Abstract
This research studies the crystalline compounds present in nopal (Opuntia ficus-indica) cladodes. The identification of the crystalline structures was performed using X-ray diffraction, scanning electron microscopy, mass spectrometry, and Fourier transform infrared spectroscopy. The crystalline structures identified were calcium carbonate (calcite) [CaCO3], calcium-magnesium bicarbonate [CaMg(CO3)2], magnesium oxide [MgO], calcium oxalate monohydrate [Ca(C2O4)•(H2O)], potassium peroxydiphosphate [K4P2O8] and potassium chloride [KCl]. The SEM images indicate that calcite crystals grow to dipyramidal, octahedral-like, prismatic, and flower-like structures; meanwhile, calcium-magnesium bicarbonate structures show rhombohedral exfoliation and calcium oxalate monohydrate is present in a drusenoid morphology. These calcium carbonate compounds have a great importance for humans because their bioavailability. This is the first report about the identification and structural analysis of calcium carbonate and calcium-magnesium bicarbonate in nopal cladodes, as well as the presence of magnesium oxide, potassium peroxydiphosphate and potassium chloride in these plants. The significance of the study of the inorganic components of these cactus plants is related with the increasing interest in the potential use of Opuntia as a raw material of products for the food, pharmaceutical, and cosmetic industries.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Opuntia ficus-indica is a family of plants which, due to their adaptative characteristics, allows cultivation in arid and semiarid regions.
Nopal cladodes have played an important role in Mexico since pre-Hispanic times. These plants were the result of a selective process through over 7,000 years by the Mesoamerican people [1]. They used the Opuntia plants in different ways, such as medicinal treatment, forage, religious celebrations, as part of construction and most importantly, as food. Nowadays, among the other mentioned uses, young cladodes are eaten as a vegetable throughout Mexico and in the southern United States [2].
In the first stages, cladodes had higher carbohydrate content, especially soluble fiber, and also a high amount of water. The main elements found in nopal ash are calcium, potassium, magnesium, sodium, and small quantities of iron and manganese. These elements are present as carbonates, chlorides, sulfates, oxalates, and phosphates in the cladodes and their composition of the cladodes varies according to the season, cultivation site, kind of soil, and age of the plant [3–5].
Many organisms (both plants and animals) produce crystals, with some minerals employing specific macromolecules in order to form homogeneous morphologies with specific orientation at particular tissue sites. This process is always a genetically defined property of a mineralized tissue [6]. The biomineralization process takes place through the interaction among the macromolecules, physicochemical conditions of the organic matrix, and the concentration of minerals. The organic matrix must have special characteristics to allow the nucleation, growth, and permanence of the mineral crystal inside the organism [7–9]. However, the mechanisms of biomineralization have not been completely explained.
Monje and Baran [10], using infrared spectroscopy, studied the presence of biominerals in three different species of the Opuntioideae subfamily; they found a complex mineral composition that includes whewellite, opal, and calcite.
Contreras-Padilla et al. [11] studied the presence of oxalates in nopal powders at different stages of development and found that this compound grows in the form of druses. At present, there are scarce studies reported worldwide about crystalline components in nopal cladodes.
The nopal cladodes are vegetables that have an increasing importance in different countries as processed food and as ingredients in food industry, because of their nutritional and functional properties; also these plants have interesting functional and medicinal properties, which allow their possible use in different industries, such as in pharmaceutical and cosmetic, among others.
The aim of this research is to investigate the presence of crystalline compounds in nopal cladodes at three different maturity stages. X-ray diffraction (XRD) was used to identify crystalline structures in nopal samples. Scanning electron microscopy (SEM) imaging was performed in order to study the morphology of crystalline structures; energy-dispersive spectroscopy (EDS), and inductively coupled plasma (ICP) mass spectrometry were used to identify chemical elements present in nopal cladodes. Fourier transform infrared (FT-IR) spectroscopy was carried out as a complementary study in order to confirm the presence of those crystalline compounds.
2 Materials and methods
2.1 Sample preparation
The nopal cladodes belonging to Opuntia ficus-indica variety redonda were cultivated and harvested at 50, 100, and 150 days of maturation stage, in the experimental farm “Los Lorenz” in Silao, Gto., Mexico. Afterward in the laboratory, the nopal cladodes were cleaned with distilled water and disinfected by using a commercial 10% sodium hypochlorite solution; then the spines were manually removed. Subsequently, the cladodes were cut in squares of 2 × 2 cm in order to obtain homogeneous size pieces.
2.2 Drying and milling
The cladode pieces were placed on stainless-steel trays and a drying process was carried out on a vacuum system for 12 h at 133 × 10−3 mbar and 45 °C in order to avoid damage in some of the components. Drying was conducted to reach a moisture content of 6%. In order to obtain nopal powder, dried pieces of cladodes were ground using Pulvex 200 (Mex) equipment. A sieve was used at the output of the mill with a restriction size of 0.8 mm.
2.3 Sample incineration
Nopal powders were incinerated at 168 °C by 2 h in order to remove the organic compounds. The use of this temperature was intended to prevent the formation of other new mineral compounds in the sample than fairchildite, which is formed during the incinerated process, in accordance with the results reported by Sharygin et al. [13]. They found that the formation of this component occurs during the combustion of biomass and forest material at temperatures below 600 °C.
2.4 X-ray diffraction analysis (XRD)
Both the powdered and incinerated samples of nopal cladodes, with a fine particle size (smaller diameter than mesh 60), were densely filled into an aluminum sample holder. X-ray diffraction analysis of the samples was performed on a Rigaku Miniflex diffractometer with operating conditions of 35 kV and 15 mA, with Cu-Kα radiation wavelength of λ = 1.5406 Å . Data was collected from 5 to 80° on a 2-θ scale with a step size of 0.02. Spectrum analysis software (Materials Data Inc. Jade V 5.0) was used.
2.5 Scanning electron microscopy (SEM) and energy-dispersive spectroscopy (EDS) analysis
Scanning electron microscopy (Jeol JSM 6060LV, Japan) with a micro-analyzer INCA x-sight and software INCA (Oxford Instrument, UK) was used to analyze nopal samples. The analysis conditions were: high vacuum, 20-kV electron acceleration voltage and secondary electron mode. Images and microanalysis were carried out on different dehydrated sections of the cladodes (not calcinated) by cutting small sections of 0.5-cm thickness that were placed on a sample holder. The mounted samples were sputter coated with gold.
Also, the samples were analyzed using scanning electron microscopy with a FEG Jeol JSM-7600 F microscope operated at 2.5–10 keV accelerating voltage, equipped with an X-ray energy-dispersive spectroscope (EDS, Oxford INCA).
2.6 Fourier transform infrared spectroscopy (FT-IR)
FT-IR spectroscopy was performed with a Bruker v 33 spectrometer. For each sample, 36 scans were averaged with a spectral resolution of 4 cm−1. Infrared spectra were recorded as transmittance spectra.
2.7 Mineral composition
Mineral content of nopal ashes were determined by atomic absorption spectroscopy (AAS) using AAS equipment and inductively coupled plasma mass spectrometry (ICP-MS).
The calcium (Ca), magnesium (Mg), potassium (K), and sodium (Na) ion concentrations were measured with a double-beam atomic absorption spectrometer AAnalyst 300 (Perkin-Elmer, USA) equipped with a deuterium lamp, background corrector, and a hollow cathode lamp; using the 968.08 method [12].
The mass spectrometer (ICP-MS) Thermo Jarrel Ash, Model IRIS/AP (Corp. Franklin MA USA) was used to quantified phosphorus (P), manganese (Mn) iron (Fe) and zinc (Zn). The tests were performed following the 984.27 method [12].
3 Results and discussion
3.1 X-ray diffraction
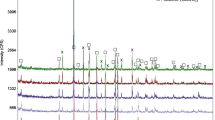
Figure 1a shows the corresponding diffractograms of nopal cladode powder for three different maturity stages (50, 100, and 150 days) before the samples were incinerated. It can be seen that the presence of the presence of organic material does not allow observation of the Bragg reflections of the crystalline compounds clearly, only it is evident the presence of calcium oxalate monohydrate which was identified with the PDF # 20–0231 of JCPDS-ICDD data base. This result is in agreement with that reported by Monje and Baran [10] and Contreras-Padilla et al. [11], which identified the presence of calcium oxalate in plants of Opuntia genera. The calcium oxalate monohydrate is considered as antinutritional compound; however according to Contreras-Padilla et al. [11], the presence of these compounds in nopal cladodes is low; therefore the calcium should be present in other compounds.
Figure 1b shows the corresponding diffractograms of the incinerated nopal samples that were obtained at 168 °C for three different maturity stages (50, 100, and 150 days). As can be observed, the major part of the organic material has been eliminated; hence, now other crystalline compounds are revealed. The analysis performed with the MDI Jade software indicates the presence of the following crystalline structures: calcium oxalate monohydrate (whewellite), calcium carbonate, magnesium oxide, calcium-magnesium bicarbonate, potassium peroxydiphosphate, and fairchildite. As mentioned before, the last compound is formed during the incinerated process, in accordance with the results reported by Sharygin et al. [13]. They found that the formation of this component occurs during the combustion of biomass and forest material at temperatures below 600 °C. Therefore, fairchildite does not correspond to a primary component in nopal cladodes. The presence of a potassium chloride type structure (sylvine) [KCl] was detected despite the lack of any observation of chlorine in the elemental analysis performed by EDS and ICP. Calcium oxalate (whewellite) was detected in the calcinated samples despite the observation of Frost and Weier that this compound suffers thermal transformation, changing to anhydrous oxalate at 161 °C [14]. This could be attributed to the fact that they studied the pure compound while the nopal sample consists of a complex vegetal matrix. Besides calcium oxalate (whewellite), Fig. 1b shows the presence of calcium carbonate ([CaCO3], PDF # 47–1743), and indicates that this is a crystalline compound that has an important presence in the incinerated nopal samples; also, calcium-magnesium bicarbonate ([CaMg(CO3)2], PDF # 36–0426), magnesium oxide ([MgO], PDF # 45–0946), potassium peroxydiphosphate ([K4P2O8], PDF # 48–1160) and potassium chloride (sylvine) [KCl], PDF# 75–0296 were identified.
These results are very important because it is well known that calcium carbonate, calcium-magnesium bicarbonate, and magnesium oxide are good sources of calcium and magnesium; moreover, these compounds have good bioavailability for the human body [15–18]. Additionally, the characterization of these crystalline structures has importance for different industries. For example, in the food industry, they can be considered as a source of calcium bioavailable for humans.
3.2 SEM and EDS analysis
The SEM image taken at 10,000× of nopal samples shows crystalline structures with dipyramidal forms (Fig. 2a), with an approximate size of 1 μm, and prismatic and dipyramidal structures (Fig. 2b) that could be the result of growth of the octahedral-like crystals shown in Fig. 2a, as will be discussed in reference to Fig. 6. Figure 2c shows faceted structures with sizes around 2 μm; these flat-shaped structures are different from those shown in Fig. 2a and b, suggesting that these flat-shaped structures correspond to another crystalline compound.
The structures cited above are very different in size and shape compared with the typical druse shape of calcium oxalate structures shown in Fig. 3a, which have been previously reported (Contreras-Padilla et al. [11]). As can be observed in the marked zone in Fig. 3a and b at higher amplification, those dipyramidal structures were found highly distributed in different zones of nopal pieces and in the three maturity stages studied. Furthermore, these observed structures present highly uniform size and shape.
Figure 4a shows the EDS analysis of the flat-shaped crystals. The results indicate a high content of carbon (40.48 wt%), oxygen (45.64 wt%), and a moderate content of calcium (9.53 wt%). Also, this analysis shows a small content of magnesium (1.72 wt%) and potassium (2.64 wt%). This composition could indicate the presence of calcium carbonate or calcium-magnesium carbonate. Moreover, the mineral structures that present exfoliation, like those shown in Fig. 2c, are characteristic of a small group of compounds. In this case, the rhombohedra exfoliation, taking into account the EDS chemical composition, indicates the presence of calcium-magnesium bicarbonate [19]. This finding is in agreement with the result obtained in X-ray diffraction analysis.
On the other hand, the presence of magnesium confirms the existence of magnesium oxide, also in accordance with the results found in the X-ray diffraction analysis.
Figure 4b shows the EDS analysis of dipyramidal crystal structure. The analysis indicates that the elements found were: carbon (46.26 wt%), oxygen (31.94 wt%), calcium (16.7 wt%), and potassium (5.1 wt%). These results suggest that these octahedral-like crystals could consist mainly of calcium carbonate. This result agrees with X-ray diffraction analysis.
Figure 5 shows EDS mapping of an area showing dipyramidal, octahedral, and prismatic crystal structures. Figure 5b, c and d show the distribution of carbon, calcium and oxygen, respectively, corresponding to the micrograph in Fig. 5a. As can be observed, the higher concentration of these elements occurs in the marked area, which corresponds to the zone in which the higher concentrations of those dipyramidal and prismatic crystal structures are present. This indicates and confirms that those crystalline structures correspond to calcium carbonate (calcite) [CaCO3] crystals and is in agreement with the X-ray diffraction results.
Figure 6 shows a low-angle backscattered electron micrograph and a secondary electron image of calcite crystal structures. Figure 6a corresponds to a sample of nopal pads at 100 days of maturation and Fig. 6b to a sample of nopal pads with 150 days of maturation. It can be observed that calcium carbonate crystals (Fig. 6a) grow into prismatic forms (Fig. 6b), suggesting that the growth of those crystal structures can be correlated with the age of the plant.
A low-angle backscattered electron micrograph and a secondary electron image of flower-shaped calcite crystals are shown in Fig. 7a and b, respectively. These flower-shaped crystals are composed of calcite bipyramidal structures, whose sizes go from the nanometer scale to about 4 μm, but its thickness always remains within the nanometer scale. As can be observed in Fig. 7b, there are some bipyramidal crystals that grow alone and bigger in size than those forming the flower-shaped crystals, but these flower structures are bigger in size than the former ones.
It has been found that biogenic minerals, or biominerals, can crystallize with different morphologies, and are significantly different in their appearance from calcite rhombohedra of their abiotic counterparts [7, 9].
From Figs. 6 and 7, it is clear that there are two different morphologies of calcite crystals, but both start from the same bipyramidal calcite crystals. This could be attributed to the concentration of nucleation sites in different areas of this plant. If the density of nucleation sites is high, flower-shaped crystal growth is favored due to the closeness and interaction between small bipyramidal crystals; meanwhile, if the density of nucleation sites is low, those calcite bipyramidal crystals are scattered and can grow to form octahedral-like crystals and then prismatic-shaped crystals.
3.3 FT-IR spectroscopy
Figure 8 corresponds to the infrared spectra of nopal powder samples at 50, 100, and 150 days; Fig. 8a corresponds to calcinated samples and Fig. 8b to dry samples. The FT-IR spectra show, in both cases, the presence of vibrational bands at 1,425, 872, and 717 cm−1 that can be attributed to the asymmetrical stretching vibration (υ3) and the carbonate out-of-plane bending vibration (υ4 and υ2 mode) of calcium carbonate (marked as C). These bands correspond to calcite according to Zhou et al. and Monje and Baran [7, 10]. The letter W indicates bands that can be attributed to calcium oxalate monohydrate (whewellite) located at 1,613, 1,317, and 780 cm−1 that correspond to the antisymmetric and symmetric carboxylate stretching, and OCO deformations, respectively [10]. Calcium magnesium bicarbonate (D) can be responsible for the vibrational bands located at 1,460, 1,049, and 890 cm−1, which correspond to asymmetric stretching vibrations (υ3) and the carbonate out-of-plane bending vibrations (υ4 and υ2 mode), respectively. Finally, the presence of magnesium oxide can be designated at the vibrational band in 485 cm−1 that can be attributed to stretching out of the plane of the Mg-O bond.
It can be observed that some whewellite bands are probably overlapped with calcite bands in the calcinated samples (Fig. 8a) due to the strong and wide vibrational band of this calcium carbonate. In the same way, some calcite and calcium magnesium bicarbonate bands are overlapped due that the vibrational bands at 1,425 (C) and 1,460 (D), 872 (C) and 890 (D) cm−1 are very near each other.
In all cases, FT-IR results are in agreement with those obtained by X-ray diffraction analysis and confirm the identification of the crystal structures observed by the SEM technique.
The existence and dissemination of these crystalline structures in plants depend on the interaction of various factors such as soil composition, location, and physicochemical characteristics of the organic matrix, etc. [9].
Among the more than 60 different biogenic minerals known so far, calcium carbonate is the most usually found in biological structures. This mineral can exist in four different structural forms: calcite, aragonite, vaterite, as well as amorphous calcium carbonate [8, 20].
Calcium-based minerals present in plants have an essential role in diverse functions of these organisms. The plants adopted calcium (Ca2+) as a versatile second messenger in various responses to abiotic and biotic stimuli, including light, high and low temperature, mechanical disturbance, drought, salt and osmotic stresses, plant hormones, and pathogen elicitors, etc. [21]. Magnesium is an essential nutrient for plants; a key function of this element is well known in the process of photosynthesis, as it is a basic component of the chlorophyll molecule. Magnesium has also a crucial role in cell proliferation, cellular function, and protein synthesis.
4 Conclusions
The crystalline compounds present in nopal cladodes have been identified and characterized successfully. The identification of the crystalline structures was performed using different techniques, such as X-ray diffraction, scanning electron microscopy, and Fourier transform infrared spectroscopy. Calcium carbonate (calcite) [CaCO3], calcium oxalate monohydrate (whewellite) [Ca(C2O4)•(C2O4)], calcium-magnesium bicarbonate [CaMg(CO3)2], magnesium oxide [MgO] and potassium peroxydiphosphate [K4P2O8] crystalline structures has been identified. The SEM images indicate that the calcium-magnesium bicarbonate structure presents rhombohedral exfoliation, calcite crystals grow to dipyramidal, octahedral-like, prismatic, and flower-like structures, and calcium oxalate is present in drusenoid morphology. Finally, a great distribution of calcite crystals has been observed in the tissue of nopal cladodes studied.
Calcium and magnesium have essential importance in the biochemical functions of plants and also for animals. For that reason, the presence of calcium carbonate and magnesium oxide is of great interest for humans.
This is the first report that shows SEM images of calcite and calcium-magnesium bicarbonate in nopal cladodes as well as the presence of magnesium oxide in these plants.
As mentioned in the introduction, the nopal cladodes are vegetables that have an increasing importance in different countries as processed foods because of their nutritional and functional properties. Moreover, the importance of the study of the components of these cactus plants is related to the increasing interest in the potential use of Opuntia as a raw material of products for food, pharmaceutical, and cosmetic industries.
References
Reyes-Agüero, A.J., Aguirre-Rivera, R.J., Hernández, H.M.: Systematic notes and a detailed description of Opuntia ficus-indica (L.) Mill. (Cactaceae). Agrociencia 39, 395–408 (2005)
Granados, D., Castañeda, A.D.: El Nopal: Historia, fisiología, genética e importancia, 2nd edn. Trillas, México (1996)
Saenz, H.C.: Cladodes: a source of dietary fiber. J. Prof. Assoc. Cactus Dev. 2, 117–123 (1997)
Rodríguez- García, M.E., De Lira, C., .B. Hernández, E., Cornejo V, M.A., Palacios F. A. J., Rojas, M. I., Reynoso, R., Quintero, .L.C., Del Real, A., Zepeda, T. A., Muñoz, C. T.: Physicochemical characterization of nopal pads (Opunita ficus-indica) and dry vacuum nopal powders as a function of the maturation., Plant Food Hum. Nutr. 62, 107–112 (2007)
Hernández-Urbiola, M.I., Contreras-Padilla, M., Pérez-Torrero, E., Hernández-Quevedo, G., Rojas-Molina, J.I., Rodríguez-García, M.E.: Study of nutritional composition of nopal (Opuntia ficus-indica cv. Redonda) at different maturity stages. Open Nutr J. 4, 1–6 (2011)
Aizenberg, J., Hanson, J., Ilan, M., Leiserowitz, L., Koetzle, T.F., Addadi, L., Weiner, S.: Morphogenesis of calcitic sponge spicules — a role for specialized proteins interacting with growing crystals. FASEB J. 9, 262–268 (1995)
Zhou, G.T., Guan, Y.B., Yao, Q.Z., Fu, Q.S.: Biomimetic mineralization of prismatic calcite mesocrystals: relevance to biomineralization. Chem. Geol. 279, 63–72 (2010)
Weiner, S., Addadi, L.J.: Design strategies in mineralized biological materials Mater. Chem. 7, 689–702 (1997)
Weiner, S.: Biomineralization: a structural perspective. J. Struct. Biol. 163, 229–234 (2008)
Monje, P.V., Baran, E.J.: Complex biomineralization pattern in cactaceae. J. Plant Physiol. 161, 121–123 (2004)
Contreras-Padilla, M., Pérez-Torrero, E., Hernández-Urbiola, M.I., Hernández-Quevedo, G., del Real, A., Rivera-Muñoz, E.M., Rodríguez-García, M.E.: Evaluation of oxalates and calcium in nopal pads (Opuntia ficus-indica var. redonda) at different maturity stages. J. Food Compos. Anal. 24, 38–43 (2012)
AOAC, Official Methods of Analysis. 17th ed., Association of Official Analytical Chemists, Gaithersburg, MD, USA, 2000
Sharygin, V.V., Zhitova, L.M., Nigmatulina, E.N.: Fairchildite K2Ca(CO3)2 in phoscorites from Phalaborwa. South Africa: the first occurrence in alkaline carbonatite complexes. Russ. Geol. and Geophys. 52, 208–219 (2011)
Frost, R.L., Weier, M.L.: Thermal treatment of whewellite: a thermal analysis and Raman spectroscopic study. Thermochim. Acta. 409, 79–85 (2004)
Lagarto, A., Bellma, A., Tillán, J., Gabilondo, T., Guerra, I., Ocanto, Z., Couret, M., González, R.: Effect of dolomite oral exposure in Wistar rats during organogenesis period of pregnancy. Exp. Toxicol. Pathol. 60, 499–504 (2008)
Straub, D.A.: Calcium supplementation in clinical practice. Nutr. Clin. Pract. 22, 286–296 (2007)
Rudea, R.K., Gruber, E.J.H.: Magnesium deficiency and osteoporosis: animal and human observations. Nutr. Biochem. 15, 710–716 (2004)
Tucker, K.L., Hannan, M.T., Chen, H., Adrienne, L.P., Cupples, W.F., Kiel, D.P.: Potassium, magnesium, and fruit and vegetable intakes are associated with greater bone mineral density in elderly men and women. Am J Clin Nutr. 69, 727–736 (1999)
Hatzor, Y. H., Zur, A., Mimran Y. H.: Microstructure effects on microcracking and brittle failure of dolomites. Tectonophysics. 281, 141-16l (1997)
Wei, H., Shen, Q., Zhao, Y., Wang, D., Xu, J.D.: Crystallization habit of calcium carbonate in the presence of sodium dodecyl sulfate and/or polypyrrolidone. Cryst. Growth 260, 511–516 (2004)
Hashimoto, K., Kudla, J.: Calcium decoding mechanisms in plants. Biochimie 93, 2054–2059 (2011)
Acknowledgments
The authors would like to thank Dra. Beatriz Millán-Malo, Dra. Genoveva Hernández Padrón and Dr. Rodrigo Esparza-Muñoz (UNAM-CFATA, Mexico) for their technical assistance in XRD, FTIR, and SEM analysis, respectively. Margarita Contreras Padilla would also like to thank CONACYT-Mexico for the financial support of her postdoctoral position at CFATA-UNAM.
Conflict of interest
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Contreras-Padilla, M., Rivera-Muñoz, E.M., Gutiérrez-Cortez, E. et al. Characterization of crystalline structures in Opuntia ficus-indica . J Biol Phys 41, 99–112 (2015). https://doi.org/10.1007/s10867-014-9368-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10867-014-9368-6