Abstract
The poor outcomes in acute myeloid leukemia (AML) necessitate new treatments. In this work, we identified that anisomycin is a potential selective anti-AML candidate, particularly for those with FLT3-ITD mutation. We found that anisomycin potently inhibited proliferation and induced apoptosis in multiple AML cell lines. Anisomycin was effective in targeting progenitor cells isolated from all tested pediatric AML patients, while sparing normal counterparts. Using AML xenograft mouse models, anisomycin exhibited inhibitory effect on tumor growth throughout the whole duration without causing toxicity in mice. The combination of anisomycin with standard of care drugs is synergistic and selective in AML cell culture system and mouse model. In addition, FLT3-ITD cells were more sensitive to anisomycin than FLT3 WT cells. Mechanistic studies revealed that anisomycin acted on AML in a p38-independent manner. We found that anisomycin decreased mitochondrial respiration by disrupting complex I activity, leading to intracellular oxidative stress. AML ρ0 cells that lack of mitochondrial respiration exhibited resistance to anisomycin. Finally, we showed that mitochondrial biogenesis contributes to differential sensitivity of FLT3-ITD and FLT3 WT cells to anisomycin. Our work is the first to systematically demonstrate that anisomycin is a useful addition to the treatment armamentarium for AML. Our findings highlight the therapeutic value of mitochondrial respiration inhibition in AML patients harboring FLT3-ITD mutation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute myeloid leukemia (AML) is haematological malignancy characterized by abnormal clonal expansion and aberrant differentiation of immature clonal myeloid cells. The core therapeutic principles are chemotherapy, allogenic hematopoietic stem cell transplantation and palliative care (Roboz 2011). The prognosis of acute myeloid leukaemia (AML) is poor with five-year survival rates of less than 30% (Thol and Ganser 2020). Mutations of the receptor tyrosine kinase fms-like tyrosine kinase 3 (FLT3), such as FLT3-ITD, resulting in constitutive signalling are common in AML and lead to a high relapse rate (Kiyoi et al. 2020). FLT3 inhibitors, such as midostaurin, have been approved for the treatment of AML with FLT3 mutation. The combination of current FLT3 inhibitors with cytotoxic drugs has not demonstrated an improvement in overall survival. Hence, patients with refractory or relapsed AML that fail to respond to standard therapy still require novel treatment strategies to manage their disease.
Anisomycin is an antibiotic that inhibits protein synthesis in protozoa and yeast (Grollman 1967). It is also identified as an agonist of p38-mitogen-activated protein kinases (MAPK) and c-Jun N-terminal kinase (JNK) in mammalian cells (Barros et al. 1997; Liu et al. 2014). In addition, anisomycin has been recently revealed as a potential anti-cancer drug. It is active against a panel of cancers in pre-clinical models, including osteosarcoma, colorectal cancer and renal carcinoma (Ushijima et al. 2016; Li et al. 2017; Cao et al. 2017). Of note, low-dose anisomycin sensitizes glucocorticoid-resistant T-acute lymphoblastic leukemia (T-ALL) (Liu et al. 2014). Anisomycin has been recently shown to selectively targets chronic myeloid leukemia (CML) cells (Li et al. 2018). The mechanisms of anti-cancer action of anisomycin include GATA-6 degradation, induction of mitochondrial dysfunction and activation of p38 MAPK/JNK (Ushijima et al. 2016; Cao et al. 2017; Liu et al. 2013). Given the potent efficacy of anisomycin in T-ALL and CML, we hypothesized that anisomycin may be effective in AML. Using AML xenograft mouse models and primary patients’ cells, we 1) evaluated in vitro and in vivo efficacy of anisomycin; 2) determined the combinatory effect of anisomycin and standard of care (SOC) and 3) identified the underlying mechanism of action of anisomycin. Our results clearly demonstrate that anisomycin is active and selective against AML, particularly those with FLT3-ITD mutation, and acts synergistically with SOC, via inhibiting mitochondrial respiration.
Materials and methods
Primary CD34+ cells, cell lines, cell culture and drugs
Frozen AML mononuclear cells (MNC) were obtained from the Department of Tissue Repository in Xiangyang No.1 People’s Hospital. Frozen human normal bone marrow MNC were purchased from Stemcell Technologies. CD34+ cells were isolated from AML or NBM MNC by immunomagnetic CD34 microbeads (Miltenyi Biotech). FLT3-ITD positive lines MV4-11, MOML13 and MOML14; and FLT3 wildtype (WT) lines SKNO-1 and OCI-AML2 were obtained from American Type Culture Collection or DSMZ. CD34+ cells were maintained using StemSPAN complete medium (STEMCELL Technologies) containing human FLT3-ligand Stem cell factor, Interleukin-6 and Interleukin-3 (R&D Systems). Cell lines were cultured and expanded using RPMI1640 medium supplemented with 10% fetal bovine serum (FBS; Hyclone). Mitochondria DNA-deficient AML ρ0 was established using the same method as described (Hashiguchi and Zhang-Akiyama 2009). SKNO-1 cells were growing in the above culturing medium and selected using 1 μg/ml ethidium bromide, 50 μg/ml uridine and 1 mM sodium pyruvate (Sigma) for 8 weeks. The lack of mitochondrial respiration of ρ0 cells was confirmed using Mito Stress assay. Supplementation of pyruvate and uridine was halted during anisomycin treatment. Cytarabine, midostaurin and anisomycin were purchased from Selleckchem and reconstituted in dimethyl sulfoxide (DMSO). Drugs were stored in -200C as aliquots. Specific condition for each experiment is described in the figure legends.
Proliferation assay and combination index (CI)
8000 cells/well were seeded onto 96-well plate. After 3 days drug treatment, cell proliferation was determined by measuring bromodeoxyuridine (BrdU) incorporation using BrdU Cell Proliferation assay kit (Abcam). Combination study was conducted using the method as described (Chou 2010). Briefly, the half maximal inhibitory concentration (IC50) of was firstly determined using proliferation assay. The cells were then treated with an equipotent constant-ratio combination of two drugs, and single drug alone. The Calcusyn median effect model was used to calculate the CI values and evaluate whether the combination was synergistic, antagonistic or additive. CI values of < 1 indicate synergism, CI = 1 indicate additivity and CI > 1 indicate antagonism (Chou and Talalay 1984).
Flow cytometry of Annexin V
5 × 10^5 cells/well were seeded onto 6-well plate. After 3 days drug treatment, cells were collected for Annexin V-FITC and 7-AAD staining (Beckman Coulter). Stained cells were then processed for flow cytometry analysis on Beckman Coulter FC500. Annexin V + cells were considered as apoptotic cells.
Colony formation assay
1000 CD34+ cells, drug at different concentrations and HSC-CFU methylcellulose medium (Miltenyi Biotec) were homogeneously mixed and plated onto 6-well plate. The plate was incubated in 370C, 5% CO2 atmosphere for 10 days. The number of colonies were observed and counted under light microscope.
AML xenograft mouse model
This study was carried out in strict accordance with guidelines and protocol approved by the Care and Use of Laboratory Animals of Hubei University of Medicine. SCID mice (4–6 weeks old, 20 ± 2 g) were purchased from Hunan SJA Laboratory Animal Co., Ltd. One million MOLM-13 or OCI-AML2 cells in PBS were subcutaneously injected into the flank of each SCID mouse. After development of palpable tumor, the mice were randomly grouped and treated with vehicle control (50%/50% DMSO/saline), drug alone or combination. Specific drug dose and administration routes were indicated in figure legend. Tumor size were measured every 3 days. After 2 weeks treatment, mice were euthanized using CO2 inhalation.
Metabolic assays
After 24 h of treatment different concentrations of anisomycin, oxygen consumption rate (OCR) was measured using the Seahorse XF24 extracellular flux analyser as previously described (Varum et al. 2011). Treated cells were transferred in XF24 well plates coated with BD Cell-Tak (BD Biosciences). One hour before performing OCR measurement, media was replaced by XF assay medium and incubated at 370C in a CO2-free atmosphere. OCR were measured at as per the XF24 analyzer standard protocol (Seahorse Bioscience). Mitochondrial respiratory complex I activity and intracellular reactive oxygen species (ROS) were measured using Mitochondrial Complex I Activity Assay kit (Novagen) and CM-H2DCFDA (Life Technologies) as per the manufacturers’ protocol, and quantified by measuring the absorbance on Spectramax M5 microplate reader (Molecular Devices).
Measurement of mitochondrial biogenesis
Mitochondrial DNA (mtDNA) copy number and mitochondrial mass were measured using the same protocol as previously reported (Skrtic et al. 2011). Briefly, the relative mtDNA copy number was determined by quantification of ND1 using a real-time PCR, and compared relative to nuclear DNA HGB-1. Mitochondrial mass was estimated by staining cells with Mitotracker Green (Invitrogen) followed by flow cytometry analysis of the median fluorescence intensity. ATP levels were measured by ATP assay kit (Abcam) according to the manufacturer’s instructions.
Statistical analyses
All data were obtained from at least three independent experiments with duplicate or triplicate, and were expressed as mean and standard deviation. For in vitro experiments, analysis of variance (ANOVA) and unpaired Student t test were utilized. For in vivo experiments, a total of 10 mice were used to assess efficacy. A significance level (α) of 0.05 was adopted in all cases.
Additional methods can be found in the supplemental information.
Results
Effect of anisomycin and SOC treatment on FLT3-ITD and FLT3 WT AML cell proliferation and apoptosis
We investigated the effects of the anisomycin alone, and in combination with cytarabine or midostaurin, on multiple FLT3-ITD and FLT3 WT AML cell lines. MV4-11 harbors homozygous FLT3-ITD whereas MOML-13 and -14 harbor heterozygous FLT3-ITD (Chen et al. 2010). SKNO-1 and OCI-AML2 are cell lines with FLT3 WT. We found that exposure to anisomycin at low μg/ml concentration range dose-dependently inhibited proliferation in AML cells by BrdU incorporation method (Fig. 1A). The IC50 of anisomycin on MV4-11, MOLM-13, MOLM14, OCI-AML2 and SKNO-1 are ~ 0.09 μg/ml, ~ 0.13 μg/ml, ~ 0.16 μg/ml, ~ 0.8 μg/ml and ~ 2 μg/ml, respectively. Anisomycin also induced apoptosis by flow cytometry of Annexin V staining in a concentration-dependent manner (Fig. 1B). It is of interest to note that FLT3-ITD cells are more sensitive to anisomycin than FLT3 WT cells.
The inhibitory effects of anisomycin in AML cell lines. Anisomycin dose-dependently inhibits proliferation (A) and induces apoptosis (B) in FLT3-TID and FLT3 WT AML cell lines. Isobologram analysis show that combination of anisomycin and standard of care (SOC) is synergistic in inhibiting proliferation of MV4-11 (C), MOLM-14 (D) and SKNO-1 (E) cells. CI was calculated using the Calcusyn software. CI of less than 1 indicates synergism
To determine the combination effects of anisomycin and SOC (cytarabine for FLT3 WT and midostaurin for FLT3-ITD cells), we designed combination studies according to the Calcusyn median effect model (Chou 2010). Combination indices (CI) at 25%, 50% and 75% growth inhibition were shown in Fig. 1C, D and E. Synergy, as defined by a CI between anisomycin and SOC, was observed at all effect levels in all tested AML cell lines. This clearly indicates that the combination of anisomycin and SOC is synergistic in targeting AML cells.
Effect of anisomycin and SOC treatment on FLT3-ITD and FLT3 WT AML and NBM progenitor growth, differentiation and apoptosis
To determine the effect of anisomycin on growth and differentiation of AML progenitor cells, we plated CD34+ cells purified from individual AML patients’ bone marrow or peripheral blood onto semi-solid hematopoietic stem cell-colony formation unit methylcellulose. The total number of colonies, including colony-forming unit-granulocyte (CFU-G), colony-forming unit-macrophages (CFU-M) and colony-forming unit-granulocyte macrophages (CFU-GM) was counted two weeks later. The typical morphology of CFU-G and CFU-M was shown in Supplementary Fig. 1. The patients’ characteristics are summarized in supplementary Table 1. To determine the possible selective anti-AML activity of anisomycin, NBM CD34+ cells were used as normal control. We observed the remarkable reduction of colony formation in AML CD34+ cells exposed to anisomycin (Fig. 2A). We found that anisomycin decreased colony formation in all tested AML samples (n = 8) and the effective concentration was as low as 0.25 μg/ml (Fig. 2B). In contrast, anisomycin at only highest concentration significantly decreased colony formation of NBM CD34+ cells. Similarly, anisomycin at the same concentration induced significantly more apoptosis in AML than NBM CD34+ cells (Fig. 2C). Consistent with cell lines, we observed a correlation between FLT3-ITD and anisomycin’s efficacy, that FLT3-ITD CD34+ cells were more sensitive to anisomycin compared to FLT3 WT cells (Supplementary Table 1). Of note, the combination of anisomycin and SOC is significantly more effective in inhibiting colony formation and inducing apoptosis in AML progenitor cells while sparing normal counterparts (Fig. 2D and E). Our results demonstrate that the combination of anisomycin and SOC preferentially inhibits AML compared to NBM progenitor cells.
Selective inhibitory effects of anisomycin in AML CD34+ cells. (A) Colony formation of AML CD34+ cells in the presence of control (DMSO) and anisomycin (2 μg/ml). The differential inhibitory effects of anisomycin on colony formation (B) and survival (C) of CD34+ cells isolated from individual FLT3-ITD and FLT3 WT AML patients and healthy normal bone marrow donors. The number of apoptotic cells was indicated by the percentage of Annexin V staining. Combination of anisomycin with standard of care (SOC) significantly further inhibits colony formation (D) and induces apoptosis (E) in AML CD34+ cells (n = 3) but not NBM CD34+ cells (n = 2). Cytarabine at 100 nM was used for FLT3 WT AML and NBM samples and midostaurin at 50 nM was used for FLT3-ITD samples. Anisomycin at 0.5 μg/ml was used in combination studies. *, p < 0.5, compared to control; #, p < 0.5, compared to SOC
Effect of anisomycin and SOC treatment on FLT3-ITD and FLT3 WT AML xenograft tumor growth
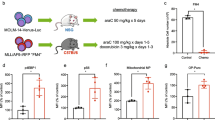
To further confirm the effect of anisomycin in AML, we established two independent AML xenograft mouse models using OCI-AML2 and MOLM-13 cells. We subcutaneously implanted cells into mouse flank. After development of palpable tumors, mice were given anisomycin, SOC alone or the combination. We have monitored toxicity signs during the whole treatment duration, including body weight, appearance (eg, skin and fur), nausea, vomiting, diarrhea, limb weakness, muscle tremors and neurologic signs. We did not observe body weight change (Supplementary Fig. 2) and other overt signs of toxicity in any treatment group, demonstrating that mice are tolerable to the dose of each drug administrated. Anisomycin delayed tumor growth beginning at 3 days of the initial treatment and its inhibitory effect was observed throughout the duration of treatment in both FLT3 WT and FLT3-ITD AML xenograft mouse models (Fig. 3). SOC drugs, such as cytarabine and midostaurin, moderately delayed OCI-AML2 and MOLM-13 tumor growth. Importantly, the efficacy of the combination of anisomycin and SOC was significantly more than single drug alone. The combination completely arrested AML tumor growth. Consistent with the in vitro findings, TUNEL staining demonstrated an increase in tumor cell apoptosis in SOC-treated group or anisomycin-treated group compared to control (Fig. 4). In addition, we observed a marked increase in individual cell apoptosis in the combination treatment group as compared to single drug groups.
Anisomycin inhibits AML growth in mice and is synergistic with standard of care drug. Combination of anisomycin and standard of care drug decreases size and weight of OCI-AML2 (A and B) and MOLM-13 (C and D) xenografts. The mice were given anisomycin at 10 mg/kg via intraperitoneal injection, 50 mg/kg cytarabine via intraperitoneal injection and 50 mg/kg midostaurin via oral gavage. *, p < 0.05, compared to SOC
Anisomycin induces AML apoptotic in mice and is synergistic with standard of care drug. Representative TUNEL staining of tumor sections show that combination of anisomycin and standard of care drug further increases apoptosis in OCI-AML2 (A) and MOLM-13 (B) xenografts. The mice were given anisomycin at 10 mg/kg via intraperitoneal injection, 50 mg/kg cytarabine via intraperitoneal injection and 50 mg/kg midostaurin via oral gavage
Anisomycin acts on AML via inhibiting mitochondrial respiration
As a well-known agonist of p38-MAPK, anisomycin exposure results in a rapid activation of p38 (Barros et al. 1997; Liu et al. 2014). In agreement with previous reports, we also observed the increased phosphorylation of p38 in MOLM-13 cells after anisomycin treatment (Supplementary Fig. 3A). However, p38 depletion did not rescue the pro-apoptotic effect of anisomycin in MOLM-13 cells (Supplementary Fig. 3B and C), indicating that anisomycin acts on AML cells in a p38-independent manner. In contrast, we found that both MOLM-13 (FLT3-mutant) and OCI-AML2 (FLT3 WT) cells treated with anisomycin displayed a reduced baseline OCR and were non-responsive to uncoupling of mitochondrial oxidative phosphorylation via FCCP (Fig. 5A and B), suggesting that anisomycin disrupts mitochondrial respiration and mitochondrial respiratory capacity in AML. We performed time course analysis of apoptosis and mitochondrial respiration after anisomycin treatment to determine which event occurs first. We found that anisomycin at 2 μg/ml disrupted mitochondrial respiration as early as 1-h treatment and induced apoptosis as early as 12-h treatment (Supplementary Fig. 4). This data clearly indicate that inhibition of mitochondrial respiration is not the consequence of apoptosis induction in anisomycin-treated AML cells. In contrast, anisomycin disrupts mitochondrial respiration, followed by apoptosis induction in AML cells.
The mechanism of action of anisomycin in AML is the inhibition of mitochondrial respiration. Anisomycin dose-dependently decreases basal and maximal OCR in OCI-AML2 (A) and MOLM-13 (B) cells. ORC was measured using treated cells without and in the presence of oligomycin, FCCP and Antimycin A and Rotenone combination. Compounds were injected into the wells at the time indicated by arrows. Anisomycin dose-dependently decrease mitochondrial complex I activity in OCI-AML2 (C) and MOLM-13 (D) cells. (E) Anisomycin significantly increases intracellular ROS level in MOLM-13 cells. (F) Anisomycin is significantly less effective in inducing apoptosis in AML ρ0 cells than AML cells. *p < 0.05, compared to control
Consistent with the previous work (Cao et al. 2017; Chambers and LoGrasso 2011), we further found that anisomycin dose-dependently reduced mitochondrial complex I activity in MOLM-13 and OCI-AML2 cells (Fig. 5C and D). As expected, increased intracellular ROS was observed in anisomycin-treated AML cells (Fig. 5E). To confirm that the ability of anisomycin in inducing apoptosis was associated with its capacity to inhibit mitochondrial respiration, we generated AML ρ0 cells that are incapable of performing mitochondrial respiration (Hashiguchi and Zhang-Akiyama 2009). We showed that these ρ0 cells exhibited a minimal level of basal OCR and completely non-responsive to FCCP stimulation (Supplementary Fig. 5), indicative of defective mitochondrial respiration. We further showed that anisomycin is ineffective in inducing apoptosis in ρ0 cells but not parental AML cells (Fig. 5F). This clearly demonstrates that disruption of mitochondrial respiration is the predominant mechanism of anisomycin’s action in AML.
FLT3-ITD cells have increased mitochondrial biogenesis and basal oxygen consumption compared to FLT3 WT cells
To investigate whether mitochondrial biogenesis contributes to differential sensitivity of FLT3-ITD and FLT3 WT cells to anisomycin, we measured mitochondrial characteristics of both cell types. MV4-11, MOLM-13 and MOLM-14 displayed higher mitochondrial mass, increased copy number of mitochondrial DNA (mtDNA), higher OCR and ATP levels than SKNO-1 and OCI-AML2 (Fig. 6). These results suggest that FLT3-ITD AML cells are likely to be more metabolically active and dependent on mitochondrial respiration than FLT3 WT AML cells.
Discussion
Advances in genomics have revealed the extensive heterogeneity and complex mutational landscape in de novo and relapsed AML (Kishtagari et al. 2020). There are at least 11 genetic classes and 20 subsets, taking into account differentiation states in leukemic blast cells (Papaemmanuil et al. 2016; Arber et al. 2016). Therefore, targeting common and essential among different molecular subclasses of AML represents an alternative therapeutic strategy for AML. Warburg’s aerobic glycolysis of reprogramming energy metabolism plays important role in cancer and has therapeutic value. However, studies have suggested that mitochondrial oxidative phosphorylation is likely more important than Warburg’s paradigm for blood cancer, particularly for leukemia stem cell. AML stem cells have increased mitochondrial biogenesis and basal oxygen consumption compared to normal hematopoietic cells (Skrtic et al. 2011). AML stem cell-enriched primary populations are metabolically dormant and are dependent on oxidative respiration rather than glycolysis for energy generation (Lagadinou et al. 2013). In addition, ALL cells have increased mitochondrial biogenesis whereas glycolytic activity is comparable than normal counterparts (Fu et al. 2017). Targeting mitochondrial respiration for leukemia therapy has therefore been garnered because mitochondria respiration is not only an effective but also selective target in leukemia. Consistent with the emerging evidence showing that AML stem cell rely on mitochondrial respiration for maintenance and survival (Skrtic et al. 2011; Lagadinou et al. 2013), we identify that anisomycin selectively eliminates AML bulk and CD34+ progenitor cells through disruption of mitochondrial respiration.
The anti-cancer activities of anisomycin have been shown in various types of cancer. In particular, a recent study shows that anisomycin selectively inhibits CML CD34+ cell survival, colony formation and self-renewal (Li et al. 2018). Our findings on the inhibitory effects of anisomycin in AML CD34+ cells correlate well with the previous work by showing that anisomycin with similar IC50 range is effective in inhibiting both CML and AML progenitor cells, and acts synergistically with SOC drugs (Fig. 2). The synergism between anisomycin and anti-cancer drugs were also reported in solid tumors (Liu et al. 2014; Cao et al. 2017; Jin et al. 2013; Abayasiriwardana et al. 2007). Our findings that the combination of anisomycin with midostaurin or cytarabine synergistically eliminated AML CD34+ cells without affecting normal hematopoietic CD34+ cells, suggesting the therapeutic window of anisomycin and SOC combination in AML. This is further confirmed in AML xenograft mouse models that the combination completed arrests tumor growth without causing significant toxicity in mice (Fig. 3 and Supplementary Fig. 2). It is worthy of investigating the effects of anisomycin using AML patient-derived xenotransplant mice model. We speculate that anisomycin is highly likely to be effective in targeting primary AML samples in vivo, particularly given our observations on the inhibitory effects of anisomycin as single drug alone and its combination with standard of care on AML primary CD34+ cells derived from patients. Interestingly, our findings further reveal that AML cells with FLT3-ITD are more sensitive to anisomycin compared to those that are FLT3 WT (Figs. 1 and 2, and Supplementary table 1). FLT3-ITD occurs in approximately 30% of all AML cases and confers a poor prognosis (Daver et al. 2019). The potent efficacy of anisomycin on FLT3-ITD cells adds anisomycin to the list of candidates to treat AML with FLT3-ITD.
Anisomycin acts on CML cells via suppressing Wnt/β-catenin without affecting p38 activity (Li et al. 2018). Although anisomycin increases p38 activity in AML cells, we found that anisomycin acted on AML cells via p38-independent manner (Supplementary Fig. S3). We found that anisomycin disrupted mitochondrial respiration via suppressing complex I and induced oxidative stress. The rescue study confirmed that mitochondrial respiration inhibition is the mechanism of anisomycin’s action in AML (Fig. 5). It seems that the mechanisms of action of anisomycin in cancer are cancer cell type specific. In addition, our work further revealed the basis of hypersensitivity of FLT3-ITD cells to anisomycin that the varying levels of mitochondrial biogenesis between FLT3-ITD and FLT3 WT AML cells (Fig. 6). This highlights the therapeutic value of inhibition of mitochondrial respiration in targeting FLT3-ITD AML. It is worthy of pursing in silico experiment on the link between increased mitochondrial biogenesis factors and worse clinical outcome in AML patients.
In conclusion, our work demonstrates anisomycin is active and selective against AML through inhibition of mitochondrial respiration. Our work also demonstrate that mitochondria-regulated biochemical pathways is a selective target in AML and warrants further therapeutic exploitation, particularly in AML patients with FLT3-ITD mutation.
Data availability
Data will be made available on reasonable request.
References
Abayasiriwardana KS, Barbone D, Kim KU, Vivo C, Lee KK, Dansen TB, Hunt AE, Evan GI, Broaddus VC (2007) Malignant mesothelioma cells are rapidly sensitized to TRAIL-induced apoptosis by low-dose anisomycin via Bim. Mol Cancer Ther 6:2766–2776
Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, Bloomfield CD, Cazzola M, Vardiman JW, The, (2016) revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 127(2016):2391–2405
Barros LF, Young M, Saklatvala J, Baldwin SA (1997) Evidence of two mechanisms for the activation of the glucose transporter GLUT1 by anisomycin: p38(MAP kinase) activation and protein synthesis inhibition in mammalian cells. J Physiol 504(Pt 3):517–525
Cao C, Yu H, Wu F, Qi H, He J (2017) Antibiotic anisomycin induces cell cycle arrest and apoptosis through inhibiting mitochondrial biogenesis in osteosarcoma. J Bioenerg Biomembr 49:437–443
Chambers JW, LoGrasso PV (2011) Mitochondrial c-Jun N-terminal kinase (JNK) signaling initiates physiological changes resulting in amplification of reactive oxygen species generation. J Biol Chem 286:16052–16062
Chen W, Drakos E, Grammatikakis I, Schlette EJ, Li J, Leventaki V, Staikou-Drakopoulou E, Patsouris E, Panayiotidis P, Medeiros LJ, Rassidakis GZ (2010) mTOR signaling is activated by FLT3 kinase and promotes survival of FLT3-mutated acute myeloid leukemia cells. Mol Cancer 9:292
Chou TC (2010) Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res 70:440–446
Chou TC, Talalay P (1984) Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul 22:27–55
Daver N, Schlenk RF, Russell NH, Levis MJ (2019) Targeting FLT3 mutations in AML: review of current knowledge and evidence. Leukemia 33:299–312
Fu X, Liu W, Huang Q, Wang Y, Li H, Xiong Y (2017) Targeting mitochondrial respiration selectively sensitizes pediatric acute lymphoblastic leukemia cell lines and patient samples to standard chemotherapy. Am J Cancer Res 7:2395–2405
Grollman AP (1967) Inhibitors of protein biosynthesis. II. Mode of action of anisomycin, J Biol Chem 242:3226–3233
Hashiguchi K, Zhang-Akiyama QM (2009) Establishment of human cell lines lacking mitochondrial DNA. Methods Mol Biol 554:383–391
Jin CY, Park C, Hong SH, Han MH, Jeong JW, Xu H, Liu H, Kim GY, Kim WJ, Yoo YH, Choi YH (2013) Synergistic induction of TRAIL-mediated apoptosis by anisomycin in human hepatoma cells via the BH3-only protein Bid and c-Jun/AP-1 signaling pathway. Biomed Pharmacother = Biomedecine & pharmacotherapie 67:321–328
Kishtagari A, Levine RL, Viny AD (2020) Driver mutations in acute myeloid leukemia. Curr Opin Hematol 27:49–57
Kiyoi H, Kawashima N, Ishikawa Y (2020) FLT3 mutations in acute myeloid leukemia: Therapeutic paradigm beyond inhibitor development. Cancer Sci 111:312–322
Lagadinou ED, Sach A, Callahan K, Rossi RM, Neering SJ, Minhajuddin M, Ashton JM, Pei S, Grose V, O’Dwyer KM, Liesveld JL, Brookes PS, Becker MW, Jordan CT (2013) BCL-2 inhibition targets oxidative phosphorylation and selectively eradicates quiescent human leukemia stem cells. Cell Stem Cell 12:329–341
Li Y, Wu X, Jin X, Wang J, Togo Y, Suzuki T, Hashimoto T, Yamada Y, Nakanishi Y, Kanematsu A, Nojima M, Kakehi Y, Yamamoto S (2017) Enhancement of death receptor 4-mediated apoptosis and cytotoxicity in renal cell carcinoma cells by anisomycin. Anticancer Drugs 28:180–186
Li Y, Hu J, Song H, Wu T (2018) Antibiotic anisomycin selectively targets leukemia cell lines and patient samples through suppressing Wnt/beta-catenin signaling. Biochem Biophys Res Commun 505:858–864
Liu Y, Ge J, Li Q, Gu L, Guo X, Ma ZG, Zhu YP (2013) Anisomycin induces apoptosis of glucocorticoid resistant acute lymphoblastic leukemia CEM-C1 cells via activation of mitogen-activated protein kinases p38 and JNK. Neoplasma 60:101–110
Liu Y, Ge J, Li Q, Guo X, Gu L, Ma ZG, Li XH, Zhu YP (2014) Low-dose anisomycin sensitizes glucocorticoid-resistant T-acute lymphoblastic leukemia CEM-C1 cells to dexamethasone-induced apoptosis through activation of glucocorticoid receptor and p38-MAPK/JNK. Leuk Lymphoma 55:2179–2188
Papaemmanuil E, Gerstung M, Bullinger L, Gaidzik VI, Paschka P, Roberts ND, Potter NE, Heuser M, Thol F, Bolli N, Gundem G, Van Loo P, Martincorena I, Ganly P, Mudie L, McLaren S, O’Meara S, Raine K, Jones DR, Teague JW, Butler AP, Greaves MF, Ganser A, Dohner K, Schlenk RF, Dohner H, Campbell PJ (2016) Genomic Classification and Prognosis in Acute Myeloid Leukemia. N Engl J Med 374:2209–2221
Roboz GJ (2011) Novel approaches to the treatment of acute myeloid leukemia. Hematology Am Soc Hematol Educ Program 2011:43–50
Skrtic M, Sriskanthadevan S, Jhas B, Gebbia M, Wang X, Wang Z, Hurren R, Jitkova Y, Gronda M, Maclean N, Lai CK, Eberhard Y, Bartoszko J, Spagnuolo P, Rutledge AC, Datti A, Ketela T, Moffat J, Robinson BH, Cameron JH, Wrana J, Eaves CJ, Minden MD, Wang JC, Dick JE, Humphries K, Nislow C, Giaever G, Schimmer AD (2011) Inhibition of mitochondrial translation as a therapeutic strategy for human acute myeloid leukemia. Cancer Cell 20:674–688
Thol F, Ganser A (2020) Treatment of Relapsed Acute Myeloid Leukemia. Curr Treat Options Oncol 21:66
Ushijima H, Horyozaki A, Maeda M (2016) Anisomycin-induced GATA-6 degradation accompanying a decrease of proliferation of colorectal cancer cell. Biochem Biophys Res Commun 478:481–485
Varum S, Rodrigues AS, Moura MB, Momcilovic O, Easley CAT, Ramalho-Santos J, Van Houten B, Schatten G (2011) Energy metabolism in human pluripotent stem cells and their differentiated counterparts. PLoS One 6:e20914
Acknowledgements
This work was supported by Xiangyang Health and Family Planning Commission (XY15A04).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Authors declare no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Chuang Zhang and Qian Deng are co-first authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, C., Deng, Q., Bao, S. et al. Anisomycin is active in preclinical models of pediatric acute myeloid leukemia via specifically inhibiting mitochondrial respiration. J Bioenerg Biomembr 53, 693–701 (2021). https://doi.org/10.1007/s10863-021-09918-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10863-021-09918-z