Ethics declarations
Competing interests
The coauthor has no objection to publish this research work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
The first study reporting the measurements of light alkenes in the forested Western Ghats of India
Large biogenic emissions of BVOCs in the form of alkenes from tropical evergreen forests
The strong dependence of isoprene emission on local weather conditions
Implications of tropical biogenic emissions on regional atmospheric chemistry
Rights and permissions
About this article
Cite this article
Tripathi, N., Sahu, L.K., Patel, K. et al. Ambient air characteristics of biogenic volatile organic compounds at a tropical evergreen forest site in Central Western Ghats of India. J Atmos Chem 78, 139–159 (2021). https://doi.org/10.1007/s10874-021-09415-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10874-021-09415-y