Abstract
Phytoplankton is a key component in the functioning of marine ecosystems, phytoplankton community structures are very sensitive to their environment. This study was conducted in the central Bohai Sea in the spring and early summer of 2015. Spatial variations in phytoplankton functional groups were examined through high-performance liquid chromatography pigment–CHEMTAX analysis. Results suggested that the phytoplankton biomass (chlorophyll a [Chl a]) in spring was mainly derived from the diatom community and was 3.5-fold higher than that in the summer. Meanwhile, the phytoplankton in the early summer sustained more diverse marker pigments than that in the spring. Despite the overwhelming predominance of microsized phytoplankton in the spring, some smaller phytoplankton (pico- or nanosized), including flagellates, such as prasinophytes, chlorophytes, and cryptophytes, highly contributed to the total Chl a in the summer. Various physico-chemical variables were recorded, and their correlations with phytoplankton density were established by redundancy analysis. Temperature, water stratification, nutrient availability, and even nutritive proportion influenced the succession of phytoplankton functional groups from diatom dominance in the spring to flagellate (mainly haptophytes and prasinophytes) dominance in the early summer. In conclusion, our work comprehensively evaluated the phytoplankton diversity and dynamics in the central Bohai Sea and suggests the need for long-term monitoring for further investigation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Primary productivity in the sea accounts for ~ 50% of the total global annual production (Longhurst et al. 1995). Phytoplankton act as primary producers in bio-geochemical processes and serve as a direct food source for aquatic animals. Phytoplankton functional groups (PFGs) are indicators of primary production and are identified by their morphological, physiological, and ecological attributes rather than common phylogeny. Within particular groups, phytoplankton species possess similar morphologies, environmental sensitivities, and tolerances (Reynolds 2006). Changes in the community structures and abundances of phytoplankton are largely influenced by environmental fluctuations, which may occur with variable frequency and intensity. Accordingly, biota vary in qualitative and quantitative aspects and hence show selected PFGs through competitive processes that favor these groups (Gemelgo et al. 2009). These features encourage interest in studying PFGs, biodiversity, and responses to environmental factors.
Traditionally, phytoplankton communities are studied by microscopy with identification, cell counting, cell size measurement, and volume-to-biomass conversion (Agirbas et al. 2015). However, phytoplankton diversity is low in microscopy-based research, which restricts species into limited functional groups, such as diatoms and dinoflagellates (Chen et al. 2016). In addition, microscopy is a laborious and time-consuming technique that requires high-level expertise because of the morphological similarities between phytoplankton species (Abad et al. 2016). High-performance liquid chromatography (HPLC), which relies on the relative concentration of phytoplankton pigments, serves as an alternative that would overcome the limitations of microscopy-based analysis (Mendes et al. 2015). HPLC is used to detect various other pigments characterized by distinct functional groups and accurately quantify chlorophyll a (Chl a). For example, fucoxanthin (Fuco), peridinin (Peri), Chl b, alloxanthin (Allo), and zeaxanthin (Zea) are typically produced by diatoms, dinoflagellates, chlorophytes, cryptophytes, and cyanobacteria, respectively (Madhu et al. 2014). However, pigment data interpretation can be difficult because some pigments are present in several algal groups (Mendes et al. 2011). A common solution is the statistical software CHEMTAX. By calculating the ratios of marker pigments to Chl a, CHEMTAX estimates the biomasses of various PFGs (Mackey et al. 1996). This software package has been widely used to determine the distribution of PFGs in different regions of the world oceans (Agirbas et al. 2015; Goela et al. 2014; Jiang et al. 2016; Mendes et al. 2011; Wright et al. 2010).
The Bohai Sea is the largest inner sea along Chinese coasts. In the past several decades, the marine environment of the Bohai Sea has changed greatly because of anthropogenic pressure (Jiang et al. 2005; Guo 1993; Guo et al. 2014). With increasing nitrogen and decreasing phosphorus loading, the N/P ratio rose significantly, and phytoplankton community changed accordingly. Previous studies suggested that diatoms dominated the phytoplankton before the 1990s (Guo et al. 2014). In recent years, a large shift from diatom dominance to diatom and dinoflagellate co-dominance was observed in the Bohai Sea (Guo et al. 2014; Wei et al. 2004). More recently, under the influence of anthropogenic disturbance (e.g., aquaculture activity), spring–summer blooms of a newly recorded picophytoplankton species (Aureococcus anophagefferens, ~ 2 μm) have been more frequently reported in the Qinhuangdao Sea area, northwest of the Bohai Sea (Kong et al. 2012; Zhang et al. 2012). These phenomena suggest that non-diatom species may currently play important roles in the Bohai Sea.
The abundance and species composition of phytoplankton in the Bohai Sea have been widely investigated by microscopy in previous studies (Fei et al. 1991; Sun et al. 2001; Zhang et al. 2004; Lin et al. 2006; Guo et al. 2014; Liu and Chen 2014). However, former investigations mainly focused on diatoms and dinoflagellates due to the limitations of the microscopic method. Some other important PFGs, for example prasinophytes, chlorophytes, and cyanobacterias, have been rarely reported in this area. The principal aim of present study was to elucidate succession of PFGs from spring to early summer in the cental Bohai sea using HPLC pigments–CHEMTAX analysis, and the relationship between the phytoplankton community structure and physical–chemical processes.
2 Method
2.1 Study site
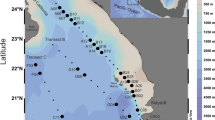
The Bohai Sea is composed of the following four parts: the Bohai Bay, Liaodong Bay, Laizhou Bay, and central Bohai Sea. The Bohai Sea covers 7.7 × 104 km2, with an average depth of 18.7 m (Zhang et al. 2004). The study area (38.21°–39.01°N, 118.98°–120.83°E) is located at the Bohai Sea center (Fig. 1), which comprises a shallow basin with a deep center (~ 30 m) and some surrounding shallow areas (~ 18 m). Yellow River, the most turbid river in the world, carries large nutrient loads to the central Bohai Sea and significantly influences the phytoplankton structures in the area (Wei et al. 2004).
2.2 Sample collection
Two cruises were undertaken in the spring (April 3–5) and early summer (June 4–6) of 2015. Surface seawater samples (0.5 m in depth) were collected into Niskin bottles from 21 stations in the spring and 27 stations in the early summer for nutrient and phytoplankton pigment analysis. Samples were not collected in some stations because of poor weather. Temperature, salinity, pH, and dissolved oxygen (DO) were recorded in situ by YSI 556 multiparameter (Yellow Springs Instruments, USA) at three depths (0.5 m, 10 m layer, and 1 m above the bottom floor).
2.3 Nutrient analysis
At each sampling station, 100 mL seawater was collected, filtered by a GF/F filter, and stored at − 20 °C to analyze the nitrate (NO3−), nitrite (NO3−), ammonium (NH4+), dissolved reactive phosphate (DRP), and dissolved silicate (DSi) levels. DIN refers to the sum of the nitrate, nitrite, and ammonium contents. The concentrations of these nutrients in each sample were determined in triplicate by using a Skalar San++ continuous flow analyzer (Strickland and Parsons 1972).
2.4 HPLC pigment analysis
Pigment extraction and determination were performed in accordance with a protocol adapted from Zapata et al. (2000). Exactly 1.0 L seawater samples were filtered through 0.7 μm filters (GF/F, Whatman; 47 mm diameter). The filters were immediately deep frozen in liquid nitrogen and stored at − 80 °C. Phytoplankton pigments were extracted in dim light with 3 mL of 95% HPLC-grade methanol. Samples were ultrasonicated for 5 min in an ice-water bath and then centrifuged at 1500g for 3 min at 4 °C. The supernatants were filtered through 0.22 μm PTFE membranes to remove the remains of filter and cell debris. A mixture of 200 μL extracts and 67 μL Milli-Q water were prepared for phytoplankton pigment analysis.
An Agilent series 1100 HPLC system fitted with a Waters Symmetry C8 column (150 × 4.6 mm2, 3.5 μm particle size, 100 Å pore size) and with a G1314A variable wavelength detector was used to separate the phytoplankton pigments. Pigments were identified by comparing their absorbance spectra, retention times, and concentrations detected by the photodiode array detector (e.g., 440 nm) with those of authentic standards. A total of 22 commercially available pigment calibration standards were obtained from DHI Inc. (Hørsholm, Denmark). These standards included Chl c3, Mg-2,4-divinylpheoporphyrin (MgDVP), Chl c2, Peri, pheophorbide a (Pheide a), 19-but-fucoxanthin (But-Fuco), Fuco, neoxanthin (Neo), prasinoxanthin (Pras), violaxanthin (Viola), 19ʹ-hex-fucoxanthin (Hex), diadinoxanthin (Diadino), Allo, diatoxanthin (Diato), Zea, lutein (Lut), canthaxanthin (Cantha), gyroxanthin-diester (Gyro), Chl b, Chl a, pheophythin a (Phe a), and β-carotene (β-Car).
2.5 CHEMTAX analysis of pigment data
The relative abundance of the PFGs contributing to the total Chl a biomass were calculated from pigment concentration data by using version 1.95 of the CHEMTAX chemical taxonomy software (Mackey et al. 1996; Wright et al. 1996, 2009, 2010). Initial pigment ratios were obtained from a range of values given by Mackey et al. (1996). Meanwhile, calculation procedures and processes were fully described by Wright et al. (2009) and Higgins et al. (2011). A series of 60 pigment ratio matrices were generated by multiplying each ratio from the initial matrix by a random function to optimize the matrix. The average of best six output matrices (with the lowest residual or root-mean-square error) were considered as the optimized results. Data from the spring and early summer were run separately so as to take potential variations into account in optimization of CHEMTAX procedures. The initial and final ratios are given in Table 1. The output data are presented as absolute concentrations (ng/L) of Chl a and relative proportions attributed to each phytoplankton group.
2.6 Statistical analysis
To explore the relationships between the environmental conditions and PFGs variability, two data matrices were used for each season. One included CHEMTAX derived Chl a concentrations of each phytoplankton group, and a second one included the environmental variables that may affect PFGs. The data in phytoplankton matrix were log transformed and centred by species prior to analysis. The environmental matrix included temperature, salinity, DO, pH, DIN, PO4, SiO3, N/Si, N/P and Si/P. The data were first analyzed by detrending correspondence analysis (DCA). Because the longest lengths of gradient for the two seasons were both less than 3.0, we then performed redundancy analysis (RDA) by using the CANOCO 4.5 software (ter Braak and Smilauer 2002). Statistical significances were evaluated by Monte Carlo permutation test. Only those variables with P < 0.05 were included in the analyses.
3 Results
3.1 Oceanographic conditions
In the spring, the sea surface temperature ranged from 3.9 to 6.3 °C with an average of 5.0 °C (Table 2). This surface temperature exhibited a decreasing gradient from the south to the north of the sampling area, with minima recorded at the upper northwest stations (Fig. 2a). Severe convection occurred in the water column of every station and resulted in vertical differences between the surface and bottom layers of no more than 0.5 °C (Fig. 2e). In the early summer, the sea surface temperature ranged from 13.7 °C to 19.5 °C with an average of 16.5 °C (Fig. 2b; Table 2). The surface temperature exhibited a decreasing gradient from southwest to northeast of the sampling area, with minima recorded in the same upper northeast stations as that in the spring. A summer stratification was noted in most stations, and the temperature differences between the surface and bottom layers reached as high as 7.5 °C (Fig. 2f).
Distribution of surface temperature (a, b) and salinity (c, d), as well as the differences in temperature (e, f) and salinity (g, h) between the surface and bottom area, in the central Bohai Sea. The differences in temperature and salinity between the surface and bottom areas were obtained by subtracting the bottom values from the surface values, respectively
In the spring, the freshwater discharge of the Yellow River did not influence the study area. Sea surface salinity showed slight variations and ranged from 30.9 to 31.7 psu (Fig. 2c; Table 2). No obvious vertical difference (less than 0.06 psu) was observed between the surface and bottom layers (Fig. 2g). In the early summer, the sea surface salinity decreased from the northeastern to southern parts of the study area because the influence of the Yellow River plume. The values ranged from 28.7 to 31.4 psu, with an average of 30.9 psu (Fig. 2d; Table 2). Given the summer stratification, the differences in salinity between the surface and bottom layers reached as high as 2.4 psu (Fig. 2h).
3.2 Nutrients
The difference between the DIN concentrations in the two seasons (9.91 ± 5.15 μM in the spring vs. 9.71 ± 6.71 μM in the early summer) was not significant (P > 0.05) (Table 2). The areas with high DIN values shifted over time from the central part to the southern part of the sampling region close to LaiZhou Bay because of the freshwater discharge of the Yellow River (Figs. 2d, 3b). During the sampling periods, the lowest DIN concentration was obtained in the spring (2.10 μM) and the highest in the early summer (29.82 μM). The mean DRP concentration in the spring (0.70 ± 0.30 μM) was significantly higher than that of the early summer (0.33 ± 0.10 μM) (P < 0.01; Table 2). Meanwhile, the lowest DRP concentration was observed in the early summer (0.17 μM), whereas the highest was noted in the spring (1.30 μM). The mean DSi concentration was significantly higher in the spring (6.56 ± 3.03 μM) than in the early summer (4.03 ± 1.88 μM) (P < 0.01; Table 2). The lowest and highest DSi concentrations (1.72 and 11.37 μM, respectively) were recorded in the spring.
The nutrient ratios (DIN:P, Si:P, and Si:DIN) exhibited significant variations between the spring and early summer. According to Justić et al. (1995), the criteria for defining nutrient limitation were (a) N limitation of DIN:P < 10 and Si:DIN > 1, (b) P limitation of Si:P > 22 and DIN:P > 22, and (c) Si limitation of Si:P < 10 and Si:DIN < 1. The N:P ratios were higher in the early summer than in the spring in almost all of the sampling stations, with mean values of 14.43 ± 7.98 and 30.09 ± 18.11, respectively. P limitation occurred in two stations in the early summer, whereas N limitation was noted occasionally in either season. The relative proportion of available nutrients revealed a severe Si limitation in both seasons (Fig. 4).
3.3 Phytoplankton pigment concentrations
A total of 21 pigments were identified in the central Bohai Sea (Table 2). The average Chl a concentration in the spring was 0.91 μg/L, which was significantly higher than that in the early summer (0.26 μg/L) (Table 2). The peak Chl a level in the spring was recorded in the western and northeastern parts of the study area (Fig. 5a). Fuco was the dominant accessory pigment at concentrations ranging from 0.11 to 2.77 μg/L and 0.07 to 1.13 μg/L in the spring and early summer, respectively. Similar to mean Chl a concentrations, the mean Fuco concentration in the spring was significantly higher than that in the early summer (Table 2). A high-Fuco region was noted at the western part of the sampling region, and a clear gradient of decreasing levels toward the eastern part was observed in the spring. The Fuco distribution in the early summer shared a similar pattern as that in the spring except for the high value area that shifted northward. The Peri concentrations were generally uniform throughout the two seasons at average concentrations of 33.89 and 34.45 ng/L, respectively. Moreover, significantly higher increases in Allo, Hex-Fuco, Pras, and Zea levels were found in the early summer than in the spring (P < 0.01; Table 2).
3.4 PFGs
The CHEMTAX analysis identified eight major PFGs contributing to the Chl a biomass in the central Bohai Sea. These PFGs included diatoms, dinoflagellates, cryptophytes, prasinophytes, chlorophytes, haptophytes (T3), haptophytes (T4), and Synechococcus (Fig. 6). In the spring, the diatoms were the major contributors (83.31%) to the total Chl a, followed by the dinoflagellates (5.95%) and cryptophytes (5.08%) (Fig. 7). However, in the early summer, a sharp decrease was observed in the diatom biomass, which accounted for 15.03% of the total biomass. High diatom biomasses were confined to the northeast part of the investigated region (st.A5-7 and B5-7) (Fig. 6b). By contrast, the biomasses of the haptophytes (T4) and prasinophytes increased to become the largest PFGs, with proportions of 22.38 and 19.06%, respectively. The dinoflagellates showed similar relative contributions during both cruises. The contributions of other PFGs, including cryptophytes, chlorophytes, haptophytes (T3), and Synechococcus, increased in different degrees from the spring to the early summer (Fig. 7).
3.5 Influence of environmental parameters on PFGs
Redundancy analysis was conducted to investigate the response of PFGs to environmental parameters (Fig. 8). A Monte Carlo test showed that seven environmental variables (temperature, salinity, DIN, PO4, SiO3, N/Si, and N/P) significantly contributed to the spatial distribution of PFGs. The RDA explained 93.0 and 93.8% of the variance associated with the phytoplankton–environment relationship in the spring and early summer, respectively. The first two canonical axes explained 68.7 and 72.6% of the total spatial distributions of PFGs in the spring and early summer, respectively. In the spring, the distribution of diatoms (Diat), prasinophytes (Pras), chlorophytes (Chlo), and Synechococcus (Syne) was positively associated with the N:Si and N:P ratios and characterized by low temperature. The two types of haptophytes (Hapt) and cryptophytes (Cryp) were negatively associated with DIN, PO4, and SiO3 concentrations. The dinoflagellates (Dino) were found to positively correlate with high nutrient concentrations. In the early summer, the diatoms were dominant in the northeast part of the sampling area (st.A5-7, B7), which was characterized by high salinity, as well as low temperature and nutrient concentration. Converse to the diatom abundance, the dinoflagellate abundance was positively associated with temperature and nutrient concentrations and negatively associated with salinity. Most other flagellates (haptophytes, prasinophytes, chlorophytes, and cryptophytes), as well as Synechococcus, which were abundant in the central part of the study area, were intermediately positioned in relation to other variables.
Redundancy analysis biplots of phytoplankton functional groups (PFGs) and major environmental variables in the central Bohai Sea. The following abbreviations were used for the different PFGs: Diat, Diatoms; Dino, Dinoflagellates; Cryp, Cryptophytes; Pras, Prasinophytes; Chlo, chlorophytes; Hapt, haptophytes; and Syne, Synechococcus
4 Discussion
4.1 PFGs as revealed by pigment analysis in the central Bohai Sea
The temporal changes in Chl a levels are usually associated with changes in the phytoplankton community composition (Bouman et al. 2005b; Rodrı́guez et al. 1998) and variations in the relative concentrations of accessory pigments (Sathyendranath et al. 2006). Notably, the mean Chl a was 3.5 folds (Table 2) greater in the spring than in the early summer. Cooler temperatures and generally higher nutrient levels than those in the early summer prevailed in the spring and led to elevated Chl a concentrations and the Fuco prominence over the other diagnostic carotenoids. However, the levels of marker pigments varied with the changing environment from spring to early summer. Fuco concentrations reduced significantly, whereas Chl b, Allo, But-Fuco, Hex-Fuco, Pras, and Zea levels increased markedly (P < 0.01) (Table 2). The variations in these pigments corresponded with the increase in number of diatoms and the decrease in chlorophytes, cryptophytes, haptophytes, cryptophytes, prasinophytes, and cyanobacteria. The abundance of Zea in the early summer was related to the abundance of Synechococcus because divinyl Chl a, the exclusive biomarker of prochlorophytes, was not detected in this study. Therefore, the HPLC pigment analysis provided immediate insight into the phytoplankton community structure in the Bohai Sea.
Despite the convenience of HPLC methods, the quantitative composition of complex phytoplankton populations require further determination through other methods, such as CHEMTAX analysis (Agirbas et al. 2015). Previous studies documented that the phytoplankton community composition in the Bohai Sea was dominated by diatoms (99.8% in the spring and 38.5% in the summer) and dinoflagellates (61.5% in the summer), and only limited species of other PFGs were observed by optical microscopy (Chen et al. 2016). In the present study through CHEMTAX analysis, the PFGs varied widely between stations and seasons in the central Bohai Sea. The phytoplankton biomass was generally greater in the spring than in the early summer in the central Bohai Sea (Fig. 6). The diatoms largely dominated the phytoplankton community in the spring and accounted for 83.3% of the total Chl a. However, during the early summer, diverse PFGs coexisted and flagellates dominated (74.1% in total), followed by diatoms (15.0%) and Synechococcus (11.0%) (Figs. 6, 7). To our best knowledge, PFG determination by HPLC pigment–CHEMTAX analysis in the Bohai Sea has not been reported. CHEMTAX appears as an effective method to ascertain the PFG distribution in this sea area.
Since 1958, a few comprehensive investigations were conducted in the Bohai Sea and revealed marked changes in the phytoplankton composition (Fei et al. 1991; Wei et al. 2004; Guo et al. 2014; Chen et al. 2016). Obviously, the diatoms tended to be replaced gradually by dinoflagellates over decades. This change is presumed to be due to the changes in nutrient discharge caused by the intensification of the industry, agriculture, and aquaculture (Wei et al. 2004). However, previous studies mainly focused on the long-term shift of dinoflagellates because of the limitation of the microscopic method. This study suggests that other flagellated phytoplankton species, i.e., prasinophytes, haptophytes, chlorophytes, and cryptophytes, increased in abundance more than that of the dinoflagellates in the summer. The long-term changes in the abundances of these flagellated phytoplankton species cannot be achieved because lack of historical data on the last several decades. Further attention should be paid to acquire future data from the Bohai Sea.
The present results also confirmed that small-sized phytoplankton, such as prasinophytes, chlorophytes, and cryptophytes, substantially contribute to the total Chl a pool, especially in the summer. Indeed, brown tides caused by the picophytoplankton A. anophagefferens have been regularly observed in the northwest part of the Bohai Sea (sea areas of Qinghuangdao) recently (Xu et al. 2017). However, the historical changes in the phytoplankton community composition of the central Bohai Sea remain unclear because of the inadequacy of the earlier results based on microscopic identification to account for the small-sized organisms. To date, we cannot ascertain whether this discovery is due to the restrictions of traditional methods or the tendency of the phytoplankton species to assume small sizes. This aspect thus merits further investigation.
4.2 Environmental factors influencing PFGs
Temperature is an important environmental parameter that influences biological processes in the ocean. Various studies have demonstrated that phytoplankton community structure varies on a regular, predictable pattern with temperature (Bouman et al. 2003, 2005a; Mendes et al. 2015). In general, microplankton, such as diatoms, usually flourish at low temperatures and low light intensity, which are typical of early spring in temperate latitudes (Nelson 1963). Meanwhile, cyanobacteria, such as Synechococcus (Agawin et al. 1998; Kemp et al. 2005; Marshall et al. 2009) and flagellates, such as haptophytes (Bouman et al. 2003, 2005a), are prevalent in the summer when the water temperature is high and the seasonal thermal stratification limits the nutrient supply to the upper layer (Agawin et al. 1998). The results presented here clearly showed the similar influence of seawater temperature on the community structure in the central Bohai Sea (RDA in Fig. 8).
Some other water temperature related abiotic factors, such as water stratification, nutrient availability, and structure, may have also played a significant part in regulating the distribution of phytoplankton communities along the study area. Several authors have proposed the general rule that sea areas with stratification are more favorable to the growth of flagellates than to that of diatoms (Smith et al. 1983; Mann 1993; Tilstone et al. 2000; Mendes et al. 2011). Additionally, flagellates can move vertically and hence have access to nutritive resources in deep water and light energy near the sea surface (Monaco and Prouzet 2014). These organisms are thus better adapted to low-mixing conditions than other organisms (Goela et al. 2014). Notably, in several stations (e.g., A5, A6, A7, B7, and C7) located near the center of a widely reported well-mixed warm water column (Fig. 2b, d) (Lin et al. 2006), the diatoms accounted for the largest proportion among all the PFGs in the summer (Fig. 7).
Similar to the present study, previous studies have suggested silicate as a limiting factor for diatom growth during the summer because of its strong consumption during the spring (Brown and Landry 2001; Drira et al. 2014; Erga et al. 2014). Likewise, a negative correlation between diatom biomass and silicate concentration was found in our study (Fig. 8). This result confirmed that silicate served as a limiting nutrient and caused the cessation of diatom prominence because of its importance to diatom growth (Lewin and Ralph 1962; Goela et al. 2014). The current result also showed that despite the diatom dominance in the spring, the diatom biomass did not correlate with silicate levels (Fig. 8). In fact, the sea is already under extensive silicate limitation in the spring (Fig. 4), and this limitation may explain the non-correspondence. The high diatom biomass was probably due to the vertical exchanges of the surface and bottom waters. These changes may have provided sufficient silicate levels to sustain the diatom growth. Despite the silicate limitation, the phosphorus limitation began to appear in some stations in the early summer. This P limitation may have promoted the succession from diatoms to flagellates. According to Goela et al. (2014), fluctuations in nutrient regimes are related to such succession pattern, with P limitation favoring flagellate dominance. Although the diatoms were proven to be most sensitive class to nutrient enrichment (Loureiro et al. 2008), they were more apparently affected than flagellates by the decreased nutrient supply (Egge 1998).
Numerous studies have demonstrated the succession of diatoms to flagellates under elevated N:P ratios. At high N:P ratios, diatom growth is more severely limited than flagellate growth (Egge 1998). Increased N:P ratios and P limitation promoted the P-related growth decline in diatoms (Skjoldal 1993). Other sea areas of China, such as the Yellow Sea (Lin et al. 2005), Changjiang River Estuary and East China Sea (Li et al. 2009), and the Pearl River Estuary and South China Sea (Yin et al. 2000), experienced rising N:P ratios and subsequent increases of flagellates blooms and decrease of diatom dominance. Given the slump in Yellow River input, the present study area is undergoing the same process. Since 1960, the nitrate concentrations in the central Bohai Sea have increased by a factor of 10, whereas the phosphate concentrations have decreased by a factor of 2 (Zhang et al. 2004). The nitrogen limitation in the central Bohai Sea has gradually changed to phosphate and silicate deficiency (Yu et al. 2001). Such a nutrient status in the sea is of great importance in determining the structure and function of phytoplankton communities. The mesocosm experiments implemented by Schollhorn and Graneli (1993), Sommer (1995), Watanabe et al. (1995), and Escaravage et al. (1999) also showed that elevated N:Si ratios in the surroundings lead to elevated flagellate:diatom ratios, which is consistent with our results (Fig. 8). In the present study, phytoplankton succession was related to the nutrient variation patterns.
5 Conclusion
HPLC pigment–CHEMTAX analysis in the central Bohai Sea showed that the spring was almost invariably dominated by diatoms at the entire sampling area, whereas the early summer revealed a highly diverse phytoplankton population dominated by flagellates. These results indicated a relatively pronounced seasonal succession. The seasonal variation of PFGs was influenced by temperature, water stratification, nutrient availability, and even nutritive proportions. Compared with previous studies by microscopy, our work provided thorough understanding of the phytoplankton community in the central Bohai Sea. In particular, the small phytoplankton (pico/nanophytoplankton) comprised the major share of the phytoplankton community structure, especially in the summer. This research may help future investigations ascertain and evaluate the changes in structure of the central Bohai Sea ecosystem. Also, it is recommended that microscopy and flow cytometry data combined with HPLC phytoplankton pigments data be used in the future work to better understand the marine ecosystem in the central Bohai Sea.
References
Abad D, Albaina A, Aguirre M, Laza-Martínez A (2016) Is metabarcoding suitable for estuarine plankton monitoring? A comparative study with microscopy. Mar Biol 163(7):1–13
Agawin N, Duarte CM, Agustí S (1998) Growth and abundance of Synechococcus sp. in a Mediterranean Bay: seasonality and relationship with temperature. Mar Ecol Prog Ser 170(4):45–53
Agirbas E, Kopuz U, Feyzioglu AM, Llewellyn CA (2015) Phytoplankton community composition in the south-eastern Black Sea determined with pigments measured by HPLC–CHEMTAX analyses and microscopy cell counts. J Mar Biol Assoc UK 95(1):35–52
Bouman HA, Trevor P, Shubha S, Li WKW, Venetia S, Cesar FY, Heidi M et al (2003) Temperature as indicator of optical properties and community structure of marine phytoplankton: implications for remote sensing. Mar Ecol Prog Ser 258(1):19–30
Bouman H, Platt T, Sathyendranath S, Stuart V (2005a) Dependence of light-saturated photosynthesis on temperature and community structure. Deep Sea Res Part I 52(7):1284–1299
Bouman H, Sathyendranath S, Fuentes-Yaco C, Platt T, Devred E (2005b) Physical forcing and phytoplankton distributions. Sci Mar 69(1):55–73
Brown SL, Landry MR (2001) Microbial community structure and biomass in surface waters during a Polar Front summer bloom along 170°W. Deep Sea Res Part II 48(19–20):4039–4058
Chen YH, Gao YH, Chen CP, Liang JR, Lin S, Yu Z, Ling Q (2016) Seasonal variations of phytoplankton assemblages and its relation to environmental variables in a scallop culture sea area of Bohai Bay. China Mar Pollut Bull 113(1):362–370
Drira Z, Elloumi J, Guermazi W, Hassen MB, Hamza A, Ayadi H (2014) Seasonal changes on planktonic diatom communities along an inshore-offshore gradient in the Gulf of Gabes (Tunisia). Acta Ecol Sin 34(1):34–43
Egge JK (1998) Are diatoms poor competitors at low phosphate concentrations? J Mar Syst 16(3–4):191–198
Erga SR, Ssebiyonga N, Hamre B, Frette Ø, Rey F, Drinkwater K (2014) Nutrients and phytoplankton biomass distribution and activity at the Barents Sea Polar Front during summer near Hopen and Storbanken. J Mar Syst 130(130):181–192
Escaravage V, Prins TC, Smaal AC, Peeters JCH (1999) The response of phytoplankton communities to phosphorus input reduction in mesocosm experiments. J Exp Mar Biol Ecol 198(1):55–79
Fei Z, Mao X, Zhu M, Li B, Li B, Guan Y, Zhang X (1991) The study of primary production in the Bohai Sea: chlorophyll a, primary productivity and potential fisheries resources. Mar Fish Res 12:55–69
Gemelgo MC, Mucci JL, Navaspereira D (2009) Population dynamics: seasonal variation of phytoplankton functional groups in Brazilian reservoirs (Billings and Guarapiranga, Sao Paulo). Braz J Biol 69(4):1001–1013
Goela PC, Danchenko S, Icely JD, Lubian LM, Cristina S, Newton A (2014) Using CHEMTAX to evaluate seasonal and interannual dynamics of the phytoplankton community off the South-west coast of Portugal. Estuar Coast Shelf Sci 151(5):112–123
Guo Y (1993) Primary productivity and phytoplankton in China Sea. In: Zhou D, Bo LY, Zeng CK (eds) Oceanology of China seas, vol I. Kluwer, Dordrecht, pp 227–242
Guo SJ, Yan-Qiao LI, Zhang CX, Zhai WD, Huang T, Wang LF, Wei MA et al (2014) Phytoplankton community in the Bohai Sea and its relationship with environmental factors. Mar Sci Bull 33(1):95–105
Higgins HW, Wright SW, Schlüter L (2011) Quantitative interpretation of chemotaxonomic pigment data. In: Roy S, Llewellyn CA, Egeland ES, Johnsen G (eds) Phytoplankton pigments: characterization, chemotaxonomy and applications in oceanography. Cambridge University Press, Cambridge, pp 257–313
Jiang H, Cui Y, Chen BJ, Chen JF, Song YL (2005) The variation trend of nutrient salts in the Bohai Sea. Mar Fish Res 26(6):61–67
Jiang T, Chen F, Yu Z, Lu L, Wang Z (2016) Size-dependent depletion and community disturbance of phytoplankton under intensive oyster mariculture based on HPLC pigment analysis in Daya Bay, South China Sea. Environ Pollut 219:804–814
Justić D, Rabalais NN, Turner RE, Dortch Q (1995) Changes in nutrient structure of river-dominated coastal waters: stoichiometric nutrient balance and its consequences. Estuar Coast Shelf Sci 40(3):339–356
Kemp WM, Boynton WR, Adolf JE, Boesch DF, Boicourt WC, Brush G, Cornwell JC et al (2005) Eutrophication of Chesapeake Bay: historical trends and ecological interactions. Mar Ecol Prog Ser 303(1):1–29
Kong FZ, Yu RC, Zhang QC, Yan T, Zhou MJ (2012) Pigment characterization for the 2011 bloom in Qinhuangdao implicated “brown tide” events in China. Chin J Oceanol Limnol 30(3):361–370
Lewin RA (1962) Physiology and biochemistry of algae. Academic Press, New York
Li J, Glibert PM, Zhou M, Lu S, Lu D (2009) Relationships between nitrogen and phosphorus forms and ratios and the development of dinoflagellate blooms in the East China Sea. Mar Ecol Prog Ser 383(1):11–26
Lin C, Ning X, Su J, Lin Y, Xu B (2005) Environmental changes and the responses of the ecosystems of the Yellow Sea during 1976–2000. J Mar Syst 55(3–4):223–234
Lin X, Xie SP, Chen X, Xu L (2006) A well-mixed warm water column in the central Bohai Sea in summer: effects of tidal and surface wave mixing. J Geophys Res Oceans 111(C11):1009–1026
Liu FF, Chen XE (2014) Simulation on seasonal variation of the phytoplankton biomass in the Bohai Sea. J Ocean Univ China 44(2):17–26
Longhurst A, Sathyendranath S, Platt T, Caverhill C (1995) An estimate of global primary production in the ocean from satellite radiometer data. J Plankton Res 17(6):1245–1271
Loureiro S, Icely J, Newton A (2008) Enrichment experiments and primary production at Sagres (SW Portugal). J Exp Mar Biol Ecol 359(2):118–125
Mackey MD, Mackey DJ, Higgins HW, Wright SW (1996) CHEMTAX—a program for estimating class abundances from chemical markers: application to HPLC measurements of phytoplankton. Mar Ecol Prog Ser 144(1):265–283
Madhu NV, Ullas N, Ashwini R, Meenu P, Rehitha TV, Lallu KR (2014) Characterization of phytoplankton pigments and functional community structure in the Gulf of Mannar and the Palk Bay using HPLC–CHEMTAX analysis. Cont Shelf Res 80(727):599–605
Mann KH (1993) Physical oceanography, food chains, and fish stocks: a review. ICES J Mar Sci 50(2):105–119
Marshall HG, Lane MF, Nesius KK, Burchardt L (2009) Assessment and significance of phytoplankton species composition within Chesapeake Bay and Virginia tributaries through a long-term monitoring program. Environ Monit Assess 150(1–4):143–155
Mendes CR, Sá C, Vitorino J, Borges C, Garcia VMT, Brotas V (2011) Spatial distribution of phytoplankton assemblages in the Nazaré submarine canyon region (Portugal): HPLC–CHEMTAX approach. J Mar Syst 87(1):90–101
Mendes CRB, Kerr R, Tavano VM, Cavalheiro FA, Garcia CAE, Dessai DRG, Anilkumar N (2015) Cross-front phytoplankton pigments and chemotaxonomic groups in the Indian sector of the Southern Ocean. Deep Sea Res Part II 118:221–232
Monaco A, Prouzet P (2014) Eutrophication of the marine environment. Wiley, New York, pp 3304–3309
Nelson DM (1963) Plankton and productivity in the oceans. In: Raymont JEG (ed) Phytoplankton, vol 1. Pergamon Press, Oxford
Reynolds CS (2006) The ecology of phytoplankton (ecology, biodiversity and conservation). Cambridge University Press, Cambridge
Rodrı́guez J, Blanco JM, Jiménez-Gómez F, Echevarrı́a F, Gil J, Rodrı́guez V, Ruiz J et al (1998) Patterns in the size structure of the phytoplankton community in the deep fluorescence maximum of the Alboran Sea (southwestern mediterranean). Deep Sea Res Part I 45(10):1577–1593
Sathyendranath S, Stuart V, Platt T, Bouman H, Ulloa O, Maass H (2006) Remote sensing of ocean colour: towards algorithms for retrieval of pigment composition. Indian J Mar Sci 34(4):333–340
Schollhorn E, Graneli E (1993) Is the increase of flagellates in coastal waters caused by changes in ratios of N: P and Si: N Toxic phytoplankton blooms in the sea. Elsevier, Amsterdam, pp 811–817
Skjoldal HR. (1993) Eutrophication and algal growth in the North Sea. In: Symposium Mediterranean Seas 2000, Italy, 1991, pp 1–30
Smith WO, Heburn GW, Barber RT, O’Brien JJ (1983) Regulation of phytoplankton communities by physical processes in upwelling ecosystems. J Mar Res 41(3):539–556
Sommer U (1995) Eutrophication related changes in phytoplankton species composition: is there a role of nutrient competition. In: ICES annual science conference
Strickland JDH, Parsons TR (1972) A practical handbook of seawater analysis. Bulletin, 167
Sun J, Liu DY, Qian SB (2001) Preliminary study on seasonal succession and development pathway of phytoplankton community in the Bohai Sea. Acta Oceanol Sin 20(2):251–260
ter Braak CJF, Smilauer P (2002) CANOCO reference manual and CanoDraw for Windows user's guide: software for canonical community ordination (version 4.5). http://www.canoco.com/
Tilstone GH, Míguez BM, Figueiras FG, Fermín EG (2000) Diatom dynamics in a coastal ecosystem affected by upwelling: coupling between species succession, circulation and biogeochemical processes. Mar Ecol Prog Ser 205(1):23–41
Watanabe M, Kohata K, Kimura T, Takamatsu T, Yamaguchi SI, Ioriya T (1995) Generation of a Chattonella antiqua bloom by imposing a shallow nutricline in a mesocosm. Limnol Oceanogr 40(8):1447–1460
Wei H, Sun J, Moll A, Zhao L (2004) Phytoplankton dynamics in the Bohai Sea—observations and modelling. J Mar Syst 44(3–4):233–251
Wright SW, Thomas DP, Marchant HJ, Higgins HW, Mackey DJ (1996) Analysis of phytoplankton of the Australian sector of the Southern Ocean: comparisons of microscopy and size frequency data with interpretations of pigment HPLC data using the ‘CHEMTAX’ matrix factorisation program. Euphytica 144(1):285–298
Wright SW, Ishikawa A, Marchant HJ, Davidson AT, Enden RL, Nash GV (2009) Composition and significance of picophytoplankton in Antarctic waters. Polar Biol 32(5):797–808
Wright SW, Enden RLVD, Pearce I, Davidson AT, Scott FJ, Westwood KJ (2010) Phytoplankton community structure and stocks in the Southern Ocean (30–80°E) determined by CHEMTAX analysis of HPLC pigment signatures. Deep Sea Res Part II 57(9):758–778
Xu X, Yu Z, Cheng F, He L, Cao X, Song X (2017) Molecular diversity and ecological characteristics of the eukaryotic phytoplankton community in the coastal waters of the Bohai Sea, China. Harmful Algae 61:13–22
Yin KD, Qian PY, Chen JC, Hsieh D, Harrison PJ (2000) Dynamics of nutrients and phytoplankton biomass in the Pearl River estuary and adjacent waters of Hong Kong during summer: preliminary evidence for phosphorus and silicon limitation. Mar Ecol Prog Ser 194(3):295–305
Yu ZG, Mi TZ, Yao QZ, Xie BD, Zhang J (2001) Nutrients concentration and changes in decade-scale in the central Bohai Sea. Acta Oceanol Sin 20(1):65–75
Zapata M, Rodriguez F, Garrido JL (2000) Separation of chlorophylls and carotenoids from marine phytoplankton: a new HPLC method using reversed phase C8 column and pyridine-containing mobile phases. Mar Ecol Prog Ser 195:29–45
Zhang J, Yu ZG, Raabe T, Liu SM, Starke A, Zou L, Gao HW et al (2004) Dynamics of inorganic nutrient species in the Bohai seawaters. J Mar Syst 44(3):189–212
Zhang QC, Qiu LM, Yu RC, Kong FZ, Wang YF, Tian Y, Gobler CJ et al (2012) Emergence of brown tides caused by Aureococcus anophagefferens Hargraves et Sieburth in China. Harmful Algae 19(9):117–124
Acknowledgements
The authors would like to thank Mr. J. F. Chen, D. S. Ding, X. Z. Zhang and Ms. C. X. Liu, F. Guo for assistance in field investigation and sample analysis. This work was supported by Shandong Provincial Natural Science Foundation, China (Grant number ZR201702200204), the Programs of the Qingdao National Laboratory for Marine Science and Technology (Grant number 2016ASKJ02), the National Natural Science Foundation of China (Grant number 41676103); the Special Fund for Environment monitoring and assessment of Bohai Sea from the Ministry of Agriculture, People’s Republic of China (Grant number 13-Q52201302).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lu, L., Jiang, T., Xu, Y. et al. Succession of phytoplankton functional groups from spring to early summer in the central Bohai Sea using HPLC–CHEMTAX approaches. J Oceanogr 74, 381–392 (2018). https://doi.org/10.1007/s10872-018-0469-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10872-018-0469-x