Abstract
In the last 15 years, a debate has emerged about the validity of the famous Hodgkin-Huxley model for nerve impulse. Mechanical models have been proposed. This note reviews the experimental properties of the nerve impulse and discusses the proposed alternatives. The experimental data, which rule out some of the alternative suggestions, show that while the Hodgkin-Huxley model may not be complete, it nevertheless includes essential features that should not be overlooked in the attempts made to improve, or supersede, it.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In high school biology courses, a standard experiment shows how a small voltage applied to a dead-frog muscle can induce its contraction. Actually, it reproduces the first observation made by Luigi Galvani in the eighteenth century. In 1850, Hermann von Helmholtz designed an experiment to measure the velocity of the signal that propagates along the sciatic nerve of a frog [1,2,3]. A quantitative description of the propagation of an electrical signal in a nerve was proposed in 1952 by A.L. Hodgkin and A.F. Huxley [4] after a careful series of experimental investigations. For a long time, this Hodgkin-Huxley model, recognized in 1963 by the Nobel Prize for Physiology or Medicine, stayed as the unquestioned basic model of this phenomenon, which launched a new field of research [5, 6]. According to this model, the nerve impulse is due to voltage-controlled flows of sodium and potassium ions through the axon membrane.
However, the phenomena are more complex. Experiments also detect heat transfer and a slight deformation of the axon together with the electrical signal. In the last 15 years, this led some scientists to raise questions about the Hodgkin-Huxley model and even to propose an alternative picture in which the propagation of a mechanical signal is the main feature [7, 8].
This article reviews the main experimental data accumulated over more than a century on the propagation of the nerve impulse, paying attention to some aspects which are particularly relevant for the ongoing discussion on the validity of the Hodgkin-Huxley model. Then, it discusses the proposals to replace, or complete, this model. The final discussion contains some comments on these attempts.
2 Experimental studies of the electrical properties of axons
The German school of “organic physicists” played a major role in creating modern physiology in the second half of the nineteenth century [9]. Hermann von Helmoltz (1821–1894), who worked in Heidelberg, was the first to measure the velocity of the signal along a nerve, and Julius Bernstein (1839–1917), who was trained under von Helmholtz, designed a clever apparatus which allowed him to record the shape of the nerve impulse [10]. Then, he related the potential difference across the membrane to the Nernst theory in a paper which founded the theoretical analysis of the phenomenon [11]. However, in his studies, Bernstein focused his attention on the negative part of the pulse, which is associated with a potassium flux. It was Ernest Overton (1865–1933), working in Würzburg, who pointed out the essential role of sodium for the excitation of a muscle [12].
Following this earlier period, the most significant results came from England, with the work of Alan Lloyd Hodgkin (1914–1998) (Trinity College, Cambridge) and his co-workers, particularly Andrew Fielding Huxley (1917–2012), and from the USA where Kenneth Stewart Cole (1900–1984) and Howard James Curtis (1906–1972) (Columbia University, New York) developed powerful experimental methods. After a first visit there in 1939, Hodgkin kept collaborating with this group, which significantly influenced his research. These results are important in the present context of the discussion of the Hodgkin-Huxley model because they, unambiguously, demonstrated that a flow of ions across the axon membrane determined the shape of the voltage pulse which carries information along a nerve. The course of the work, which is a mixture of careful planning and accidental observations, has been vividly reported at a conference by Hodgkin in 1976 [13].
2.1 Evidence for electrical transmission in nerve (Hodgkin 1937)
The goal of this early study [14] was to determine whether a local excitation was able to excite a neighboring region, i.e., to get evidence that a signal was actually propagating along the nerve. Hodgkin created a blocking region by freezing the axon over 3 to 5 mm, or by pressing it between two blocks of ebonite. Although the electrical pulse could not pass through, he noticed that some signal was nevertheless leaking through the blocking domain, and was making the nerve highly excitable on the other side. Following the arrival of a pulse on one side of the blocked region, on the other side a new signal could be generated by a much smaller electrical excitation than in an unperturbed region of the axon. Although Hodgkin did not discuss any mechanical response of the axon, in the context of the present discussions on the nature of the signal, this example shows that freezing the mechanical displacements in some region of the axon does not block the transmission of some signal. This argues against a purely mechanical driving mechanism for the nerve impulse.
2.2 Searching for the mechanism of the nerve impulse
Hodgkin alone or with the help of different collaborators in Cambridge, as well as Curtis and Cole in the USA, carried an impressive series of systematic experiments which provided the basis for the development of the Hodgkin-Huxley model. The results of these measurements should not be overlooked in any theory of the nerve impulse.
In an attempt to detect local effects of the membrane excitation, Hodgkin was able to isolate a single unmyelinated nerve fibre of a crab (with a great piece of luck as he says himself [13]) and this allowed him to detect sub-threshold potentials, i.e., signals which are not sufficient to launch a nerve impulse but nevertheless locally modify the properties of the membrane [15]. This was the first evidence of a local response in a nerve fiber.
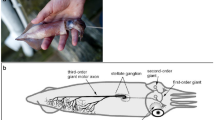
A decisive step was made by Cole and Curtis [16], inspired by the “membrane theory” of Bernstein [11] who postulated a decrease of the membrane resistance during a pulse. They managed to measure the impedance of the membrane of the giant axon of a squid during the passage of a pulse created several centimeters away, using a highly sensitive electrical bridge. The choice of the squid axon was an element of their success because it has a diameter which can reach 0.5mm and segments 3–8 cm long could be prepared. The other important element was to perform the measurements at low temperature (4–8 °C) which reduced the conduction velocity and enhanced the signal captured by the bridge.
The measurements only detected a small change of the membrane capacity but a big drop of its resistance when the voltage pulse passed. The change of the capacity depends on the frequency but never exceeds 10%, with an average of 2%. In the rest state, the membrane of the axon is non-conducting, but, during the passage of a pulse, Cole and Curtis found that its resistance dropped by a factor of about 36. The time of rise of the conductance could not be precisely determined but it was estimated to be less than 100 μs.
The experiment of Cole and Curtis did not investigate the role of specific ions. This was further studied in a series of experiments which varied the ionic content of the medium around the nerve fibers.
Hodgkin [17] showed that the velocity of the pulse decreases by about 30% when the outside medium changes from sea water to oil, which is an important indication for the models.
Curtis and Cole [18] made measurements with one electrode inside the axon and another outside. For the first time, it gave a precise view of the membrane potential. The resting potential (Vinside − Voutside in Hodgkin-Huxley notation) is about − 50mV, while the action potential is positive and differs by about 110mV from the resting potential. Replacing the Na+ ions of the outside medium by K+ causes a dramatic drop of the action potential. The values of the resting and action potentials were later confirmed by Hodgkin and Huxley [19], but, as recognized by Huxley himself [20], the explanations that they proposed for the sign reversal within the pulse were wrong. It is only soon after that they began to consider an increase in membrane permeability specific for sodium ions, which was confirmed by a study by Hodgkin and Katz [21]. All these experiments are very challenging because the electrodes can become polarized during the measurement, and thus alter the potentials to be measured. Hodgkin and Katz developed specific electrodes that they could introduce inside the axon and their paper describes several tests that they made to validate their results. Using various external solutions, they demonstrated the crucial role of the sodium, and the sharp rise of membrane permeability to sodium when the action potential arrives. In agreement with Overton’s observations [12], they could show that lithium ions also show an effect very similar to sodium although, on the long term, lithium damages the axon. Therefore, all data started to fit together nicely to set the stage for the Hodgkin-Huxley model proposed in 1952 [4].
This picture, inferred from experiments immersing axons in various solutions, was later confirmed by a direct observation of the flow of ions through the membrane in a series of papers by Hodgkin and Keynes in 1955 using radioactive tracers [22, 23]. The experiments showed that, during the nerve impulse, both Na+ and K+ move down concentration gradients, i.e., their transport is passive, contrary to the slower transport which brings the axon back to its rest state in the recovery process. It uses metabolic energy, is highly temperature dependent, and can be inhibited by dinitrophenol contrary to the passive transport during the pulse. In this voltage clamp experiment, which can impose a fixed potential difference even if the concentration of the ions is modified, Hodgkin and Keynes managed to show that the potassium flux is not proportional to concentration but increases more steeply [23]. This convinced them that the ions do not move independently from each other, and they showed that all their observations could be well reproduced by a model in which the K+ ions move along a chain of potassium selective sites which stretch through the membrane, and that all n sites in each chain are occupied by a potassium. It is quite remarkable that a careful analysis of macroscopic experiments managed to determine the features of the ion channels which were detected only years later.
Another study by Hodgkin and Katz [24] is also very important in the context of current discussions on the basic mechanism of the nerve impulse. It is their investigation of the effect of temperature on the electrical activity of the giant axon of the squid from − 1 to 40 °C. The resting potential was practically constant up to 20 °C and dropped at higher temperature. The action potential showed a gradual evolution, with a slight decrease in amplitude up to 20 °C and then a faster drop above 30 °C. Over the whole temperature range, the change in the time scale of the pulse is gradual, but very significant. At lower temperature, the nerve pulse becomes very broad, and moreover the rise and fall times have different temperature dependencies. The fall time grows much more than the rise time when temperature decreases. This led Hodgkin and Katz to suggest different mechanisms for these two processes, which is consistent with the current knowledge that they involve different ion channels.
3 The nerve impulse is not only an electrical signal
Although a large part of the efforts to understand the transmission of signals along nerves focused on the electrical aspects, the phenomena are more complex as shown by calorimetric and mechanical measurements.
3.1 Thermal effects
In 1848, Helmholtz failed to detect any heat effect associated to the nerve impulse, and the data remained controversial for several decades [25]. It was only more than 80 years later that reliable evidences of a very weak heat effect associated with the nerve impulse could be obtained but repeated stimulation was necessary so that the relative timing between the heat release or absorption and the electrical pulse could not be determined [25]. A very small resting heat production could also be detected by putting a frog nerve in a nitrogen atmosphere. Depriving the nerve from oxygen appears to stop some oxidative processes, leading to a slow decline of the resting heat production.
More modern measurements managed to follow the details of the heat exchanges for a single impulse in non-myelinated nerves [26, 27]. The pulse causes first an emission of heat, and, in a second stage, an absorption which almost compensates the emission. The measurements show a gradual evolution between 4 and 15 °C. The magnitude of the positive heat decreases and the interval between the stimulus and the negative heat increases. Replacing sodium by lithium does not change the overall picture but the heat emission is reduced by about 20% while the absorption increases by a similar amount. In the whole temperature range, there is a close agreement between the heat emission and the rising phase of the the action potential, and between the absorption and the falling phase of the potential.
It is tempting to connect the heat effects to the energy needed to charge or discharge a capacitance, but the quantitative analysis shows that this simple “condenser model” does not account for all the observed heat exchanges. The authors of these measurements speculated that a great part of the heat exchanges could come from changes in the entropy of the nerve membrane when it is depolarized and repolarized.
However, the “condenser model” is clearly oversimplified. For a membrane in a conducting fluid, the charge distribution is not only located at the surface. Very recently, a new thermodynamics analysis has been carried out [28]. A full electrostatic model of the charged bilayer has been established. Assuming that the equilibration of the diffuse layer is sufficiently fast compared with the dynamics of the action potential, the paper uses a Poisson-Boltzmann approach to derive the charge distribution, and then compute the electrostatic energy. The entropy associated with the electric field takes into account the polarization of the water dipoles in the diffuse layers which reduces entropy, as well as the entropy changes inside the lipid membrane which can be deduced from the temperature dependence of the membrane capacitance. The results support the idea that the heat exchanges measured when the nerve impulse propagates have an electrostatic origin. The results heavily depend on the membrane surface charge and only a calorimetric measurement performed together with the recording of the transmembrane potential with an electrode inside the axon might fully confirm the electrostatic origin of the heat of nervous conduction, but, as discussed in detail in [28], this looks highly plausible.
3.2 Mechanical and structural changes
Early measurements showed signs that the excitation potential is accompanied by some mechanical and structural changes in the axon [29]. Small changes in turbidity and birefringence were observed. Axons immersed in anilinonaphthalene sulfonic acid, the fluorescence of which is extremely sensitive to conformational changes of various macromolecules, also showed fluorescence changes associated with nerve excitation [29].
Laser interferometry managed to detect rapid changes in the diameter of an axon, which take place when an action potential progresses along the giant axon of the crayfish [30]. The recorded deformation starts 250μs after the excitation by the pulse and starts by a contraction which peaks 400μs later. Then, the diameter returns to normal before showing a slow expansion to finally recover its initial size after about 4ms. The simultaneous recording of the electrical and mechanical pulses shows that, besides the delay of the mechanical deformation, one also observes that this deformation occurs on a significantly longer time scale. The overall displacement is very small, ranging from 3 to 25Å in different nerve preparations. It decreases when the nerve deteriorates. These experiments could also show that the deformation is directly linked to the action potential. If the electrical stimulation of the axon is reduced to 90% of the value that triggers a pulse, the mechanical deformation of the axon is not observed.
This result was later confirmed by optical measurements using the near field at the end of an optical fiber brought in close proximity of the axon and by piezo-electric measurements of the pressure at the axon surface [31]. The mechanical displacement reaches 50 to 100Å for crab nerves [32]. Clues to the mechanism of the swelling of axons were provided by studying volume transitions observed in synthetic and natural ionic gels by varying the ratio of monovalent and bivalent ions [33]. Changing the concentration in Na+, Li+, and K+ ions of a medium containing gel beads showed sharp transitions of the bead diameters, associated to thermal effects, which could be understood as structural transitions in the gel. Similar observations were made with the squid giant axon [34, 35], suggesting that the change in ionic concentrations around the membrane, induced by the nerve impulse, could lead to transitions in the membrane structure responsible for the mechanical deformation associated to the pulse. Heat exchanges, which accompany these transitions, could contribute to the thermal effects recorded when a pulse passes.
4 The debate on the Hodgkin-Huxley model
4.1 The Hodgkin-Huxley model
In the famous article which introduced their model [4], Hodgkin and Huxley explain that it is built upon a series of measurements of the flow of electric currents through the surface membrane of the squid giant axon. From their experiments, they deduced that the main features of the nerve impulse result from a transient increase of the sodium conductance of the membrane, which leads to a strong flow of Na+ ions towards the inside of the axon generated by the gradient of sodium concentration across the membrane. This step, leading to a rise of the action potential V = Vinside − Voutside, is followed by a slower but maintained increase in potassium conductance. In the rest state, the potassium concentration is greater inside the axon than outside, so that potassium tends to flow out of the axon, causing a decrease of the action potential, which, after a small overshoot, finally comes back to its resting value. However, Hodgkin and Huxley went well beyond this qualitative picture. From their measurements, they managed to propose a set of differential equations which model the sodium and potassium conductances. These equations could not be solved analytically but Hodgkin and Huxley relied on a pioneering approach to obtain solutions that they could quantitatively compare with their experiments. They performed what was probably the first numerical simulation in biological physics. However, as the the EDSAC (electronic delay storage automatic calculator), built in the Cambridge Mathematical Laboratory, which they later used for their studies, was not immediately available, they had to carry lengthy calculations on a manual calculator [6]. Their article [4] contains a detailed section on the numerical methods, which explains for instance how an iterative scheme could be used to determine one unknown parameter, the propagation speed of the impulse. This approach allowed a thorough test of the model, not only for the pulse but also for subthreshold responses.
The model is clearly only focused on the electrical aspects of signal propagation along the nerves. Hodgkin and Huxley took great care to discuss the limitations of their model, but did not discuss other physical phenomena, such as thermal effects, although they were certainly aware of their existence. Presumably, they considered the action potential to be the dominant phenomenon. Moreover the model has not been established from the basic principle of physics and chemistry, which would have naturally introduced other phenomena, for instance through a thermodynamics analysis. In the discussion of the paper, the authors wrote that “the agreement [with experiments] must not be taken as evidence that our equations are anything more than an empirical description of the time course of the changes in permeability to sodium and potassium.” Therefore, it is not surprising that the model can be discussed and completed. However, if the model stood out as the main model to describe the nerve impulse for about 70 years, it is because Hodgkin and Huxley based their conclusions on a large set of detailed experiments that they thoroughly analyzed. This is probably why the model, established before the knowledge of the existence and structure of ionic channels, could propose equations for the variation of the membrane conduction which turned out to match structural data of the ionic channels that were discovered much later. For instance, for potassium channels, Hodgkin and Huxley noticed that, for the opening, the conductance versus time needed a third- or fourth-order equation to be fitted, while the closing could be described by a first-order equation. This led them to a model in which the potassium channel opening is controlled by 4 sites which should simultaneously occupy a certain position in the membrane. Molecular dynamics simulations of a voltage gated potassium channel, carried out 60 years later [36], are perfectly consistent with this view because they confirm that 4 voltage-sensitive domains must be up before the pore can reopen after closure. Thus, although Hodgkin and Huxley took great care in stressing that “the interpretation given is unlikely to provide a correct picture of the membrane,” they managed to extract a lot from their voltage clamp measurements, which strengthens the credibility of the model that they proposed. The structure and basic function of sodium and potassium ionic channels were discovered about 30 years after the proposal of Hodgkin and Huxley [37], and this confirmed their insight.
Of course, the Hodgkin-Huxley model is not perfect; however, the precise analysis of the voltage clamp techniques that underlie its equations leads to a wide acceptance of the hypothesis of channels mediating ionic flow across the membrane and the Hodgkin-Huxley model kinetics describes most of the classic experiments fairly well although some experiments showed that the picture may be oversimplified. In spite of its successes, as noticed in Section 3, the model does not describe all phenomena associated to nerve signalling, so that some scientists noticed that “given the many experimental features not explained within the Hodgkin-Huxley theory, it is surprising that it remains an unchallenged dogma” [7].
4.2 A mechanical model for the propagation of the nerve impulse
And indeed the dogma has been challenged, in particular with proposals that the dominant effect in nerve signalling might be mechanical rather than electrical [7]. T. Heimburg and A. Jackson suggest that the nerve impulse could actually propagate as a localized deformation of the axon [7]. In most physical systems, a localized deformation, which, in Fourier space, is an infinite combination of signals of different wavelengths, tends to spread as it propagates due to dispersion because the different wavelengths propagate at different speeds. However, in some systems, the effect of dispersion can be compensated by nonlinearity, leading to solitary waves, which may have particle-like properties, which gave them their name of “solitons” [38]. Heimburg and Jackson noticed that lipid membranes generally display order–disorder phase transitions in a temperature range which is not far from physiological temperatures. Heating can destroy the lateral order of the molecules, which absorbs heat and leads to a swelling of the structure. This structural change modifies the volume and area compressibility of the membrane. As a result, in the vicinity of the transition, the speed of sound depends on ρA, its mass per unit area. Therefore, the equation for the propagation of a mechanical disturbance ΔρA contains a nonlinear contribution associated to the variation of the lateral compressibility near the transition. And because the thermal exchanges occurring at the transition are slow processes, the speed of sound, which is also a function of the specific heat as shown by the thermodynamics Maxwell relations, depends on the frequency (and wavelength) of the mechanical disturbance, so that the equation for the propagation of the mechanical disturbance also includes dispersion. Heimburg and Jackson show that the signs of the contributions are such that nonlinearity can compensate dispersion. Using standard expansions, they derive an equation for ΔρA which has some similarities with the Boussinesq equation, a standard equation in soliton theory [38].
This analysis suggests that, in the vicinity of the order-disorder transition of lipid membranes, a mechanical perturbation can therefore propagate as a quasi-soliton. Owing to the exceptional properties of solitons, in particular their ability to move by preserving their shapes in the presence of perturbations, or even in collisions with other solitons, it is tempting to conclude that the main phenomenon that lies behind the propagation of the nerve impulse is the compensation between nonlinearity and dispersion which allows the motion of narrow mechanical disturbances. In this view, the electrical signal studied by Hodgkin and Huxley is not the dominant mechanism but a secondary effect that would be slaved to the mechanical disturbance. The deformation of the membrane could affect the proteins that form the ion channels, and therefore induce the ionic flow through the membrane.
The idea of solitons propagating along lipid membranes is interesting and it would deserve studies to confirm it in some experiments. This is probably why it sounded sufficiently attractive to appear as an alternative to the Hodgkin-Huxley model of nerve impulse. It is supported by the experiments that recorded a variation of the diameter of the axon in the region of the pulse [30,31,32]. The heat exchange at the transition also appeared as a candidate to explain the discrepancy between the measured thermal exchanges that accompany the nerve impulse and the evaluations deduced from the condenser theory [26, 27]. Moreover, an experiment which showed that action potential launched towards each other in some axons which allow orthodromic (normal) and antidromic (inverse) propagation can pass through each other [39], in analogy with a remarkable property of solitons, sounds like a strong argument supporting the mechanical soliton picture for the nerve impulse.
However, while it might play a role in cell mechanics, the theory proposed by Heimburg and Jackson does not stand up to close scrutiny regarding the propagation of nerve impulse when it is confronted by experiments.
-
(i)
The link to a phase transition in the axon membrane, occurring at a particular temperature Tc close to physiological temperature, is a serious constraint. First, while such a transition has been observed in unilamellar vesicles, bovine lung surfactant, and two bacterial membranes, it has not been reported for the axon membrane [7]. But the main problem is that it gives a specific role to a temperature Tc while experimental investigations of the effect of temperature on the nerve impulse, from − 1 to 40 °C [24], do not show any discontinuity or qualitative change around a specific temperature, but rather a gradual evolution (Section 2.2).
-
(ii)
The argument of a disagreement between the measured thermal effects and the evaluations of the condenser theory that could be explained by the thermal exchanges associated to the latent heat of the transition is not very strong because the condenser theory is oversimplified and more careful evaluations of the energy exchange associated to ion transfers [28] do not conclude to such a disagreement, although the conclusion on this point may still be open.
-
(iii)
Similarly, the conclusion drawn from the possibility of nerve pulses to pass through each other should be taken with caution because other experiments, using different axons, contradict this result [40]. Moreover, the Hodgkin-Huxley model does not preclude such a crossing of the pulses for some values of its parameters [41], which could explain why some axons show the survival of colliding nerve pulses while others do not.
-
(iv)
In [7], the mechanical properties of the axon membrane are those of a lipid layer, but actually the membrane is much more complex. As shown in [42], actin, spectrin, and associated proteins form a cytoskeletal structure in axons. This makes the membrane much more rigid than a simple lipid bilayer, drastically reducing nonlinear effects in its mechanical distortions.
-
(v)
The strongest argument against the theory of Heimburg and Jackson [7] is actually provided by the measurements of the deformation of the axon [30], which they present as supporting their idea (Section 3.2). As they used an interferometric method and simultaneously record the electrical signal, Hill et al. could determine the precise timing of the two phenomena. The mechanical signals begin by a contraction which starts 250μs after the arrival of the electrical pulse; and therefore, it cannot be the mechanical signal that causes the electrical signal. Owing to the time scale of the electrical nerve impulse, the delay of 250μs is really significant. Moreover, the time traces displayed in Fig. 3 of [30] show that the mechanical signal lasts significantly longer than the electrical pulse. Rather than supporting the idea that a mechanical could induce the electrical pulse, as proposed in [7], the data of [30] support instead the proposal that Tasaki [33] presented after a series of measurements [31, 32, 34, 35]. The swelling of the axon might be related to a structural transition, but, instead of a transition induced by a temperature change, it would be a transition caused by the change of the ionic environment around the membrane, which follows the electrical pulse.
Thus, although the idea of a soliton-like propagation of the nerve impulse might look attractive, a precise confrontation with experimental facts cannot support this proposal.
Another recent view of the nerve impulse [43] considers another type of soliton, belonging to the class of envelope solitons [38] in which a carrier wave is modulated by a localized envelope function to generate a localized wavepacket. In this model, the carrier wave would be an oscillation of the dipolar orientation of the water molecules in the vicinity of the membrane. The dipolar interactions would lead to forces applied to the membrane, generating a coupling between a mechanical disturbance and the dipolar reorientations so that the dipolar signal would “surf on the capillary waves propagating along the axon.” This picture appears to suffer from several weaknesses:
-
(i)
The picture is only qualitative, and no quantitative evaluation has been made to explain how such a combined dipolar-mechanical signal could stay localized. Capillary waves are dispersive and would tend to spread. A quantitative mechanism has to be presented to show, in a convincing way, that the coupling tends to maintain the necessary localization, and that it has the strength to do it.
-
(ii)
The dissipation in the mechanical signal would presumably be very high. At the macroscale, capillary waves can propagate with a rather small dissipation. But, as shown by Purcell in a a beautiful article “Life at low Reynolds number” [44], at the microscopic scale phenomena are very different, and the viscosity of water plays a much stronger role. Moreover, considering the frequencies of the order of 100 kHz considered as plausible in [43] for the capillary waves, at the scale of the axon disturbances, dissipation would damp out the motion very quickly due to water viscosity but also the losses within the membrane itself.
-
(iii)
But the main objection to the scheme proposed in [43] is that it assumes that, once the signal is launched by some ion transfers, the action potential moves on without charge currents. This contradicts the observations of local ionic currents through the membrane [45] and the evidence of the ionic flows using radioactive tracers [22, 23] (Section 2.2).
4.3 Completing the Hodgkin-Huxley model
While the picture of a nerve pulse dominated by a mechanical signal does not stand up in front of a critical examination, it does not mean that the Hodgkin-Huxley model cannot be completed to include some phenomena that were deliberately left out by Hodgkin and Huxley, who focused their attention on the electrical phenomena only. Several attempts have been made around this idea, and they probably point to a direction that could improve our understanding of nerve signalling.
A. El Hady and B.B. Machta [46] studied the mechanical surface waves which accompany the propagation of the action potential. Their viewpoint is completely different from those of Heimburg and Jackson [7] and Kotthaus [43]. They do not consider that the mechanical signal is the main signalling pathway. Instead, they assume that the axon carries an electrical pulse, defining the action potential, without making any hypothesis on the origin of this pulse, which could be the Hodgkin-Huxley pulse, or has a different origin. This pulse drives the membrane deformation and they compute the response of the axon to this driving. In this approach, the axon is viewed as an elastic and dielectric tube filled by a viscous fluid. The elastic energy is stored in the deformation of the tube, and the kinetic energy is carried by the motion of the axoplasmic and extracellular viscous fluid, which moves according to the Navier-Stokes equation. The displacement of the membrane is expressed as a linear function of the forces due to the action potential. Therefore, this model does not consider any mechanical nonlinearity, which is probably legitimate if the membrane is actually strengthened by a cytoskeleton, as observed in [42]. However this approach does not require nonlinearity to localize the mechanical distortion because the shape of the signal is imposed by that of the action potential, which is spatially localized. Besides the energy exchanges due to charge transfer through the membrane, this model predicts an additional heat effect due to the isothermal distortion of the membrane because its free energy depends on its area, which is locally modified. Using parameters estimated from what we know of the axon, the calculation leads to results in the range of the experimental observations. And, in contrast to the model of [7], as the mechanical distortion is a consequence of the electrical pulse, it is natural that it follows the action potential arrival with a small delay as observed in [30].
The approach of Engelbrecht et al. [47] takes a similar viewpoint that the electrical signals are the carriers of information in nerves and trigger all other processes, but it is more ambitious because it tries to describe the coupling between the mechanical aspects (fluid flows and membrane deformation) and the action potential, instead of assuming that the mechanical component is slaved to the electrical one. In their view, the channels in the membrane can be open and closed not only under the influence of the electrical signals but also by mechanical inputs. The shape of the action potential is therefore not assumed, but instead described by a simplified version of the Hodgkin-Huxley model, the FitzHug-Nagumo model, initially proposed by FitzHugh as a model for the axon [48] and then built and further studied by Nagumo et al. [49] as a transmission line using tunnel diodes, for possible applications in electronics and signal processing. The mechanical signal is described by an equation which includes nonlinearity and dispersion, in the spirit of [7], and a phenomenological coupling between the electrical and mechanical components is added. This coupling is expressed as a function of the variation of the fields rather than their instantaneous values, which puts emphasis on dynamical effects.
The motivation of this approach is interesting because it should provide some understanding of the mechanisms that link the electrical and mechanical signals. However, the experimental data on this coupling, which appears to be too complex to be described from first principles of physics and chemistry, are still sparse, and little is known on the effect of mechanical constraints to control the ion channels. As a result, the assumptions made by Engelbrecht et al. are difficult to validate so that, in its present stage, this approach does not actually bring a further understanding of nerve signalling.
Finding the actual mechanisms behind the coupling between the electrical and mechanical components is a challenge to reach a meaningful extension of the Hodgkin-Huxley model. An interesting suggestion has been made by Krichen and Sharma [50]. Piezoelectricity is a well-known coupling between mechanical strain and electrical polarization, but, for uniform strains it only applies to materials which lack a mirror symmetry. However, as pointed out by Krichen and Sharma, if the strain itself does not have a mirror symmetry, even a centro-symmetric medium such as a fluid or a membrane can exhibit an electromechanical coupling. This is flexoelectricity. Moreover, as membranes are highly flexible, they can show very large strain gradients. Even if the coupling coefficient is small, the resulting effect can be large. Chen et al. [51] used this idea to propose an axon model which couples the distortion of the axon and the action potential. It is a two-way coupling because a strain gradient can induce an electrical polarization, and the action potential can cause a local distortion. Their approach can treat myelinated and unmyelinated axons. This is an attractive idea which would deserve further experimental and theoretical studies to be fully validated.
5 Discussion
In spite of alternatives introduced in the last 15 years, the answer to the question raised in the title “How is information transmitted in a nerve?” still appears to be “as an electrical pulse,” approximately described by the Hodgkin-Huxley model proposed in 1952. Of course the answer that it provides is oversimplified. This model was developed mostly from data recorded on the giant squid axon, and it should be amended to describe other axons, but it is nevertheless very likely to describe the essence of the phenomena involved in nerve signalling.
From the start the model was not designed to be complete as Hodgkin and Huxley only investigated the electrical component of the signal. Experiments have shown that, as for most biological phenomena, nerve signalling is complex, also involving a local deformation of the axon and thermal exchanges. This led some authors to challenge the main ideas of the Hodgkin-Huxley model and even to put forward mechanisms in which the electrical signal is not dominant, such as a mechanical soliton as the carrier of information. However, as shown in Section 4.2, this idea cannot stand in front of a careful examination of the experimental facts. In the case of a phenomenon as complex as the nerve impulse, a first-principle model is still out of reach and one must rely on phenomenological models. This can nevertheless be fruitful if this approach is built on a detailed analysis of the experimental observations, without neglecting some data which, at a first glance, may seem non-essential. This is exactly what Hodgkin and Huxley did. They established their model after years of experiments and thoughts, which allowed them to predict phenomena or properties which had not yet been observed, such as some features of the ion channels. Instead, the models assuming a dominant mechanical signal, although they were motivated by the observed change of the diameter of the axon that accompanies the action potential, neglect some elements (Section. 4.2). The deformation starts after the rise of the electrical pulse so that causality excludes that it could generate it.
The mechanisms of anesthesia have been discussed as a possible test of the models for the propagation of the nerve impulse. One could wonder whether their study could help decide between the two alternatives, electrical or mechanical. This is questionable because anesthetics probably exert their action on synaptic transmission rather than axonal conduction [52]. Nevertheless, there are many evidences that anesthetics act on ion channels [53], which is a hint that nerve signals are electrical rather than mechanical. Various studies have shown that anesthetics act directly on proteins rather than on lipids [52, 54]. There are however cases in which the membrane is involved, as demonstrated recently for inhaled anesthetics [55], but the actual target is nevertheless an ion channel and the membrane lipids only play an intermediate role.
Thermal effects have also been suggested as a means to solve the dilemma between the electrical and mechanical views of nerve signaling. This has two aspects, first whether the pulse is adiabatic, i.e., exothermic and endothermic contributions are equal, and second what is the magnitude of thermal effects. Adiabaticity cannot make the difference between the two alternatives. The view that the Hodgkin-Huxley action potential must be dissipative because it involves currents through resistors is oversimplified. Experiments [26, 27] show that exothermic effects are followed by an endothermic process of about the same magnitude. In the Hodgkin-Huxley model, this is understandable because during the rise of the action potential the Na+ ions move down the potential gradient giving rise to heat release, while in the second stage the K+ ions move up the potential gradient but down their concentration gradient, converting heat into capacitive energy [46]. In the soliton picture, thermal effects come from the latent heat of a reversible phase transition in the membrane, so that the overall thermal effect vanishes. The second aspect, the magnitude of the thermal effect, could, in principle, lead to a conclusion because the thermal effect due to a phase transition in the membrane should be significantly greater than the magnitude measured by experiments, ruling out the soliton model. However, the measurements are very difficult and, for the fastest pulses, they may not have a sufficient temporal resolution to catch the full magnitude of the thermal exchanges [56]. Nevertheless, as experiments observe that replacing sodium by lithium modifies the magnitude of the thermal effect, and as theories that go beyond the simple condenser model conclude that a proper thermodynamic analysis of the processes involved in the Hodgkin-Huxley model is compatible with the experimental observations, the studies of the thermal effects appear to favor the electrical view. As pointed out in [46], an additional contribution to thermal effects could nevertheless come from the stretching of the membrane which accompanies the action potential in recent models. Its order of magnitude is well below the contribution of latent heat in the soliton picture.
This does not mean that the proposals challenging the Hodgkin-Huxley model have been useless. They stimulated further thoughts and lead to models that combine electrical and mechanical effects. The goal of a model is not to reproduce experimental facts but to show what are the underlying phenomena which lead to these observations and allow further developments. Including mechanical distortion in a nerve-impulse model makes sense if it actually contributes to the process, which would be the case if ion channels are not only sensitive to voltage but also to forces or membrane distortions, as suggested by some studies [57]. However, establishing a reliable model of the axon coupling electrical and mechanical signals will certainly need further experimental investigations at the scale of ion channels.
A model may also be useful to understand additional phenomena. For nerve signalling, the role of the myelin layer deserves attention. Within the Hodgkin-Huxley model, or its simplified version the FitzHugh-Nagumo model, it is easy to show how an extra layer reducing the capacitance of the membrane can speed up the signal, but the role of myelin also introduces constraints on the ionic flow so that its effect is not straightforward to predict [58].
The Hodgkin-Huxley model has many parameters, and they could vary from cell to cell or with changes in the external medium. A promising line of investigations is to try to reduce the parameter space by looking how some parameter combinations may be enough to determine the main features of the model [59], and what is the stability of the results when the parameters or environment conditions change. Recent investigations [59] suggest that viewing the Hodgkin-Huxley model in a low-dimensional space may bring a deeper understanding of cell excitability.
The validity of a model also depends on the scale of interest. The Hodgkin-Huxley model smooths out the effect of individual ion channels in its continuous equations. When the noise due to individual channels becomes relevant, discrete stochastic models may be more appropriate [60]. On the other hand, the Hodgkin-Huxley model has also been challenged for studies at the scale of a full neural network [61]. Therefore, this model is certainly not the ultimate model for nerve signalling. It can be completed to include additional physical phenomena or modified for special purposes, but it does not deserve to be discarded simply because it does not describe all the complexities of the nerve impulse.
References
Von Helmholtz, H.: Messungen über den zeitlichen Verlauf der Zuckung animalischer Muskeln und die Fortpflanzungsgeschwindigkeit der Reizung in den Nerven. Archiv für Anatomie, Physiologie und wissenschaftliche Medicin 276–364 (1850)
Von Helmholtz, H.: Note sur la vitesse de propagation de l’agent nerveux dans les nerfs rachidiens. C. R. Acad. Sci. (Paris) XXX, 204–-206 (1850)
Von Helmholtz, H.: Deuxième note sur la vitesse de propagation de l’agent nerveux. C. R. Acad. Sci. (Paris) XXXIII, 262–-265 (1851). available at: https://www.academie-sciences.fr/archivage_site/activite/hds/textes/tsf_Debru1.pdf
Hodgkin, A.L., Huxley, A.F.: A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. 117, 500–544 (1952)
Vandenberg, J.I., Waxman, S.G.: Hodgkin and Huxley and the basis for electrical signalling: a remarkable legacy still going strong. J. Physiol. 590.11, 2569–2570 (2012)
Schwiening, C.J.: A brief historical perspective: Hodgkin and Huxley. J. Physiol. 590.11, 2571-–2575 (2012)
Heimburg, T., Jackson, A.D.: On soliton propagation in biomembranes and nerves. Proc. Natl. Acad. Sci. U.S.A. 102, 9790–9795 (2005)
Holland, L., de Regt, H.W., Drukarch, B.: Thinking about the nerve impulse: The prospects for the development of a comprehensive account of nerve impulse propagation. Front. Cell. Neurosci. 13, art. 208 (2019)
Seyfarth, E.-A.: Julius Bernstein (1839–1917): pioneer neurobiologist and biophysicist. Biol. Cybern. 94, 2–8 (2006)
Bernstein, J.: Ueber den zeitlichen Verlauf der negativen Schwankung des Nervenstroms. Pflügers Archiv. 1, 173–207 (1868)
Bernstein, J.: Untersuchungen zur Thermodynamik der bioelectrischen Ströme. Pflügers Archiv. 92, 521–562 (1902)
Overton, E.: Beiträge zur allgemeinen Muskel- und Nervenphysiologie. II Ueber die Unentbehrlichkeit von Natrium- (oder Lithium-)Ionen fü,r den Contractionsact des Muskels. Pflügers 92, 346–386 (1902)
Hodgkin, A.L.: Chance and design in electrophysiology: an informal account of certain experiments on nerve carried out between 1934 and 1952. J. Phys. 263, 1–21 (1976)
Hodgkin, A.L.: Evidence for electrical transmission in nerve. Part I. J. Phys. 90, 183–210 (1937)
Hodgkin, A.L.: The subthreshold potentials in a crustacean nerve fibre. Proc. Roy. Soc. London B 126, 87–121 (1938)
Cole, K.S., Curtis, H.J.: Electric impedance of the squid giant axon during activity. J. Gen. Physiol. 22, 649–670 (1939)
Hodgkin, A.L.: The relation between conduction velocity and the electrical resistance outside a nerve fibre. J. Phys. 94, 560–570 (1939)
Curtis, H.J., Cole, K.S.: Membrane resting and action potential from the squid giant axon. J. Cell. Comp. Physiol. 19, 135–144 (1942)
Hodgkin, A.L., Huxley, A.F.: Resting and action potentials in single nerve fibres. J. Physiol. 104, 176–195 (1945)
Huxley, A.F.: Hodgkin and the action potential. J. Physiol. 538, 2 (2002)
Hodgkin, A.L., Katz, B.: The effect of sodium ions on the electrical activity of the giant axon of the squid. J. Physiol. 108, 37–77 (1949)
Hodgkin, A.L., Keynes, R.D.: Active transport of cations in giant axons from Sepia and Loligo. J. Physiol. 128, 28–60 (1955)
Hodgkin, A.L., Keynes, R.D.: The potassium permeability of a giant nerve fibre. J. Physiol. 128, 61–88 (1955)
Hodgkin, A.L., Katz, B.: The effect of temperature on the electrical activity of the giant axon of the squid. J. Physiol. 109, 240–249 (1949)
Feng, T.P.: The heat production of nerve. Ergeb. Physiol. Biol. Chem. Exp. Pharmakol. 38, 73–132 (1936)
Howarth, J.V., Keynes, R.D., Ritchie, J.M.: The origin of the initial heat associated with a single impulse in mammalian non-myelinated nerve fibres. J. Physiol. 194, 745–793 (1968)
Howarth, J.V., Keynes, R.D., Ritchie, J.M., vin Muralt, A.: The heat production associated with the passage of a single impulse in olfactory nerve fibres. J. Physiol. 249, 349–368 (1975)
de Lichtervelde, A.C.L., de Souza, J.P., Bazant, M.Z.: Heat of nervous conduction: a thermodynamic framework. Phys. Rev. E 101, 022406 (2020)
Tasaki, I., Watanabe, A., Sandlin, R., Carnay, L.: Changes in fluorescence, turbidity and birefringence associated with nerve excitation. Proc. Natl. Acad. Sci. U.S.A. 61, 883–888 (1968)
Hill, B.C., Schubert, E.D., Nokes, M.A., Michelson, R.P.: Laser interferometer measurement of changes in crayfish axon diameter concurrent with action potential. Science 196, 426–428 (1977)
Iwasa, K., Tasaki, I.: Mechanical changes in squid giant axons associated with production of action potential. Biochem. Biophys. Res. Commun 95, 1328–1331 (1980)
Iwasa, K., Tasaki, I., Gibbons, R.C.: Swelling of nerve fibers associated with action potentials. Science 210, 338–339 (1980)
Tasaki, I., Byrne, P.M.: Discontinuous volume transitions in ionic gels and their possible involvement in the nerve excitation process. Biopolymers 32, 1019–1023 (1992)
Tasaki, I.: Rapid structural changes in nerve fibers and cells associated with their excitation processes. Jap. J. Physiol. 49, 125–138 (1999)
Tasaki, I.: Evidence for phase transition in nerve fibers, cells and synapses. Ferroelectrics 220, 305–316 (1999)
Jensen, M.Ø., Jogini, V., Borhani, D.W., Leffler, A.E., Dror, R.O., Shaw, D.E.: Mechanism of voltage gating in potassium channels. Science 336 (6078), 229–233 (2012)
Catterall, W.A.: From ionic currents to molecular mechanisms: The structure and function of voltage-gated sodium channels. Neuron 26, 13–25 (2000)
Dauxois, T., Peyrard, M.: Physics of Solitons. Cambridge University Press, Cambridge (2006)
Gonzalez-Perez, A., Mosgaard, L.D., Budvytyte, R., Nissen, S., Heimburg, T.: Penetration of action potentials during collision in the median and lateral giant axons of invertebrates. Phys. Rev. X 4, 031047 (2014)
Tasaki, I.: Collision of two nerve impulses in the nerve fibre. Biochim. Biophys. Acta 3, 494–-497 (1949)
Aslanidi, O.V., Mornev, O.A.: Can colliding nerve pulses be reflected?. JETP Lett. 65, 579–-585 (1997). (Pis’ma Zh. Éksp. Teor. Fiz. 65, No. 7, 553–558 10 April 1997)
Xu K., Zhong, G., Zhuang, X.: Actin, spectrin, and associated proteins form a periodic cytoskeletal structure in axons. Science 339, 452–456 (2013)
Kotthaus, J.P.: A Mechatronics view at nerve conduction. arXiv:1909.06313 [physics.bio-ph] (2019)
Purcell, E.M.: Life at low Reynolds number. Am. J. Phys. 45, 3–11 (1977)
Neher, E., Sakmann, B.: Single-channel currents recorded from membrane of denervated frog muscle fibres. Nature 260, 799–802 (1976)
El Hady, A., Machta, B.B.: Mechanical surface waves accompany action potential propagation. Nat. Commun. 6, 6697 (2015)
Engelbrecht, J., Peets, T., Tamm, K.: Electromechanical coupling of waves in nerve fibres. Biomech. Model Mechanobiol. 17, 1771–-1783 (2018). arXiv:1802.07014v2
FitzHugh, R.: Impulses and physiological states in theoretical models of the nerve membrane. Biophys. J. 1, 445–466 (1961)
Nagumo, J., Arimoto, S., Yoshizawa, S.: An active pulse transmission line simulating nerve axon. In: Proceedings of the IRE, pp 2061–2070 (1962)
Krichen, S., Sharma, P.: Flexoelectricity: a perspective on an unusual electromechanical coupling. J. Appl. Mech. 83, 030801–1-6 (2016)
Chen, H., Garcia-Gonzalez, D., Jérusalem, A.: Computational model of the mechanoelectrophysiological coupling in axons with application to neuromodulation. Phys. Rev. E. 99, 032406 (2019)
Franks, N.P., Lieb, W.R.: Molecular and cellular mechanisms of general anaesthesia. Nature 367, 607–614 (1994)
Yakamura, T., Bertaccini, E., Trudell, J.R., Harris, R.A.: Anesthetics and ion channels: Molecular models and sites of action. Annu. Rev. Pharmacol. Toxicol. 43, 23–51 (2001)
El-Din, T.M.G., Lanaeus, M.J., Zheng, N. , Catterall, W.A.: Fenestrations control resting-state block of a voltage- gated sodium channel. Proc. Natl. Acad. Sci. U.S.A. 51, 13111–13116 (2018)
Pavel, M.A., Petersen, E.N., Wang, H., Lerner, R.A., Hansen, S.B.: Studies on the mechanism of general anesthesia. Proc. Natl. Acad. Sci. U.S.A. 117, 13757–13766 (2020)
Heimburg, T.: The important consequences of the reversible heat production in nerves and the adiabaticity of the action potential. arXiv:2002.06031v2 [physics.bio-ph] (2020)
Beyder, A., Rae, J.L., Bernard, C., Strege, P.R., Sachs, F., Farrugia, G.: Mechanosensitivity of Na v 1.5, a voltage-sensitive sodium channel. J. Physiol. 588, 4969–4985 (2010)
FitzHugh, R.: Computation of impulse initiation and saltatory conduction in a myelinated nerve fiber. Biophys. J. 2, 11–21 (1962)
Ori, H., Marder, E., Marom, S.: Cellular function given parametric variation in the Hodgkin and Huxley model of excitability. Proc. Natl. Acad. Sci. U.S.A. 115, E8211–E8218 (2018)
Strassberg, A.E., DeFelice, L.J.: Limitations of the Hodgkin-Huxley formalism: Effects of single channel kinetics on transmembrane voltage dynamics. Neural Comput. 5, 843–855 (1993)
Meunier, C., Segev, I.: Playing the Devil’s advocate: is the Hodgkin–Huxley model useful? Trends Neurosci. 25, 558–563 (2002)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that he has no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Peyrard, M. How is information transmitted in a nerve?. J Biol Phys 46, 327–341 (2020). https://doi.org/10.1007/s10867-020-09557-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10867-020-09557-2