Abstract
From April 2011 to January 2012, seasonal variation of the aragonite saturation state (Ωar) was observed for the first time in Tokyo Bay, in order to understand the current state of ocean acidification in a highly eutrophicated bay in Japan. Ωar in the bay ranged between 1.55 and 5.12, much greater than observed in offshore waters. At the surface, Ωar was high during summer as a result of photosynthesis with some conflicting effect of freshwater input. At the bottom, Ωar was low during summer due to remineralization of organic matter. Based on an assumption that our observations represent current conditions in Tokyo Bay, it is estimated that the emission of anthropogenic CO2 has already decreased Ωar by 0.6 since the preindustrial period and will further decrease by 1.0–1.6 by the end of this century if emission of CO2 is continued at a high level [representative concentration pathway (RCP) 8.5 scenario]. With other conditions remaining the same, bottom waters of the bay will reach seasonal aragonite undersaturation by 2060–2070. However, because coastal regions have a large interannual variability, we need further observations to evaluate our estimations and future predictions presented here. Nevertheless, it should be safe to say that the larger seasonal variation in Ω causes the Tokyo Bay to reach aragonite undersaturation earlier than offshore regions and such conditions have negative consequences on the variety of calcifying organisms living in Tokyo Bay. Ocean acidification could thus give an additional stress to the ecosystem of the bay, which is now suffering from eutrophication and hypoxia.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The uptake of anthropogenic carbon dioxide (CO2) by the oceans alters carbonate chemistry of seawater to make the world’s oceans more acidic (e.g., Caldeira and Wickett 2003). This so-called “ocean acidification” is expected to profoundly affect marine ecosystems and may threaten some organisms, including commercially important shellfish, corals and fishes (e.g., Gattuso and Hansson 2011; Branch et al. 2013). Of particular concern is the impact of ocean acidification on calcifying organisms, such as clams, mussels, oysters, sea urchins, abalones, corals etc., because the saturation state of calcium carbonate (CaCO3) in seawater decreases as a result of ocean acidification. The CaCO3 saturation state (Ω) with respect to either aragonite or calcite (Ωar or Ωca), the two major types of CaCO3 produced by marine organisms, is expressed by the product of carbonate and calcium ions ([CO3 2−] and [Ca2+]) in seawater relative to the stoichiometric solubility product (K′SP, for aragonite or calcite) at a given temperature, salinity and pressure (Mucci 1983):
When CO2 is dissolved in seawater, carbonate ion (CO3 2−) is consumed via a series of chemical reactions, summarized as follows:
This results in a decrease in Ω to make the seawater less favorable to precipitate CaCO3. When Ω is <1, seawater is corrosive and therefore CaCO3 will begin to dissolve in the absence of protective mechanisms. Indeed, studies have shown a reduction in survival, calcification, growth, development and abundance when calcifying organisms such as mollusks (bivalves) were exposed to elevated CO2 and decreased Ω conditions (Kroeker et al. 2013 and references there in). It has also been revealed that larval stages of mollusks are extremely sensitive to acidification (Talmage and Gobler 2009; Kroeker et al. 2013). Some organisms, however, may be able to regulate their metabolism and calcification to compensate for increased seawater acidity, but at a substantial energetic cost (Wood et al. 2008). Therefore, this regulation is unlikely to be sustainable in the long term and may reduce energy for other important metabolic processes of calcifying organisms (Wood et al. 2008; Kroeker et al. 2013). Ocean acidification also positively or negatively affects reproduction, behavior or photosynthesis of a range of organisms, while effects are overall negative for calcifiers (Gattuso and Hansson 2011; Kroeker et al. 2013). Although the overall effects of ocean acidification on marine ecosystems need to be further investigated, ocean acidification and the decrease in Ω can have substantial consequences on fisheries and marine ecosystem services (e.g., Cooley and Doney 2009; Narita et al. 2012; Branch et al. 2013).
The coastal area is the main field for aquaculture and capture fisheries, especially for shellfish, as well as other ecosystem services. In coastal regions, however, distribution and seasonal variation of Ω is not well known, as it is strongly locally regulated by other natural and anthropogenic environmental processes, such as freshwater and carbon inputs from land, intensive mixing, eutrophication and resulting bottom hypoxia (Salisbury et al. 2008; Wootton et al. 2008; Feely et al. 2010; Provoost et al. 2010; Cai et al. 2011; Melzner et al. 2013; Duarte et al. 2013). Therefore, observations of Ω with other environmental parameters are essential for understanding and prediction of carbonate chemistry with increasing atmospheric CO2 and the impact of ocean acidification on regional ecosystems. In the present paper, we first show observational results of seasonal variation in Ω in Tokyo Bay, a highly eutrophicated bay in Japan. We will focus on Ωar because aragonite is more soluble and thus has lower Ω values than calcite, and because aragonite constructs the larval shell of mollusks, including commercially important bivalves such as clams and oysters. Studies have shown that a decrease in Ωar in seawater or at the sediment–water interface results in an increase in mortality and decrease in growth of larval and juvenile bivalves (Green et al. 2004; Talmage and Gobler 2009). Based on observations, we will discuss processes affecting Ω in the bay. We also make an estimation of future changes in Ω in order to provide information on future progress of ocean acidification in the bay.
2 Study area
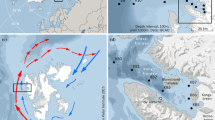
Tokyo Bay is a semi-enclosed bay located in central Japan, with an area of 922 km2 and a mean depth of 19 m (Fig. 1). The bay is surrounded by highly urbanized areas and receives freshwater and nutrients from rivers with discharges from sewage treatment plants. This makes the bay one of the most eutrophicated coastal environments in the world. The annual primary production in Tokyo Bay is reported as one of the highest among the world’s estuarine-coastal ecosystems (Cloen et al. 2014), even though nutrient inputs to the bay and concentrations in surface bay waters have significantly decreased since the 1980s (Furukawa and Okada 2006; Kanda et al. 2008; Kodama et al. 2010; Kodama and Horiguchi 2011). Because of high primary production and surface stratification, respiration of organic matter by bacteria often causes hypoxia/anoxia in the bottom water of the Tokyo Bay during summer (e.g., Ishii et al. 2008). The annual catches of fish and shellfish in the bay decreased from ~100,000 tons in the 1960s to ~20,000 tons in the 2000s, due to excessive eutrophication, hypoxia/anoxia, coastal reclamation and competition with newly introduced species (Furukawa and Okada 2006). At present, shellfish correspond to more than 60 % of the total catch. The nearshore areas of the bay are also popular fields for recreational clam gathering.
Ocean acidification in Tokyo Bay is expected to cause additional stresses on organisms living in this eutrophicated bay, with socioeconomic consequences. Furthermore, it has been pointed out that eutrophication can amplify ocean acidification (Cai et al. 2011; Melzner et al. 2013). Eutrophication in coastal waters increases heterotrophic remineralization of organic matter and produces more CO2 in bottom water, which lowers Ω and also reduces the ability of seawater to buffer changes in pH. Therefore, we expect that Tokyo Bay is one of the regions in Japan most vulnerable to ocean acidification. However, there is no observational information on the state of Ω and its seasonal variation in Tokyo Bay or other coastal areas of Japan. It is speculated that Ω in the bay has large seasonal variability, due to freshwater input and extensive biological activities during summer and cooling during winter. The large seasonal variability should lead the bay water to reach to the threshold, e.g. Ω = 1, earlier than the open ocean, at least seasonally.
In the open ocean off Japan, some observational data of Ω are available. Distribution of surface Ωar in the open ocean region of the northeastern Pacific Ocean (Fig. 2) shows that Ωar is lower in the north, especially in the cyclonic western subarctic gyre, and is over 3.0 in the region south of 35.5°N where Tokyo Bay is located. This spatial variation reflects the fact that low temperature water has lower Ω (CaCO3 and CO2 dissolve more in colder water) and also upwelling of deeper water brings CO2-enriched low Ω water toward the surface. A detailed and long-term observation in the subtropical North Pacific off the southern coast of Japan showed that Ωar of surface water is ~3.5 during the summer, but decreases to lower than 3.0 during the winter (at 33°40′N–34°00′N; Ishii et al. (2011)). In the western subarctic gyre further north, a similar seasonal pattern in surface Ωar was observed, but with a larger variation and lower values for both summer (~2.5) and winter (~1.3) (at ~45°N; Wakita et al. 2013). From these observations, the Ωar of waters in Tokyo Bay would be ~3 with a maximum value in summer, if there is no alternation of water properties by processes within the bay.
Surface Ωar in the northeastern Pacific Ocean calculated from chemical data (DIC, TA and nutrients) in the GLODAP database (Key et al. 2004). Note that there are no data in the coastal region of Japan with depths shallower than 1000 m, and observations are from May to September only. A black rectangle in the map shows the location of Tokyo Bay
3 Observation and analysis
To estimate Ω of seawater, samples for dissolved inorganic carbon (DIC) and total alkalinity (TA) were collected in 8-L Niskin bottles mounted on a CTD rosette on the R/V Seiyo-maru of Tokyo University of Marine Science and Technology, monthly from April 2011 to January 2012 (except August 2011) at two stations in Tokyo Bay (Fig. 1). Station F3 and F6 are located at 35.51°N, 139.83°E and 35.42°N, 139.79°E, respectively. Bottom depths are 23 and 26 m for F3 and F6, respectively. Seawater samples were also collected for the analysis of nutrient and chlorophyll a concentrations. DIC of sample water was determined using a CO2 extraction-coulometry method. TA was measured by using a spectrophotometric method (Yao and Byrne 1998). The DIC and TA values were calibrated against certified reference material (Batch 105) provided by Prof. A. G. Dickson (Scripps Institution of Oceanography). Pooled standard deviations of duplicate samples were 2.3 µmol kg−1 (n = 10) and 2.8 µmol kg−1 (n = 13) for DIC and TA, respectively. Unfortunately, we missed a sample and could not measure DIC and TA of bottom water at F6 in September. Nutrient concentrations were determined with an autoanalyzer system, a Quaatro-Marine 5ch (SEAL Analytical, Ltd, Fareham, UK), following the protocols for the Joint Global Ocean Flux Study (JGOFS 1996) and Kanda (1995) for ammonium analysis. Measured values were calibrated against standard reference materials for nutrient analysis from General Environmental Technos Co., Ltd., Japan. The detection limit of the system is 0.05 µmol L−1 for phosphate, 0.37 µmol L−1 for silicate, 0.10 µmol L−1 for nitrate, and 0.15 µmol L−1 for ammonium. Water samples for chlorophyll a measurement were filtered through precombusted (450 °C for 3 h) Whatmann GF/F filters. After the filtration, chlorophyllous pigments were extracted in N, N-dimethylformamide (DMF). Concentrations of chlorophyll a were determined by the fluorometric method (Suzuki and Ishimaru 1990) using a Turner Design Model TD-700 fluorometer.
Temperature, salinity and pressure were measured by a CTD (Falmouth Scientific Inc., Bourne, MA, USA). Sensor data have been scaled with pre-cruise calibrations from the sensor manufacturer. Changes in values between calibrations were less than 0.02, 0.02 °C, and 2 dbar for salinity, temperature, and pressure, respectively. Dissolved oxygen (DO) concentration was measured by a RINKO sensor (JFE Advantech Co., Ltd.), except in April 2011. Calibrations of the RINKO sensor performed for each cruise showed that the error between cruises was less than 3 %.
Observations of DIC, TA, nutrients, temperature, salinity and pressure were used to calculate Ω and partial pressure of CO2 (pCO2 with in situ temperature and pressure) by using the CO2sys program (Lewis and Wallace 1998) with constants of Lueker et al. (2000) for K 1 and K 2 and Dickson (1990) for KSO4. The uncertainty in both DIC and TA measurements can lead to an error of <0.06 in Ω and <32 µatm in pCO2. In these calculations, we did not account for the contribution of dissolved organic matter (DOM) to TA: it has been shown that a bulk DOC acts as a proton acceptor and influences the acid–base system of seawater (e.g., Cai et al. 1998; Hernández-Ayon et al. 2007; Kim and Lee 2009). In open oceans, this contribution is generally much smaller than that of inorganic compounds, and is therefore negligible. In coastal waters, however, high concentrations of DOM due to high primary production and inputs from rivers can significantly contribute TA. For example, Cai et al. (1998) estimated organic contributions to TA (TAorg) of <50 µmol kg−1 for waters in the coastal zone of Georgia (USA) with dissolved organic carbon (DOC, as a measure of DOM) concentrations of 300–750 µmol kg−1. They also observed higher TAorg values up to 120 µmol kg,−1 but only in a river estuary with very high DOC concentrations (>1000 µmol kg−1). In the Baltic Sea, TAorg was estimated to be ~30 µmol kg−1 in waters with DOM of ~300 µmol kg−1 (Kuliński et al. 2014). Accordingly, the assumption of TAorg = 0 can result in errors in calculations of Ω and pCO2 in coastal waters. To estimate the order of this error, we assumed TAorg = ~20 µmol kg−1 in Tokyo Bay, based on the relationship between DOC and TAorg in the above-mentioned observations in coastal oceans and DOC concentrations of 80–180 µmol kg−1 at F3 and F6 stations (Kubo et al. 2015), although acid–base properties of DOM are regionally and temporally variable. A comparison between estimations with and without TAorg (TA-TAorg) shows that 20 µmol kg−1 of TAorg results in an overestimation of Ω by ~8 % and an underestimation of pCO2 by ~10 %, consistent with error estimations by Koeve and Oschlies (2012).
4 Results and discussions
4.1 Temperature, salinity, DO and chlorophyll a
Observations of temperature and salinity showed a strong stratification during summer (Fig. 3a, b). The salinity of the upper layer decreased from spring to summer by inputs of river runoff and precipitation, and reached a minimum of 15.9 and 27.3 in September at F3 and F6, respectively (Fig. 3b). At the bottom, salinity was 33.0–34.3, somewhat fresher than the water flowing into the bay from the open ocean (33.5–34.5) (Okada et al. 2007). Temperature increased from spring to summer at all depths and reached ~26.5 °C at the surface in early September at both stations. From October, atmospheric cooling and winds caused vertical mixing and resulted in a well-mixed water column with a temperature of ~10 °C in January. DO and chlorophyll a concentrations also showed distinct seasonal variations (Fig. 3c, d). Waters in the upper layer increased in DO and had high chlorophyll a concentration (7–40 µg L−1) from June to November (also in May at F3), indicating active primary production. DO decreased in December and increased again in January. This coincides with the changes in chlorophyll a concentration, which decreased to 2 µg L−1 in December and increased again to 15–20 µg L−1 in January. High chlorophyll a in winter is sometimes observed in the inner part of the bay and has been attributed to the transport of phytoplankton cells from other parts of the bay, episodic stratification and favorable light conditions (Shibata and Aruga 1982; Nomura and Yoshida 1997). Nutrients are not considered to be the limiting factor for primary production in Tokyo Bay in winter (Shibata and Aruga 1982; Bouman et al. 2010). In the bottom layer, DO was low throughout the year except in January, due to respiration and decomposition of organic matter produced in the upper layer and transported from land. The bottom water at F3 reached hypoxic condition (≤2.0 ml L−1) in July and severe hypoxia (≤0.2 ml L−1) in September. At F6, the minimum DO concentration was 2.1 ml L−1 observed in September. These observations showed that in 2011, F3 had a larger seasonal variability in surface salinity and bottom DO than F6. Previous studies have also shown larger seasonal variations at F3 than at F6 in salinity, nutrients, and chlorophyll a concentrations (Matsumura et al. 2001; Kanda et al. 2008). This is consistent with the fact that F3 is located in the innermost region of the bay, which directly receives massive amounts of freshwater, nutrients and organic matter from the land (e.g., Ninomiya et al. 1996; Kubo et al. 2015). Accordingly, the innermost region is characterized by large seasonal variations, strong stratification, high primary production, and frequent occurrence of hypoxic/anoxic waters in the water column (Shibata and Aruga 1982). Also, the seafloor of the innermost region is covered by silt and clay with high COD (chemical oxygen demand) and little benthic biomass, compared to the central region of the bay where F6 is located (Furukawa and Okada 2006; Kodama and Horiguchi 2011).
4.2 TA, DIC and pCO2
Seasonal variations of carbonate parameters (Fig. 4) should be explained by the above-mentioned physical and biological conditions. At station F3, TA and DIC ranged from 1523 to 2290 µmol kg−1, and from 1207 to 2160 µmol kg−1, respectively. At F6, TA was between 2092 and 2283 µmol kg−1, and DIC was between 1763 and 2092 µmol kg−1. Note that normalization of TA and DIC to a constant salinity was not applied in this study because of the significant inputs of TA and DIC from rivers into Tokyo Bay. Variations are much larger than those in the open ocean (e.g., Wakita et al. 2013; Yasunaka et al. 2013). The lowest values observed at the surface of the F3 station in September correspond to the lowest salinity. Both TA and DIC correlated well linearly with salinity, and the coefficient of determination is 0.85 (n = 95) and 0.81 (n = 94), respectively, indicating that the mixing between freshwater and seawater is the main factor controlling seasonal variations of these parameters in the bay (Fig. 5). The intercept of salinity–TA regression line (1076 ± 97 µmol kg−1) is close to that of Taguchi et al. (2009), who reported that salinity and TA in the bay can be expressed as a mixture of offshore water (North Pacific subtropical mode water; salinity = 34.82, TA = 2293 µmol kg−1) and freshwater end-member with TA of 1006 µmol kg−1. It is also close to TA in rivers flowing to Tokyo Bay; ~950 µmol kg−1 in Arakawa River (Kobayashi 1960) and 1094 µmol kg−1 in Tamagawa River (observed by ourselves in April 2011 at Futako Bridge, 18 km upstream from the river mouth). It should be noted that waters observed in April and May, as well as some summer surface waters, had higher TA values than expected from the regression line in the salinity-TA diagram (Fig. 5). This should reflect a seasonal variability in TA of river runoff and/or in relative contributions of river runoff and precipitation (TA = 0). Biological activity also alters TA, but to a smaller degree; assimilation of nitrate and denitrification increases TA and assimilation of ammonia and bacterial nitrification decreases TA (Brewer and Goldman 1976). The effect of these processes on TA can be evaluated by calculating the potential alkalinity (pA = TA + nitrate−ammonium). The difference between pA and TA was ~ ±20 µmol kg−1. This means that nitrogen-related biological processes alter TA in Tokyo Bay by ± 20 µmol kg−1. Formation and dissolution of calcium carbonate by calcifying organisms can also alter TA of seawater [one mole of CaCO3 formation (dissolution) results in two moles of TA decrease (increase)]. For example, Shamberger et al. (2011) observed ~100 µmol kg−1 of daily TA fluctuation on a Hawaiian coral reef and Murata et al. (2006) found ~80 µmol kg−1 lower TA than expected from salinity in blooms of coccolithophorid in the Bering Sea. In the Tokyo Bay, shellfish are the major calcifier and calcifying plankton very rarely dominate in the bay (Yamada 1997). In our observations, bottom waters observed at 23 and 26 m in October at F6 showed lower TA values at salinity of ~34 by up to ~40 µmol kg−1 compared to other months (red crosses in Fig. 5a). This may be due to calcification at the bottom. However, it is not clear at the moment whether observed lower TA was due to calcification or not. Nevertheless, we suppose that biological impacts on seasonal variation in TA are overall smaller than that of freshwater inputs, as suggested by the relatively good linear relationship between salinity and TA and the fact that it follows a mixing line between offshore water and freshwater end-member (Fig. 5a; Taguchi et al. 2009).
Relationships between salinity and TA (a), and salinity and DIC (b). Filled and open square (F3) and circles (F6) are data observed in spring (April and May) and in summer (June to September), respectively. Pluses (F3) and crosses (F6) represents data from fall and winter (October to January). Red crosses in (a) indicate bottom water (23 and 26 m) observed in November at F6. Black lines in (a) and (b) indicate regression lines of TA = 35.456 × salinity + 1076.1 or DIC = 48.849 × salinity + 398.9, respectively
One can expect that freshwater end-member for DIC is closer to that for TA (1076 ± 97 µmol kg−1), as bicarbonate ion (HCO3 −) is a dominant carbonate species in freshwater. Actually, annual mean DIC observed in the lower Tamagawa river was ~1050 µmol kg−1 (Kubo et al. 2015). Furthermore, runoff into the bay can transport excess DIC due to a large contribution of effluent from sewage treatment plants (50 % of freshwater flux into Tokyo Bay) which have high pCO2, high organic matter content and high DIC (~1600 µmol kg−1; Kubo et al. 2015). However, the intercept of salinity-DIC relationship was as low as 399 ± 158 µmol kg−1. This means that excess CO2 from runoff has been removed on the way from river mouths to the study sites, probably in very low salinity area near the coast. The process of removal should be air-sea exchange and biological drawdown of CO2. Consequently, freshening of surface water resulted in low DIC concentrations (Figs. 3b, 4b, 5b). In situ primary production further decreases DIC in surface waters. At the bottom, waters become enriched with DIC as produced organic matter settles and is respired by bacteria. This is more evident when pCO2 is estimated from TA and DIC (Fig. 4c). The seawater pCO2 was lower than that in the atmosphere (~400 µatm, Kubo, unpublished data in Tokyo Bay from 2007 to 2010) in the entire water column in January and in the upper layer throughout the year as a result of photosynthesis (in situ or in upstream region), except the upper layer in October at F3 (probably influenced by vertical mixing with deeper water) (Figs. 3c, d, 4c). On the other hand, pCO2 in the bottom layer increases from May to August because of remineralization of organic matter. The lowest pCO2 (82 µatm) was in the freshest surface water at F3 in September and the highest DIC (2160 µmol kg−1) and pCO2 (938 µatm) was observed in the anoxic bottom water at F3 in September (Fig. 4). Because of these biological impacts on CO2, observed range in DIC was 1.2 and 1.7 times larger than in TA at F3 and F6, respectively. Variations in DIC, TA and pCO2 are larger at F3 in the innermost bay than F6 in the central bay reflecting larger freshwater inputs and higher productivity/remineralization in the former region (Matsumura et al. 2001; Kanda et al. 2008; Kubo et al. 2015).
4.3 Ω
Figure 6 shows seasonal variation in Ωar in Tokyo Bay. Despite its highly eutrophicated environment, waters at two stations in Tokyo Bay were oversaturated with respect to calcium carbonate throughout the year. Seasonal variation of Ωar was large and almost mirrored pCO2. From April to November, Ωar was high in the upper layer and low in the bottom layer. A decrease in October is probably due to mixing with deeper water as increased pCO2 at the surface suggests (Fig. 6). In December, Ωar in the water column was the lowest but increased in January. These results suggest that photosynthesis and remineralization are major processes controlling seasonal variation of Ωar in Tokyo Bay: photosynthesis removes CO2 from seawater while remineralization releases CO2 into seawater to increase and decreases Ωar, respectively (Eqs. 1 and 2). The lowest value of 1.55 was observed in the anoxic and CO2-enriched bottom water at F3 in September, as a result of organic matter remineralization. The highest Ωar (5.12) was, on the other hand, observed at the surface of F3 in July, not in September when pCO2 was the lowest. This is due to the heavy freshening in September which lowers Ωar by reducing Ca2+ and CO3 2− concentrations (Salisbury et al. 2008; Yamamoto-Kawai et al. 2009, 2011). Therefore, the effect of photosynthesis was cancelled out by that of freshening in September. At F3, Ωar varied between 2.01 and 5.12 (Δ3.11) at the surface and 1.55–3.07 (Δ1.52) at the bottom. At F6, Ωar was 2.27–4.04 (Δ1.77) at the surface and was 1.68–2.85 (Δ1.17) at the bottom, although we missed a sample of bottom water in September at F6. The range of variation was larger at F3 than F6 as expected from the more active photosynthesis and remineralization in the innermost bay. When compared to the offshore subtropical waters (Ωar = 3.0–3.5; Ishii et al. 2011), waters in Tokyo Bay experiences much larger temporal variation in Ωar both at the surface and the bottom. The pattern of seasonal variation is similar to that of open ocean in the surface layer, high in spring and summer and low in winter, but there are additional significant effects of freshening (descreases Ωar), mixing with CO2-enriched bottom water (decreases Ωar), and high primary production (increases Ωar). Bottom waters showed an opposite variation, low in summer and high in winter with an occasionally low value in November at F6 when pCO2 was high (Fig. 6). Bottom water in summer showed much lower Ωar than in offshore where Tokyo Bay water originates (Figs. 2, 6). From these results, we conclude that Ω in Tokyo Bay is controlled by the freshwater input and photosynthesis at the surface and remineralization at the bottom.
Here, we try to estimate effects of human activity on Ω in the bay through regulation of freshwater and nutrient discharges. Okada et al. (2007) estimated an increase of freshwater input to the Tokyo Bay from 341 m3 s−1 in 1947–1974 to 424 m3 s−1 in 2002–2003, mostly due to introduction of freshwater to the metropolitan region from outside of the drainage basins. We roughly estimate the effect of the freshwater increase to Ω of the bay as follows. Assuming that offshore water with salinity = 34.82 and TA = 2293 µmol kg−1 (following Taguchi et al. 2009) mixes with freshwater with salinity = 0 and TA = 1076 μmol kg−1 to have salinity between 15 and 34.5, fraction of freshwater (fFW) and TA in mixed waters are estimated to be 0.56–0 and 1605–2293 µmol kg−1, respectively. We calculated Ωar of the mixed waters by assuming 2 dbar for pressure, 276 µatm for pCO2 and 18 °C for temperature, which are average values of our observations in surface water at F3 and F6. Then, we reduced fFW of the mixed water by 20 % (fFW × 0.80), based on the increase estimated by Okada et al. (2007), and recalculated salinity, TA, and then Ωar. Results show that the removal of 20 % of freshwater increases Ωar by up to 0.32. Note that effects of increases in nutrients or carbon discharges from land that should result in changes in biological activities and DO concentration are not considered here, as they should be included in the estimates of eutrophication effects as described below. Therefore, we estimate that the increase in freshwater input to the bay could have decreased Ωar by ~0.3. However, we suppose that Ωar in bay water was not actually decreased during the period from 1947–1974 to 2002–2003, because the expected decrease in salinity was not observed during this period despite an increase in freshwater discharge (Okada et al. 2007). This contradiction suggests a shorter residence time of freshwater in the bay, due to the decreased bay area, increased inflow of saline offshore water to the bay, and/or increased discharge itself (Okada et al. 2007; Ando et al. 2003). Therefore, the effect of increased freshwater input on Ωar and those of physical changes may have cancelled each other out. In other words, if the changes in physical condition are independent from the increased discharge, they could have increased Ωar by 0.3.
Anthropogenic eutrophication, on the other hand, should have largely modified Ω in the bay. For example, Ishii et al. (2008) analyzed data of DO in Tokyo Bay from 1948 to 2005 and revealed that DO in summer bottom water in the innermost bay area was higher than 2.5 ml L−1 before 1956. but decreased to ~1.0 ml L−1 or lower in and after the 1960s. This change coincides with a sharp and large increase during the 1950s in COD in 1955-1975 data (Unoki and Kishino 1977, cited in Kawabe and Kawabe 1997), as well in numbers of red tide events in 1907–1995 (Nomura 1998). These indicate that a significant and rapid eutrophication occurred in the 1950/1960s. The decrease of DO by 1.5 ml L−1 (67 μmol kg−1) corresponds to an increase by 46 μmol kg−1 of DIC when O2:C stoichiometry of remineralization reaction was assumed to be −170:117 (Anderson and Sarmiento 1994). An addition of 46 μmol kg−1 of DIC to summer bottom water lowers Ωar by about 0.45. Note that when the O2/C ratio was varied within the theoretical range of 1–1.58 (Fraga et al. 1998), the change in DIC and Ωar would be 42–67 μmol kg−1 and 0.4–0.6, respectively. At the surface, DO in summer was ~6.0 ml L−1 in the 1950s, and >7.5 ml L−1 after the 1950s due to enhanced photosynthesis, whereas the concentration in winter stayed nearly constant (Ishii et al. 2008). This should have resulted in an increase in summer surface water Ωar by about 0.45, if other conditions stayed the same. Therefore, we roughly estimate that the eutrophication after the 1950s could have expanded seasonal variation of Ωar in the innermost bay region by 15 % [0.45/(3.11−0.45)] and 40 % [0.45/(1.52−0.45)] at the surface and the bottom, respectively. Note that this can be an underestimation because we did not consider effects of denitrification in sediment or bottom water which increases DIC without changing DO and could have enhanced as a result of eutrophication, and air-sea exchange releases some of DO produced by photosynthesis in surface waters but has less impact on CO2 due to a slower gas exchange rate for the latter. Also, temporal changes in neither photo-degradation of DOM to DIC nor TAorg were considered because of the lack of information.
4.4 Ω and atmospheric CO2
In this section, we examine the effect of increased atmospheric CO2 concentration on Ω in Tokyo Bay in order to provide some information on the future progress of ocean acidification in the bay. First of all, DIC is expressed as:
where CEQ is DIC of water in equilibrium with atmospheric CO2 concentration when the water was last in contact with the atmosphere, ΔCDiseq represents air-sea disequilibrium and ΔCBio is the change in DIC due to biological activity (cf. Gruber et al. 1996; Sabine et al. 2002; Yamamoto-Kawai et al. 2013). Using the CO2sys program (Lewis and Wallace 1998), CEQ can be estimated from salinity, temperature, TA and atmospheric CO2 concentration (xCO2) (converted to pCO2 at 100 % humidity and standard atmospheric pressure). The sum of latter two terms on the right hand side of the Eq. (3) is calculated by subtracting estimated CEQ from DIC observed in 2011/2012. The Tokyo Bay is shallow enough to assume that all waters were very recently in contact with atmosphere and thus we used xCO2 = 394 ppm, the mean xCO2 at Mauna Loa for our observation period from April 2011 to January 2012 (NOAA/PMEL), for the calculation of CEQ for our observation period. We should note that xCO2 in the air over the Tokyo Bay can be different from that observed at Mauna Loa. A comparison of xCO2 data between monthly observations from 2007 to 2010 in Tokyo Bay (Kubo, unpublished data) and monthly mean at Mouna Loa was from −18 to +35 ppm (Tokyo Bay–Mauna Loa). As for annual mean, the difference was only ~10 ppm. Therefore, we decided to use observations at Mauna Loa in 2011/2012, rather than observations in Tokyo Bay in different years. Assuming that salinity, temperature, and TA remains the same over time, we can estimate CEQ of the bay water under the atmosphere with a given xCO2. Then, with an assumption that (ΔCDiseq + ΔCBio) also remains the same over time, DIC of the water under each xCO2 can be estimated. Then, Ωar was calculated from this estimated DIC and observed temperature, salinity and TA (Fig. 7).
Evolution of Ωar in surface and bottom waters in each month with increasing xCO2. Years for corresponding xCO2 are also shown in x-axis [based on representative concentration pathway (RCP)8.5 scenario; Meinshausen et al. 2011]. PI represents preindustrial period. A grey line in each figure indicates conditions in 2011–2112. A black line shows the level when seawater is in equilibrium with aragonite (Ωar = 1)
It is shown that anthropogenic CO2 has already decreased Ωar of Tokyo Bay waters by about 0.6 from values in the preindustrial era with xCO2 of 280 ppm. As discussed in the previous section, eutrophication should have also altered Ωar in summer, to counteract and to aggravate the effect of ocean acidification at the surface and the bottom, respectively. Therefore, for summer seasons, preindustrial Ωar could be lower for surface waters and higher for bottom waters than that presented in Fig. 7. If conditions other than xCO2 remain the same as today in the future, Ωar in both surface and bottom waters will be further reduced by 1.0–1.6 by the end of this century when xCO2 reaches 1000 ppm with a high CO2 emission scenario RCP8.5 in IPCC AR5 (Riahi et al. 2011). Surface waters in Tokyo Bay will remain oversaturated until the end of this century, but will be undersaturated at the beginning of the next century. Bottom water in the innermost bay region (F3) will experience seasonal undersaturation with respect to aragonite when xCO2 reaches to 600 ppm. For the middle part of the bay (F6), bottom water becomes seasonally undersaturated when xCO2 exceeds 650 ppm. xCO2 of 600 and 650 ppm can be reached as early as the years 2060 and 2070, respectively, in the RCP8.5 scenario. The year to reach undersaturation is about 50 years earlier in the bay than that expected in offshore waters. For instance, near surface waters south or southeast of Tokyo Bay are estimated to have Ωar around 1.5 even when xCO2 reaches 1000 ppm at the end of this century, and may reach undersaturation in the 2120s (Yara et al. 2012; Ishii et al. 2011). The present study suggests that this difference is caused by the large effect of remineralization on Ωar in bottom water in the bay. Accordingly, an enhancement or reduction of eutrophication should alter the timing when waters are corrosive to aragonite. As a case study, we have estimated Ωar in bottom water at F3 in September with doubled and without DIC change due to eutrophication (Fig. 8). For this calculation, 46 μmol kg−1 of DIC was assumed to represent the current eutrophication from the discussion above, and was added (doubled eutrophication) or removed (no eutrophication) in the ΔCBio term. Figure 8 indicates that enhancement or reduction of eutrophication could change the year to reach aragonite undersaturation by a few decades. It is also indicated in Fig. 8 that even if we could remove eutrophication from the bay, anthropogenic CO2 will bring these water to be corrosive to aragonite by the year 2080.
5 Summary and conclusion
Monthly observation at two stations in Tokyo Bay revealed that Ωar in the bay varies much more than in offshore waters (cf. Ishii et al. 2011; Wakita et al. 2013). The very high primary production and resulting remineralization of organic matter in this shallow and eutrophicated bay are the major causes of such large variations. It should be declared that our observations are only from 1 year and may not represent typical conditions of Tokyo Bay. However, because this study is the first to report Ωar in Tokyo Bay, we believe that it is worth trying to quantify effects of different processes on Ωar, and to provide information on future changes in Ωar. Based on this standpoint, we estimated that eutrophication since the late 1950s has increased and decreased Ωar of summer bay waters by 0.45 or more at the surface and the bottom, respectively, in the innermost bay region. In the middle bay region, the effect of eutrophication on Ωar is smaller. Ocean acidification, on the other hand, affects Ωar at any depths and anywhere in this shallow bay. It is estimated that anthropogenic CO2 has already decreased Ωar of the bay water by 0.6 since the preindustrial period. Therefore, in terms of Ωar, ocean acidification has a higher impact than eutrophication. If emission of CO2 is continued at a high level as predicted by the IPCC RCP8.5 scenario (Riahi et al. 2011), bottom waters in the innermost region of the bay will reach seasonal undersaturation with respect to aragonite as early as 2060. In the middle bay region with less effect of eutrophication, bottom waters will experience seasonal undersaturation by 2070. Even when the effect of eutrophication is removed, bottom water in the innermost bay region will reach undersaturation by 2080. Eutrophication and hypoxia in Tokyo Bay are great concerns, and many efforts have been made to remedy these problems. Our results suggest that ocean acidification should also be considered as a serious problem as it can make the bay water to be corrosive to aragonite within 45 years at earliest, and many benthic organisms, including commercially important bivalves, rely on a planktonic early life stages with shells composed of aragonite. Furthermore, even when Ωar is still higher than 1.0, a decrease in Ωar can cause enhanced mortality and delayed development of larval bivalves because of the presence of amorphous calcium carbonate in their shells, which is substantially more soluble than aragonite (Talmage and Gobler 2009). Ocean acidification also affects other organisms. For example, increased CO2 damages tissue of internal organs in some larval fish (Frommel et al. 2011) while it enhances growth of fleshy seaweeds (Kroeker et al. 2013 and references there in). An effect of ocean acidification on one species can progress to other species or to the ecosystem through the food web or by altering competitive balance between species (Kroeker et al. 2012). It is also pointed out that the concurrent effects of acidification and hypoxia are more severe than would be predicted by either individual stressor, and that even organisms insensitive to acidification alone could be negatively affected by acidification when concurrently exposed to hypoxic conditions (Gobler et al. 2014). Therefore, progress of ocean acidification could give an additional stress to the ecosystem of the Tokyo Bay, which is now suffering from eutrophication and hypoxia. Definitely we need more observations of Ω in Tokyo Bay to evaluate our estimations presented here. Also, observations in other coastal regions of Japan, together with investigations into responses of local ecosystems to ocean acidification are necessary in order to assess the impact of this ongoing global problem on local fisheries and marine ecosystem services in Japan.
References
Anderson LA, Sarmiento JL (1994) Redfield ratios of remineralization determined by nutrient data analysis. Global Biogeochem Cycles 8(1):65–80
Ando H, Kashiwagi N, Ninomiya K, Ogura H, Yamazaki M (2003) Long term trends of seawater temperature in Tokyo Bay. Oceanogr Japan 12(4):407–713 (in Japanese with English abstract)
Bouman HA, Nakane T, Oka K, Nakata K, Kurita K, Sathyendranath Sm Okatt T (2010) Environmental controls on phytoplankton production in coastal ecosystems: a case study from Tokyo Bay. Estuar Coast Shelf Sci 87:63–72
Branch TA, DeJoseph BM, Ray LJ, Wagner CA (2013) Impacts of ocean acidification on marine seafood. Trends Ecol Evol 28(3):178–186
Brewer PG, Goldman JC (1976) Alkalinity changes generated by phytoplankton growth. Limnol Oceanogr 21(1):108–117
Cai WJ, Wang YC, Hodson RE (1998) Acid-base properties of dissolved organic matter in the estuarine waters of Georgia, USA. Geochim Cosmochim Acta 62:473–483
Cai WJ et al (2011) Acidification of subsurface coastal waters enhanced by eutrophication. Nat Geosci 4(11):766–770
Caldeira K, Wickett ME (2003) Anthropogenic carbon and ocean pH. Nature 425(6956):365
Cloen JE, Foster SQ, Kleckner AE (2014) Phytoplankton primary production in the world’s estuarine-coastal ecosystems. Biogeosciences 11:2477–2501. doi:10.5194/bg-11-2477-2014
Cooley SR, Doney SC (2009) Anticipating ocean acidification’s economic consequences for commercial fisheries. Environ Res Lett 4(2):024007
Dickson AG (1990) Standard potential of the reaction: AgCl (s) + 1/2H2(g) = Ag(s) + HCl(aq), and the standard acidity constant of the ion HSO4 − in synthetic sea water from 273.15 to 318.15 K. J Chem Thermodynamics 22:113–127
Duarte MD, Hendriks IE, Moore TS, Olson YS, Steckbauer A, Ramajo L, Carstensen J, Trotter JA, McCulloch M (2013) Is ocean acidification an open-ocean syndrome? Understanding anthropogenic impacts on seawater pH. Estuaries Coasts 36:221–236
Feely RA, Alin SR, Newton J, Sabine CL, Warner M, Devol A, Krembs C, Maloy C (2010) The combined effects of ocean acidification, mixing, and respiration on pH and carbonate saturation in an urbanized estuary, Estuarine. Coastal Shelf Sci 88(4):442–449
Fraga F, Ríos AF, Pérez FF, Figueiras FG (1998) Theoretical limits of oxygen:carbon and oxygen:nitrogen ratios during photosynthesis and mineralization of organic matter in the sea. Sci Mar 1–2:161–168
Frommel AY, Maneja R, Lowe D, Malzahn AM, Geffen AJ, Folkvord A, Piatkowski U, Reusch TBH, Clemmesen C (2011) Severe tissue damage in Atlantic cod larvae under increasing ocean acidification. Nat Clim Chang 2:42–46
Furukawa K, Okada T (2006) Tokyo Bay: its environmental status-past, present and future. In: Wolanski E (ed) The Environment in Asia Pacific Harbours, Springer, pp. 15–34
Gattuso JP, Hansson L (2011) Ocean acidification. Oxford University Press, p. 311
Gobler CJ, DePasquale EL, Griffith AW, Baumannobler H (2014) Hypoxia and acidification have additive and synergistic negative effects on the growth, survival, and metamorphosis of early life stage bivalves. PLoS One 9(1):e83648. doi:10.1371/journal.pone.0083648
Green MA, Jones ME, Boudreau CL, Moore RL, Westman BA (2004) Dissolution mortality of juvenile bivalves in coastal marine deposits. Limnol Oceanogr 49(3):727–734
Gruber N, Sarmiento JL, Stocker TF (1996) An improved method for detecting anthropogenic CO2 in the oceans. Global Biogeochem Cycles 10:809–837
Hernández-Ayon JM, Zirino A, Dickson AG, Camiro-Vargas T, Valenzuela-Espinoza E (2007) Estimating the contribution of organic bases from microalgae to the titration alkalinity in coastal seawaters. Limnol Oceanogr: Methods 5:225–232
Ishii M, Hasegawa K, Kakino J (2008) Long-term fluctuations of the water quality in Tokyo Bay judged from a data set of Chiba prefecture. Bull Jpn Soc Fish Oceanogr. 72(3):189–199 (In Japanese with English abstract)
Ishii M, Kosugi N, Sasano D, Saito S, Midorikawa T, Inoue HY (2011) Ocean acidification off the south coast of Japan: a result from time series observations of CO2 parameters from 1994 to 2008. J Geophys Res. doi:10.1029/2010JC006831
Joint Global Ocean Flux Study (JGOFS) (1996) Protocols for the Joint Global Ocean Flux Study core measurements. Bergen, Centre for Studies of Environment and Resources. JGOFS Core Project Office (JGOFS Report 19)
Kanda J, Chomthaison P, Horimoto N, Yamaguchi Y, Ishimaru T (2008) Time-series variations in nutrient concentrations at two monitoring stations in Tokyo Bay. J Japan Soc Water Environ 31(9):559–564 (In Japanese with Englsih abstract)
Kawabe M, Kawabe M (1997) Temporal and spatial characteristics of chemical oxygen demand in Tokyo Bay. J Oceanogr 53:19–26
Key RM, Kozyr A, Sabine CL, Lee K, Wanninkhof R, Bullister JL, Feely RA, Millero FJ, Mordy C, Peng TH (2004) A global ocean carbon climatology: results from Global Data Analysis Project (GLODAP). Global Biogeochem Cycles 18 GB4031. doi:10.1029/2004GB00224
Kim HC, Lee K (2009) Significant contribution of dissolved organic matter to seawater alkalinity. Geophys Res Lett 36:L20603. doi:10.1029/2009GL040271
Kobayashi J (1960) Average composition and its features of Japanese rivers. Stud Agric (Nōgaku Kenkyū) 48:63–106 (in Japanese)
Kodama K, Horiguchi T (2011) Effects of hypoxia on benthic organisms in Tokyo Bay, Japan: a review. Mar Pollution Bull 63:215–220
Kodama K, Oyama M, Lee J, Kume G, Yamaguchi A, Shibata Y, Shiraishi H, Morita M, Shimizu M, Horiguchi T (2010) Drastic and synchronous changes in megabenthic community structure concurrent with environmental variations in a eutrophic coastal bay. Prog Oceanogr 87:157–167
Koeve W, Oschlies A (2012) Potential impact of DOM accumulation on fCO2 and carbonate ion computations in ocean acidification experiments. Biogeosci 9:3787–3798
Kroeker KJ, Micheli F, Gambi MC (2012) Ocean acidification causes ecosystem shifts via altered competitive interactions. Nat Clim Chang 9:156–159
Kroeker KJ, Kordas R, Crim R, Hendriks IE, Ramajo L, Singh GS, Duarte CM, Gattuso JP (2013) Impacts of ocean acidification on marine organisms: quantifying sensitivities and interaction with warming. Glob Change Biol 19(6):1884–1896
Kubo A, Yamamoto-Kawai M, Kanda J (2015) Seasonal variations in concentration and lability of dissolved organic carbon in Tokyo Bay. Biogeosciences 12:269–279
Kuliński K, Schneider B, Hammer K, Machulik U, Schulz-Bull D (2014) The influence of dissolved organic matter on the acid-base system of the Baltic Sea. J Mar Sys 132:106–115
Lewis E, Wallace DWR (1998) Program Developed for CO2 System Calculations. ORNL/CDIAC-105, Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, U.S. Department of Energy, Oak Ridge, Tennessee. doi:10.3334/CDIAC/otg.CO2SYS_DOS_CDIAC105
Lueker TJ, Dickson AG, Keeling CD (2000) Ocean pCO2 calculated from dissolved inorganic carbon, alkalinity, and equations for K1 and K2: validation based on laboratory measurements of CO2 in gas and seawater at equilibrium. Mar Chem 70:105–119
Matsumura T, Horimoto N, Y Xu, Ishimaru T (2001) Recent trends of nutrients in Tokyo Bay (1989–1998). La mer 39:19–32
Meinshausen M et al (2011) The RCP greenhouse gas concentrations and their extensions from 1765 to 2300. Clim Change 109:213–241
Melzner F, Thomsen J, Koeve W, Oschlies A, Gutowska MA, Bange HW, Hansen HP, Körtzinger A (2013) Future ocean acidification will be amplified by hypoxia in coastal habitats. Mar Biol 160(8):1875–1888
Mucci A (1983) The solubility of calcite and aragonite in seawater at various salinities, temperatures and atmosphere total pressure. Am J Sci 238:780–799
Murata A (2006) Increased surface seawater pCO2 in the eastern Bering Sea shelf: An effect of blooms of coccolithophorid Emiliania huxleyi?, Global Biogeochem. Cycles, 20, GB4006, doi:10.1029/2005GB002615
Narita D, Rdhdanz K, Tol RSJ (2012) Economic costs of ocean acidification: a look into the impacts on global shellfish production. Clim Chang 113:1049–1063
Ninomiya K, Kashiwagi N, Andoh H (1996) Seasonal characteristics of special distribution of water temperature and salinity in Tokyo Bay. J Jpn Soc water Environ 19(6):480–490 (in Japanese with English abstract)
Nomura H (1998) Changes in red tide events and phytoplankton community composition in Tokyo Bay from the historical plankton records in a period between 1907 and 1997. Umi no kenkyu 7(3):159–178
Nomura H, Yoshida M (1997) Recent occurrence of phytoplankton in the hyper-eutrophicated inlet, Tokyo Bay, central Japan. La mer 35:107–121 (in Japanese with English abstract)
Okada T, Takao T, Nakayama K, Furukawa K (2007) Change in freshwater discharge and residence time of seawater in Tokyo Bay. J Jpn Soc Civil Eng 63:67–72 (in Japanese with English abstract)
Provoost P, van Heuven S, Soetaert K, Laane RWPM, Middleburg JJ (2010) Seasonal and long-term changes in pH in the Dutch coastal zone. Biogeosci 7:3869–3878
Riahi K, Rao S, Krey V, Cho C, Chirkov V, Fischer G, Kindermann G, Nakicenovic N, Rafaj P (2011) RPC 8.5-A scenario of comparatively high greenhouse gas emissions. Clim Change 109:33–54
Sabine CL, Feely RA, Key RM, Bullister JL, Millero FJ, Lee K, Peng TH, Tilbrook B, Ono T, Wong CS (2002) Distribution of anthropogenic CO2 in the Pacific Ocean. Glob Biogeochem Cycles 16(4):1083. doi:10.1029/2001GB001639
Salisbury J, Green M, Hunt C, Campbell J (2008) Coastal acidification by rivers: a threat to shellfish? Eos Trans Am Geophys Union 89(50):513
Shamberger KEF, Feely RA, Sabine CL, Atkinson MJ, DeCarlo EH, Mackenzie FT, Drupp PS, Butterfield DA (2011) Calcification and organic production on a Hawaiian coral reef. Mar Chem 127:64–75
Shibata Y, Aruga Y (1982) Variations of Chlorophyll a concentration and photosynthetic activity of phytoplankton in Tokyo Bay. La mer 20:75–92
Suzuki R, Ishimaru T (1990) An improved method for the determination of phytoplankton chlorophyll using N. N-Dimethylformamide J Oceanogr Soc Jpn 46:190–194
Taguchi F, Fujiwara T, Yamada Y, Fujita K, Sugiyama M (2009) Alkalinity in costal seas around Japan. Bull Coastal Oceanogr 47(1):71–75
Talmage SC, Gobler CJ (2009) The effects of elevated carbon dioxide concentrations on the metamorphosis, size, and survival of larval hard clams (Mercenaria mercenaria), bay scallops (Argopecten irradians), and Eastern oysters (Crassostrea virginica). Limnol Oceanogr 54(6):2072–2080
Unoki S, Kishino M (1977) Averaged oceanographic conditions and water exchange in Tokyo Bay. Tech Rep Phys Oceanogr Lab, The Institute of Physical and Chemical Research, No. 1, p. 89 (in Japanese)
Wakita M et al (2013) Ocean acidification from 1997 to 2011 in the subarctic western North Pacific Ocean. Biogeosci 10:7817–7827
Wood HL, Spicer JI, Widdicombe S (2008) Ocean acidification may increase calcification rates, but at a cost. Proc R Soc B 275:1767–1773
Wootton TT, Pfister CA, Forester JD (2008) Dynamic patterns and ecological impacts of declining ocean pH in a high-resolution multi-year dataset. Proc Natl Acad Sci 105(48):18848–18853
Yamada Y (1997) The strange color of sea occurred in Tokyo Bay and Sagami Bay, May 1996. Bull Kanagawa Pref Fish Res Inst 2:65–75
Yamamoto-Kawai M, McLaughlin FA, Carmack EC, Nishino S, Shimada K (2009) Aragonite Undersaturation in the Arctic Ocean: effects of ocean acidification and sea ice melt. Science 326:1098–1100
Yamamoto-Kawai M, McLaughlin FA, Carmack EC (2011) Effects of ocean acidification, warming and melting of sea ice on aragonite saturation of the Canada Basin surface water. Geophys Res Lett. doi:10.1029/2010GL045501
Yamamoto-Kawai M, McLaughlin F, Carmack E (2013) Ocean acidification in the three oceans surrounding northern North America. J Geophys Res Oceans. doi:10.1002/2013JC009157
Yao W, Byrne RH (1998) Simplified seawater alkalinity analysis: use of linear array spectrometers. Deep Sea Res 45(8):1383–1392
Yara Y, Vogt M, Fujii M, Yamano H, Hauri C, Steinacher M, Gruber N, Yamanaka Y (2012) Ocean acidification limits temperature-induced poleward expansion of coral habitats around Japan. Biogeosci 9:4955–4968
Yasunaka S, Nojiri Y, Nakaoka S, Ono T, Mukai H, Usui N (2013) Monthlymaps of sea surface dissolved inorganic carbon in the North Pacific: basin-wide distribution and seasonal variation. J Geophys Res Oceans 118:3843–3850. doi:10.1002/jgrc.20279
Acknowledgments
We thank all participants, officers and crewmembers for their help in sampling during the cruise of R/V Seiyo-maru. We are grateful for the support of the Oceanographic Observation Center at the Tokyo University of Marine Science and Technology for assistance with coordination of observation, maintenance of equipment, and data processing. We also thank the two anonymous reviewers for their careful and helpful comments. This work was partly supported by a grant from Kurita Water and Environment Foundation. Some figures in this paper were illustrated using Ocean Data View software (R. Schlitzer 2008, available at http://odv.awi.de/).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yamamoto-Kawai, M., Kawamura, N., Ono, T. et al. Calcium carbonate saturation and ocean acidification in Tokyo Bay, Japan. J Oceanogr 71, 427–439 (2015). https://doi.org/10.1007/s10872-015-0302-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10872-015-0302-8