Abstract
Comprehensive atmospheric studies have demonstrated that carbonaceous particles are one of the main components of atmospheric aerosols over Europe. The aim of our study was to establish an automated elemental analyser interfaced to a stable isotope mass spectrometer (EA-IRMS) method at the Hertelendi Laboratory of Environmental Studies (HEKAL), as a suitable method of quantification of total carbon mass in individual PM2.5 aerosol samples. Total carbon (TC) mass and simultaneous stable isotopic ratios were determined for both test standard and genuine aerosol samples. Finally, the results were compared to the ones obtained independently by an alternative sealed tube combustion method developed previously at HEKAL. The TC recovery tests of standard material prepared by the sealed tube method confirmed at least a carbon recovery yield of 92% for a broad range of carbon mass (100–2000 μg). The stable isotopic results confirmed that sealed tube method is reproducible and suitable to be used as a reference to verify our new EA-IRMS method. The EA-IRMS TC measurements of genuine aerosols gave on average 3% higher carbon recovery yield, relative to the uncorrected results of the sealed tube method. The comparison of the stable isotopic results by the two methods for aerosols also showed minimal differences. Consequently, the possibility of simultaneous TC and stable isotopic analyses makes the EA-IRMS method a very attractive alternative for continuous measurement of aerosols, with an accuracy and reliability similar to other commercial devices.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Atmospheric particulate matter (PM) is the subject of intensive environmental investigation as it significantly influences the radiative balance of the Earth and, hence, its surface temperature. Furthermore, PM affects the quality of air and has adverse effects on human health (Künzli et al. 2000; Ramanathan et al. 2007; Filep et al. 2015). Comprehensive studies have demonstrated that carbonaceous material is one of the most important components both in PM2.5 and PM10 modes of the atmospheric aerosol over Europe. Thus, investigations aiming its chemical composition, mass concentration, and areal distribution are of great importance (Krivácsy et al. 2001; Putaud et al. 2010).
Total carbonaceous material consists of a number of organic material-derived carbons (OC) and highly-resistant elemental carbon (EC, soot) found in the atmosphere. Inorganic carbonates are excluded from TC according to the general carbonaceous aerosol classification (Szidat et al. 2009). Similar to other species of aerosols, carbonaceous particles can also be emitted either as primary or secondary particles into the atmosphere, and may originate from natural (only modern carbon) or anthropogenic (modern and fossil carbon) sources (Novakov et al. 1997; Cachier et al. 1989; Chow and Watson 2002). Based on our previous investigation conducted in 2011 in the city of Debrecen, Hungary, the mass concentration of total carbon of monthly PM2.5 aerosol samples varied from 2 to 15 μg m−3, deriveing predominantly (70% on average) from modern sources (Major et al. 2015).
For quantification of the different carbonaceous species, numerous techniques and devices have been developed and are presently being used worldwide (Cachier et al. 1989; Mertes et al. 2004; Utry et al. 2014). We can distinguish between real-time techniques such as the Filter Dynamics Measurement System (Green et al. 2009), and offline thermal (e.g. thermal manganese oxidation) or optical (e.g. aethalometer) methods (Hansen et al. 1984). With optical techniques, primarily the black carbon fraction possessing optical characteristics can be studied, while the translucent or vague organic carbons remain undetectable. For offline methods, sufficient aerosol material can be collected onto impactors or filters using high-volume samplers or smaller pumps with a moderate sampling rate (Mader et al. 2003). In the case of offline thermal methods, the different disintegration temperatures of the carbonaceous species are used to obtain information on the amount of OC and EC sub-fractions separately. The principle of the thermal methods that is relevant to either the EA-IRMS or sealed tube method is the conversion of TC in the aerosol into CO2 in an oxidising gas atmosphere. The combustion temperature of the sample is commonly over 800 °C i.e. the decomposition temperature of the more resistant EC. The amount of the evolved CO2 can then be quantified either manometrically or directly, using a non-dispersive infrared (NDIR) analyser (Lavanchy et al. 1999). In the International Aerosol Carbon Round Robin Test, Schmid et al. (2001) demonstrated that the TC determination is well constrained in the participating laboratories since there were no vital differences in the TC results gained by nine different thermal and optical methods.
In addition to mass and concentration data, the thermal-based EA-IRMS technique offers an opportunity which can substantially expand our current knowledge of carbonaceous particles. For example, the ratio of 13C and 12C stable isotopes (noted as δ13C) can provide important information about the potential sources and atmospheric processes influencing the characteristics of the carbonaceous particles (Kawamura and Ikushima 1993; Górka et al. 2014). Hence, the 13C results represent an important contribution to the source apportionment investigations of atmospheric carbonaceous aerosols.
The aim of our work was to establish an EA-IRMS-based preparation and measuring method at HEKAL, for simultaneous TC mass and stable isotope determinations of atmospheric carbonaceous aerosol samples.
2 Materials and methods
The sample preparation was designed to be as simple as possible, thus only a minimal prep-work is necessary prior the measurements, such as cutting discs out of the filters. The automatic combustion of samples and purification of CO2 takes place online, connecting to the IRMS mass spectrometer without any manual intervention. The main gas purifying steps take place completely automatically inside the Fisons NA 1500 type elemental analyser (combustion, oxidation and reduction steps) and the Thermo Finnigan DeltaPLUS XP IRMS performs the detection process (Polgári et al. 2016).
In the test phase, numerous pairs of laboratory standard materials and genuine aerosol samples were prepared and measured. The δ13C values of the standard test and aerosol samples were applied to evaluate the stability and reliability of the combustion and gas purification steps of the samples. In parallel with the EA-IRMS tests, we performed the preparation and recovery yield measurements of all the test and aerosol samples using an offline sealed tube combustion method and the results were eventually compared. The latter method is applied for combustion of organic material devoted to radiocarbon investigations at HEKAL.
2.1 Standard and atmospheric aerosol test samples
In recovery tests, sulphanilamide and graphite standard materials (with known carbon content and stable isotope ratio) were prepared according to the same protocol as atmospheric aerosol samples (Table 1). The uncertainty range obtained for the standard materials using the sealed tube method were also applied to the aerosol samples since, due the limited amount of the filters, we did not have the opportunity for duplicate these measurements.
Sulphanilamide (CE Instruments, Cod.338 25,100) of known composition is a reference material widely applied in the elemental analyser technique. Various compounds containing nitrogen, sulphur and oxygen can be found in ambient aerosol samples, so we used sulphanilamide to simulate the behaviour of complex, organic-like carbon species (Kertész et al. 2010). To test the combustion efficiency of EC, graphite powder with a particle size below 20 μm (Sigma-Aldrich, PCode: 101,546,139 and IAEA reference material USGS24) was used. We applied this graphite standard since one of the main questions was whether the EA combustion is effective enough to completely combust graphite-like compounds.
In the testing phase, both rural and urban atmospheric PM2.5 aerosol samples were analysed. Rural samples were collected by colleagues of the Air Chemistry Research Group of the University of Pannonia at the official European Monitoring and Evaluation Programme (EMEP) background site of K-puszta, Hungary (N 46°58′ E 19°33′, 125 m a.s.l.). K-puszta station is located in a clearing surrounded by mixed forest on the Hungarian Great Plain, in the middle of the Carpathian Basin. At this site, continuous atmospheric measurements and investigations have been performed for many decades (Gelencsér et al. 2000). Carbonaceous particulate matter was collected on pre-heated quartz filters (Whatman, QMA ø150 mm) which were replaced every 24 h between 11th January and 8th February 2013. For the sampling, a specially-designed high-volume sampler (Kálmán Systems, KS-303, sampling rate: 32 m3 hr.−1) was used within the framework of the 2013 EMEP campaign (Tørseth et al. 2012). Due to the short sampling time, only smaller amount of PM and carbon could be collected on the filters, so these samples represent the lower range of the investigated carbon mass scale.
Urban aerosol sampling took place in the city of Debrecen, Hungary (N 47°33′ E 21°38′, 121 m a.s.l.) where biweekly samples were collected by the Laboratory of Ion Beam Applications of Institute of Nuclear Research (ATOMKI). In this case, an SKC® IMPACT PM Coarse type sampler collected the PM2.5 aerosol onto pre-heated quartz fibre filters with a diameter of 47 mm, at a flow rate of 10 dm3 min−1. During these longer sampling periods, larger amounts of PM2.5 and TC were collected on the filters than at the K-puszta site, and represent the higher range of the carbon mass scale (Major et al. 2015).
2.2 Sample treatment and TC measurements by the EA-IRMS method
The coupled EA-IRMS method is an online technique, where samples are inserted into the EA directly and all the sample preparation and analyses are performed by the EA-IRMS system. One of the advantages of this method is the twenty-minute measuring time per sample, in contrast to the three-day combustion time using the sealed tube method, significantly enhancing the number of measured samples per day.
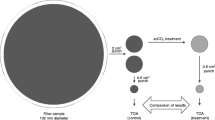
For mass determination of the aerosol filters, a Sartorius® analytical scale with a mass resolution of 2 μg was used. The balance room was maintained at constant temperature (~ 24 °C) and humidity (~50–60%). Before the measurements, the aerosol samples were stored in a freezer and allowed to condition for 24 h. For the purpose of duplicated IRMS measurements, two discs with a diameter of 5.5 mm (A = 0.24 cm2) were punched out of each filter. The discs were then packed into individual ultra clean aluminium capsules (IVA Analysentechnik e.K. Part No.: 76.9807.16) together with three times the sample mass of vanadium-pentoxide reagent (Elemental Microanalysis, EC No.: 215–239-8) to support complete combustion. Aerosol TC measurements were calibrated by control measurements of the sulphanilamide standard after every fifth filter sample. The standard samples were weighted into aluminium capsules and were treated similarly to the aerosol samples.
The main steps of the EA-IRMS analysis are sample combustion, CO2 gas purification and online mass spectrometric measurement of the pure CO2 gas. First, the packed standard and aerosol samples are loaded into the autosampler of a elemental analyser. After loading, the rapid combustion process of the wrapped samples takes place in He carrier gas flow with added high purity O2. The temperature of the oxidation column filled with tungsten-oxide and copper granules and the reduction column filled with copper granules are kept constantly at 1020 °C and 650 °C, respectively. Using a gas-flow reducing unit (open split, ConFlow), the CO2 gas is driven through the purifying columns in a He flow and is directed into the ion source of the mass spectrometer (Fig. 1). The electron impact ionization source is held at 3 kV acceleration voltage for all analysed gases. The positive CO2 + ions produced in the ion source are injected into the magnetic analyser of the MS and are separated according their molecular mass (44 and 45 amu). The collectors use deep, individually shielded Faraday cups with secondary-electron suppression. Finally, the analogue current signals of the detector are amplified and used in the data acquisition procedure.
Main stages of the online combustion and gas purification by the EA-IRMS method (Finnigan ConFlo 2003)
During the evaluation of the results, the mass spectrum of the sample is compared to the one obtained for the reference material of known carbon content and 13C/12C ratio (Finnigan ConFlo 2003). All calculations of weight percent determination refer to the so-called significant peak. Since the area under the curve is a function of carbon content and the peak for the standard is accurately known, the carbon content of the unknown sample can be calculated by means of their ratio according to the next formula:
wsample denote the carbon percentage of the sample, k is a constant gained by means of the standard, Asample and msample are the area under the peak obtained for the sample and the mass of the sample, respectively.
The carbon content and the isotopic ratio measurements take place simultaneously. The ratios of the isotopes are always measured relative to an isotopic standard in order to eliminate any bias or systematic error in the measurements. The stable isotope ratio of the samples relative to a reference material is determined based on the following formula:
Rsample and Rstandard denote respectively the isotope ratio(13C/12C) of the sample and the standard that are measured under identical conditions. The δ13C values of the samples, expressed versus an international reference as Vienna Pee Dee Belemnite (VPDB), are reported in per mil (‰) unit.
In the course of our tests two sample discs were measured successively from each unknown aerosol filter and the final TC and stable isotope result was calculated as the mean value of these two independent measurements and the uncertainty was given as their standard deviation.
2.3 Sample treatment and TC measurements using the sealed tube combustion method
In our comparison experiments, all the results from the EA-IRMS method were compared to the ones obtained for the same samples by our sealed tube method. This latter method was designed previously at HEKAL for offline combustion and gas purification of organic samples. The related purifying vacuum line was constructed with water and CO2 traps and a calibrated known-volume reservoir supplied with a pressure gauge. The amount of the CO2 gas and carbon liberated from the samples can be calculated using the ideal gas law (Janovics 2015). Before preparation of the filters, numerous combustion and gas purifying measurements using the standard materials were performed to obtain information on the applicability of the line. For example, the recovery yield was tested by combustion of sulphanilamide and graphite standard material (n = 12, 12) with increasing amounts from 100 to 2000 μg.
Sample combustion by this method was performed within sealed test tubes (ø9mm, 120 mm) made of borosilicate glass. In these experiments, test tubes were loaded with the standard material and were evacuated to <5*10−5 mbar high vacuum before being sealed with a gas torch. Beside the sample, MnO2 reagent (sample mass X 100) was also placed into the tubes in order to quantitatively oxidise the TC of the carbonaceous material to CO2 at a combustion temperature of 550 °C and duration of three days. After combustion, the resulting CO2 gas was purified and quantified using the gas-handling vacuum system (Fig. 2). The test tubes were attached to the vacuum line using a tube-cracker and after breaking the tube, the gas was driven cryogenically through the water and the CO2 traps cooled down to −78 °C, and −196 °C, respectively. The CO2 gas originating from the sample was frozen into the last trap, while other by-product gases were either captured in the water trap or were removed by pumping incondensable gases. After closing the valves of the known volume, the solid CO2 was allowed to sublimate. The quantity of the CO2 was determined by high-precision pressure measurement and finally the yield was calculated based on the initial and final masses of the sample (Janovics 2015).
The punching process for the atmospheric aerosol filter samples for offline combustion was carried out at the same time as the EA-IRMS preparation work, under identical conditions. For the offline method, a piece with an area of 7.29 cm2 was cut out of the aerosol filters using a square - shaped tool made of stainless steel. The mass of the squares was determined by a Mettler Toledo® analytical scale with an uncertainty of 10 μg. Due to the relatively large filter area, we did not have the opportunity to do duplicated combustion experiments thus only one result was available in all cases. This punched filter was then placed into a test tube and was treated in the same way as the standard test samples. The closed tubes were placed into a muffle oven at a temperature of 550 °C for three days to completely combust the total carbonaceous material and convert its carbon content to CO2 gas. Over three days, the EC of the samples was also completely combusted as had been shown by earlier combustion experiments of elemental graphite by Janovics (2015). Due to the presence of MnO2, as an oxidising agent and catalyst, the combustion of the elemental carbon already occurs at the lower temperature of 550 °C, well below its general decomposition temperature of 800 °C.
Eventually, the CO2 gas samples were transferred from the gas purification line to the IRMS system in closed glass ampoules (ampoule No. 5 in Fig. 2). The δ13C analysis of the offline liberated CO2 gases were also performed by the Thermo Finnigan DeltaPLUS XP IRMS device applying dual inlet method (Vető et al. 2004; Vodila et al. 2011).
3 Results and discussion
3.1 Recovery yield and stable isotopic results of the offline sealed tube combustion method
Recovery yield experiments of standard test materials were carried also out using the offline sealed tube method. Since we intended to use this method as a reference for the evaluation of the EA-IRMS method, it was crucial to verify its combustion and CO2 purification yield as accurately as possible. Portions of sulphanilamide and graphite standard materials with an increasing carbon mass of 100, 250, 750, 1000 and 2000 μg were combusted in the same manner as aerosol samples. Finally, the expected TC masses were compared to the ones estimated by the pressure of the liberated CO2. The broad range of carbon mass was needed due to the wide TC mass scale typical in collected ambient atmospheric aerosols.
In the combustion experiments, recovery yield is as high as 95% and 92% for sulphanilamide and the graphite, with linear regression line slopes of 0.98 and 0.95 (R2 = 0.998 in both cases), respectively. In the lower mass range (below 500 μg) of graphite samples, we experienced slightly higher scatter (±13%, n = 5) while in the 500–2000 μg range this value was reduced to ±6% (n = 14) on average. Thus, for the whole mass range (0–2000 μg) an average relative uncertainty of ±8% was generally applied for TC determination by this method.
The slightly lower recovery yield of sulphanilamide and graphite samples can partly be explained by a small amount of water absorbed at the surface of the test materials. For the combustion of graphite, a complete oxidation is expected due to the smaller elemental carbon particles (PM2.5) with larger surface existing in aerosol samples. Our experiments also confirmed that MnO2 is an excellent oxidizing agent for both the organic-like and the elemental carbon test materials (Fig. 3).
Stable isotope ratio results also confirmed the complete combustion of the standard materials. Carbon isotopic ratios of sulphanilamide (n = 8) and graphite (n = 7) materials combusted offline were measured from the evolved CO2 and then were compared to their nominal values. Results suggest that the prepared samples of standard material are completely combusted in the glass tubes and no significant isotopic fractionation can be seen. The calculated mean values of stable isotopic ratio of sulphanilamide and graphite are −26.69 ‰ and −16.01 ‰, respectively. These results differ from the nominal values with an uncertainty of 0.01 ‰, while the internal uncertainty of the instrument is ±0.1 ‰ (Fig. 4). Earlier, we assumed that the poorer yield was due to minimal water desorption taking place in the sample evacuation step and this was supported by agreement of the isotopic results. A slightly inaccurate pressure measurement in the lower mass range (< 500 μg carbon) may also be a source of some uncertainty in the recovery estimates.
Our measurements have shown that the reproducibility, accuracy and precision of the sealed tube method is sufficient to be used in the verification process of the newly established automated EA-IRMS method. Furthermore, this offline method possessing negligible isotope fractionation can also be applied successfully for preparation of aerosol samples dedicated to radiocarbon or other investigations.
3.2 Comparison of TC and δ13C results of atmospheric aerosol samples
Atmospheric aerosol samples originating from the rural and urban sampling sites were also compared after preparation by both the offline sealed tube and the automated EA-IRMS method.
In case of the offline method, TC results of rural aerosol samples (n = 22) varied from 15 to 60 μg cm−2 representing the low TC range of our aerosol test sample set. The carbon mass obtained by the EA-IRMS system ranged from 15 to 65 μg cm−2, similar to the offline combustion results. Plotting the TC results of EA-IRMS method as a function of the offline sealed tube method and fitting a linear regression line on them, we obtained a strong correlation with an R2 of 0.94 (Fig. 5). The recovery yield of the EA-IRMS is slightly (3%) higher on average than the offline method.
The reason for a slight deviation in the yield of the two methods can be that the samples, in case of the offline method, are pumped down to high vacuum with a purpose of elimination all the residual CO2 of air and over this time some removal of organic-derived VOC or water absorbed on the sample itself or the filter can not be excluded. In contrast, the small amount of CO2 gas evolved during the combustion with EA passes through the entire system in high purity He carrier gas flow that minimizes the possibility of any sample loss. We expect that in subsequent investigations freezing of the combustion tubes during the pumping step could reduce the loss of the absorbed VOC compounds.
Aerosol TC results from the urban environment of Debrecen city (n = 13) represents a wider carbon mass range due to the one-month-long integration time of sampling. The maximum values were around 500 μg cm−2 while the minimum ones were close to the value of 50 μg cm−2. Comparing of the results gained by the two methods show a strong correlation (R2: 0.944) as we observed in the case of rural samples, too (Fig. 6). In this case, the recovery yield of the EA-IRMS method is on average 6% higher relative to that of the sealed tube method.
The mean δ13C values of samples prepared by both the online EA-IRMS and the offline sealed tube combustion methods are in a good agreement within a standard deviation of ±0.3 ‰ (Fig. 7).
In the range of the measured values (from −26.5 ‰ to −25.0 ‰) some variability can be observed but it does not appear to be yield-dependent. Consequently, the samples are not affected considerably in either the procedures they undergo; i.e. samples are completely combusted. Therefore, we conclude that the diverse thermal stability of the different constituent materials does not play a significant role in the TC measurements. Considering the δ13C results, both methods give comparable and reproducible results.
4 Conclusions
In this study, the automated online EA-IRMS was tested for simultaneous TC and δ13C measurements of aerosol samples at HEKAL. Using a quantity verification procedure, this method was compared to the sealed tube combustion method being already in use at HEKAL. Comparative experiments of carbon stable isotope measurements showed good accuracy and reproducibility and no significant isotope fractionation in either case of the methods was observed. TC results measured by EA-IRMS match well with those obtained by the offline sealed tube aerosol combustion method within a mean relative uncertainty range of ±8%. The results of the two methods show a very good correlation (R2 = 0.94) and therefore both methods can be safely applied for the TC quantification of unknown aerosol samples.
The automated sample feeder and short measurement time (20 min per sample) makes the EA combustion method a very attractive alternative for the TC determination of aerosols, since other thermal or optical devices offer solely mass results and, in addition, require manual sample introduction. We therefore conclude that the EA technique can be a preferred choice in aerosol studies because δ13C values and TC data from the same sample can be determined simultaneously.
References
Cachier, H., Brémond, M.P., Buat-Ménard, P.: Determination of atmospheric soot carbon with a simple thermal method. Tellus. 41B(3), 379–390 (1989)
Chow, J.C., Watson, J.G.: PM2.5 carbonate concentrations at regionally representative Interagency Monitoring of Protected Visual Environment sites. Jour. of Geophys. Res. 107, 8344 (2002). doi:10.1029/2001JD000574
Filep, Á., Drinovec, L., Palágyi, A., Manczinger, L., Vágvölgyi, C., Bozóki, Z., Hitzenberger, R., Szabó, G.: Source specific Cyto- and genotoxicity of atmospheric aerosol samples. Aerosol and Air Quality Res. 15(6), 2325–2331 (2015)
Finnigan ConFlo III Operating Manual. Thermo Electron Corporation. Published by Product Marketing, Thermo Electron Corporation, Bremen, Germany. 6.6 paragraph 6 (19)-6 (21) (2003).
Gelencsér, A., Hoffer, A., Molnár, A., Kriváncsy, Z., Kiss, G., Mészáros, E.: Thermal behaviour of carbonaceous aerosol from a continental background site. Atmos. Environ. 34, 823–831 (2000)
Górka, M., Rybicki, M., Simoneit, B.R.T., Marynovski, L.: Determination of multiple organic matter sources in aerosol PM10 from Wroclaw, Poland using molecular and stable carbon isotope compositions. Atmos. Environ. 89, 739–748 (2014)
Green, D.C., Fuller, G.W., Baker, T.: Development and validation of the volatile correction model for PM10- an empirical method for adjusting TEOM measurements for their loss of volatile particulate matter. Atmos. Environ. 43, 2132–2141 (2009)
Hansen, A.D.A., Rosen, H., Novakov, T.: The aethalometer—an instrument for the real-time measurement of optical absorption by aerosol particles. Sci. Tot. Environ. 36, 191–196 (1984)
Janovics, R.: Development of radiocarbon-based measuring methods and their application for nuclear environmental monitoring. PhD thesis. University of Debrecen Press https://dea.lib.unideb.hu in Hungarian (2015).
Kawamura, K., Ikushima, K.: Seasonal changes in the distribution of dicarboxylic acids in the urban atmosphere. Environ. Sci. Technol. 27, 2227–2235 (1993)
Krivácsy, Z., Sávári, Z., Temesi, D., Baltensperger, U., Nyeki, S., Weingartner, E., Kleefeld, S., Jennings, S.G.: Role of organic and black carbon in the chemical composition of atmospheric aerosol at European background sites. Atmos. Environ. 35, 6231–6244 (2001)
Künzli, N., Kaiser, R., Medina, S., Studnicka, M., Chanel, O., Filliger, P., Herry, M., Horak Jr., F., Puybonnieux-Texier, V., Quénel, P., Schneider, J., Seethaler, R., Vergnaud, J.C., Sommer, H.: Public-health impact of outdoor and traffic-related air pollution: a European assessment. Lancet. 356, 795–801 (2000)
Lavanchy, V.M.H., Gäggeler, H.W., Nyeki, S., Baltensperger, U.: Elemental carbon (EC) and black carbon (BC) measurements with a thermal method and an aethalometer at the high-alpine research station Jungfraujoch, Atmos. Environment. 33(17), 2759–2769 (1999)
Mader, B.T., Schauer, J.J., Seinfeld, J.H., Flagan, R.C., Yu, J.Z., Yang, H., Lim, H.J., Turpin, B.J., Deminter, J.T., Heidemann, G., Bae, M.S., Quinn, P., Bates, T., Eatough, D.J., Huebert, B.J., Bertram, T., Howell, S.: Sampling methods used for the collection of particle-phase organic and elemental carbon during ACE-Asia, Atmos. Environment. 37(11), 1435–1449 (2003)
Major, I., Furu, E., Haszpra, L., Kertész, Z., Molnár, M.: One-year-long continuous and synchronous data set of fossil carbon in atmospheric PM2.5 and carbon-dioxide in Debrecen, Hungary. Radiocarbon. 57(5), 991–1002 (2015)
Mertes, S., Dippel, B., Schwarzenböck, A.: Quantification of graphitic carbon in atmospheric aerosol particles by Raman spectroscopy and first application for the determination of mass absorption efficiencies. J. Aerosol Sci. 35(3), 347–361 (2004)
Novakov, T., Hegg, D.A., Hobbs, P.V.: Airborne measurements of carbonaceous aerosols on the East Coast of the United States. J. Geophys. Res.-Atmos. 102, 30023–30030 (1997)
Polgári, M., Németh, T., Pál-Molnár, E., Futó, I., Vigh, T., Mojzsis, S.J.: Correlated chemostratigraphy of Mn-carbonate microbialites (Úrkút, Hungary). Gondwana Res. 29, 278–289 (2016)
Putaud, J.P., Van Dingenen, R., Alastuey, A., Bauer, H., Birmili, W., Cyrys, J., Flentje, H., Fuzzi, S., Gehrig, R., Hansson, H.C., Harrison, R.M., Herrmann, H., Hitzenberger, R., Hüglin, C., Jones, A.M., Kasper-Giebl, A., Kiss, G., Kousam, A., Kuhlbusch, T.A.J., Löschau, G., Maenhaut, W., Molnar, A., Moreno, T., Pekkanen, J., Perrino, C., Pitz, M., Puxbaum, H., Querol, X., Rodriguez, S., Salma, I., Schwarz, J., Smolik, J., Schneider, J., Spindler, G., ten Brink, H., Tursic, J., Viana, M., Wiedensohler, A., Raes, F.: A European aerosol phenomenology 3: physical and chemical characteristics of particulate matter from 60 rural, urban, and kerbside sites across Europe. Atmos. Environ. 44, 1308–1320 (2010)
Ramanathan, V., Ramana, M.V., Roberts, G., Kim, D., Corrigan, C., Chung, C., Winker, D.: Warming trends in Asia amplified by brown cloud solar absorption. Nature. 448, 575–578 (2007)
Schmid, H., Laskus, L., Abraham, H.J., Baltensperger, U., Lavanchy, V., Bizjak, M., Burba, P., Cachier, H., Crow, D., Chow, J., Gnauk, T., Even, A., ten Brink, H.M., Giesen, K.P., Hitzenberger, R., Hueglin, E., Maenhaut, W., Pio, C., Carvalho, A., Putaud, J.P., Toom-Sauntry, D., Puxbaum, H.: Results of the "carbon conference" international aerosol carbon round robin test stage I, Atmos. Environment. 35(12), 2111–2121 (2001)
Szidat, S., Ruff, M., Perron, N., Wacker, L., Synal, H.A., Hallquist, M., Shannigrahi, A.S., Yttri, K.E., Dye, C., Simpson, D.: Fossil and non-fossil sources of organic carbon (OC) and elemental carbon (EC) in Goteborg. Sweden. Atmos. Chem. and Phys. 9, 1521–1535 (2009)
Tørseth, K., Aas, W., Breivik, K., Fjæraa, A.M., Fiebig, M., Hjellbrekke, A.G., Lund Myhre, C., Solberg, S., Yttri, K.E.: Introduction to the European monitoring and evaluation Programme (EMEP) and observed atmospheric composition change during 1972–2009. Atmos. Chem. Phys. Discuss. 12, 5447–5481 (2012)
Utry, N., Ajtai, T., Pintér, M., Tombácz, M., Illés, E., Bozóki, Z., Szabó, G.: Mass specific optical absorption coefficients of mineral dust components measured by a multi wavelength photoacoustic spectrometer. Atmos. Measur. Tech. Dis. 7, 9025–9046 (2014)
Vető, I., Futó, I., Horváth, I., Szántó, Z.: Late and deep fermentative methanogenesis as reflected in the H-C-O-S isotopy of the methane-water system in deep aquifers of the Pannonian Basin (SE Hungary). Org. Geochem. 35, 713–723 (2004)
Vodila, G., Placsu, L., Futó, I., Szántó, Z.: A 9-year record of stable isotope ratios of precipitation in eastern Hungary: implications on isotope hydrology and regional palaeoclimatology. Journ. Hydro. 400, 144–153 (2011)
Kertész, Z., Szoboszlai, Z., Angyal, A., Dobos, E., Borbély, K.I.: Identification and characterization of fine and coarse particulate matter sources in a middle-European urban environment. Nucl. Inst. And Meth. B. 268, 1924–1928 (2010)
Acknowledgements
This research was supported by the European Union and the State of Hungary, co-financed by the European Social Fund in the framework of TÁMOP 4.2.4.A/2-11-1-2012-0001 ‘National Excellence Program and the project of GINOP-2.3.2.-15-2016-00009 ‘ICER’.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Major, I., Gyökös, B., Túri, M. et al. Evaluation of an automated EA-IRMS method for total carbon analysis of atmospheric aerosol at HEKAL. J Atmos Chem 75, 85–96 (2018). https://doi.org/10.1007/s10874-017-9363-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10874-017-9363-y