Abstract
Bronchopulmonary dysplasia (BPD) is characterized by impaired vascular and alveolar development, and the underlying molecular mechanisms have remained elusive. MicroRNAs are important players in various biological functions including the pathogenesis of BPD. The present study aimed to examine the expression of miR-203a-3p in the peripheral blood of BPD patients and elucidate the mechanisms underlying miR-203a-3p-mediated progression of BPD. We examined the expression of miR-203a-3p in the peripheral blood of BPD patients and found that miR-203a-3p was up-regulated in the patients. Additionally, the mRNA expression levels of vascular endothelial growth factor A (VEGFA) and hypoxia-inducible factor-1alpha were down-regulated in the BPD patients. Further in vitro studies showed that miR-203a-3p suppressed the expression of VEGFA in RLE-6TN cells by targeting the VEGFA 3′ untranslated region. Overexpression of miR-203a-3p inhibited the viability of RLE-6TN cells and induced cell apoptosis, whereas the knockdown of miR-203a-3p exerted opposite effects. VEGFA treatment significantly attenuated the increase in the RLE-6TN cell apoptotic rates induced by miR-203a-3p overexpression; while VEGFA knockdown significantly increased the cell apoptotic rates of RLE-6TN cells, which was partially reversed by the treatment with miR-203a-3p inhibitor. Furthermore, miR-203a-3p was up-regulated, whereas VEGFA was down-regulated in the lung tissues of BPD rats, and sequestration of the expression of miR-203a-3p prevented hyperoxia-induced lung damage, increased VEGFA mRNA and protein expression levels, and promoted the protein expression of ERK, PI3K, and p38 in the lung tissues of BDP rats. In summary, the findings of our study indicate that miR-203a-3p knockdown alleviates hyperoxia-induced lung tissue damage in the BPD rat model, and its effect may be associated with the up-regulation of VEGF.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Preterm infants born with immature lungs require frequent respiratory support (Bancalari and Jain 2018). However, the exposure of the immature lungs to hyperoxia often leads to the development of chronic lung disease in infancy, which is referred to as bronchopulmonary dysplasia (BPD) (Principi et al. 2018). BPD is defined as the need for supplemental O2 for >28 days of life and/or 36 weeks of corrected gestational age, and it is associated with impaired vascular and alveolar development (Gentle and Lal 2019; Sudhadevi et al. 2020). Although preterm infant mortality has decreased over the past 20 years, the incidence of BPD is relatively unchanged (Brener Dik et al. 2017). There is increasing evidence showing that different signaling pathways such as the vascular endothelial growth factor A (VEGFA) and hypoxia-inducible factor-1alpha (HIF-1α) are involved in the development of BPD (Asikainen et al. 2005; Liu et al. 2020; Yin et al. 2016). Unfortunately, the molecular mechanisms underlying BPD development remain unclear and may require further investigation.
MicroRNAs (miRNAs) are a class of small non-coding RNAs, which can post-transcriptionally regulate the expression of protein-coding genes by reducing the mRNA stability of the targeted genes (Ameis et al. 2017; Arora et al. 2018; Yang et al. 2013). Recent studies have revealed that miRNAs are involved in various biological processes such as tumorigenesis, infection, fibrosis, and respiration. There is increasing evidence indicating that miRNAs may be involved in the pathophysiology of BPD (Ameis et al. 2017; Arora et al. 2018; Yang et al. 2013). Durrani-Kolarik et al., showed that miR-29b decreased the expression of matrix proteins, which led to the improvement of alveolarization in mice exposed to maternal inflammation and neonatal hyperoxia (Durrani-Kolarik et al. 2017). Syed et al., demonstrated that hyperoxia induced miR-34a-mediated injury via angiopoietin-1 in neonatal lungs (Syed et al. 2017). The study of Lal et al., showed that exosomal miR-876-3p predicted and protected against severe BPD in extremely premature infants (Lal et al. 2018). Additionally, Zhang et al., identified the HIF-1α/miR-30a/Snail 1 axis as an important signaling pathway associated with the regulation of hyperoxic lung injury (Zhang et al. 2019). Thus, the identification of the novel miRNAs involved in the pathophysiology of BDP may be important for the development of novel therapies for BPD.
Previously, our studies compared the differentially expressed non-coding RNAs in the lung tissues of normal rats and hyperoxia-induced BPD rats, and we identified the up-regulation of miR-203a-3p in the lung tissues of BPD rats (Cheng et al. 2020), suggesting the potential role of miR-203a-3p in the pathogenesis of BPD. Therefore, the present study further examined the expression of miR-203a-3p in the peripheral blood of BPD patients, and in vitro and in vivo studies were performed to elucidate the mechanisms underlying miR-203a-3p-mediated progression of BPD.
Materials and methods
Collection of clinical samples
The study was approved by the Ethics Committee of Shenzhen People’s Hospital. Four patients with BPD, according to the National Institute of Child Health and Human Development guidelines (Day and Ryan 2017) and four non-BPD age-matched controls were enrolled from the Shenzhen People’s Hospital. Human blood samples were obtained from these patients and written informed consent was obtained from the guardians of the patients.
Cell culture
The rat AT2 cell line (RLE-6TN) was obtained from the American Type Culture Collection (ATCC; Manassas, USA). The RLE-6TN cells were grown in the Dulbecco’s modified Eagle medium supplemented with 10% fetal bovine serum and maintained in a humidified incubator with 5% CO2 at 37 °C.
MiRNAs, siRNAs, cell transfections and drug treatments
The miR-203a-3p mimics (sense, 5′-GUGAAAUGUUUAGGACCACUAG-3′ and antisense, 5′-AGUGGUCCUAAACAUUUCACUU-3′), inhibitor (5′-CUAGUGGUCCUAAACAUUUCAC-3′) and the corresponding negative controls (mimics-NC: sense, 5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense, 5′-ACGUGACACGUUCGGAGAATT-3′ and inhibitor-NC: 5′-CAGUACUUUUGUGUAGUACAA-3′) were synthesized by Ribobio (Guangzhou, China). The VEGFA siRNA 5’-GCGGAUCAAACCUCACCAA-3′ and the nonsense control siRNA (siNC) 5′- UUCUCCGAACGUGUCACGU-3′ were synthesized by RiboBio. The transfections of miRNAs into RLE-6TN cells were achieved by using the Lipofectamine 2000 reagent (Invitrogen, Carlsbad, USA) according to the manufacturer’s instructions. At 48 h after transfection, the RLE-6TN cells were processed for further functional assays. The rat recombinant VEGFA was from R&D systems (Minneapolis, MN, USA) and the RLE-6TN was treated with VEGFA at a concentration of 10 ng/mL. The HIF-1α signaling activator, dimethyloxalylglycine (DMOG) was purchased from Sigma-Aldrich. The RLE-6TN were treated with DMOG at a concentration of 500 μM.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA from RLE-6TN cells, serum, or lung tissues were extracted by using the TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. For the miRNA detection, the RNA was reversely transcribed into cDNA by using the TaqMan Advanced miRNA cDNA synthesis kit (Thermo Fisher Scientific, Waltham, USA). For VEGFA mRNA detection, the RNA was reverse transcribed into cDNA by using the SuperScript® III First-Strand Synthesis SuperMix kit (Thermo Fisher Scientific). The real-time PCR was performed using the ABI7900 Fast Real-Time PCR system (Applied Biosystems, Foster City, USA). The primers for the corresponding primers are shown in Table 1. The relative expression levels of miR-203a-3p and VEGFA were determined using the comparative Ct method. U6 and GAPDH were used as internal references for miR-203a-3p and VEGFA, respectively.
Luciferase reporter assay
VEGFA 3′ UTR fragment was amplified from the genomic DNA and cloned into NheI and SalI sites of pmirGLO vector (Promega, Madison, USA) to construct the wild type (WT) luciferase reporter vector (WT-VEGFA). The mutation of the VEGFA 3′ UTR was generated using a Site-Directed Mutagenesis kit (Stratagene, USA), and the mutated fragment was inserted into the pmiGLO vector (Promega) to construct the mutant (MUT) luciferase reporter vector (MUT-VEGFA). For the luciferase reporter assay, the RLE-6TN cells were co-transfected with WT-VEGFA or MUT-VEGFA and different miRNAs including miR-203a-3p mimics, miR-203a-3 inhibitor, mimics-NC, or inhibitor-NC. At 48 h post-transfection, the relative luciferase activity was determined with the Dual-Luciferase Reporter Assay System (Promega).
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay
Cell viability was assessed by using MTT assay. Briefly, RLE-6TN cells, after the different treatments, were incubated for 24, 48, and 72 h. Next, the RLE-6TN cells were incubated with 5 mg/ml MTT for 4 h at 37 °C. Cell viability was determined by measuring the optical density values at a wavelength of 490 nm.
Flow cytometry
For the detection of cell apoptosis, a double-staining method with propidium iodide (PI)/ fluorescein isothiocyanate (FITC)-labeled Annexin-V Apoptosis Detection kit (BD Biosciences, USA) was used. Briefly, the treated RLE-6TN cells were suspended in 100 μl of the buffer solution (10 mM HEPES/NaOH, 140 mM NaCl, 2.5 mM CaCl2, 5 mM KCl, pH 7.4) and stained with 5 μl of FITC-conjugated Annexin-V and 5 μl of PI (50 μg/ml) for 30 min at room temperature in the dark, followed by the addition of 400 μl of the binding buffer. Apoptotic cells were analyzed via flow cytometry by using the FACScan system (BD Biosciences).
Double immunofluorescence staining to determine the expression of miR-203a-3p and VEGFA in RLE-6TN cells
The treated cells were fixed with 4% paraformaldehyde (PFA) for 10 min at room temperature followed by washing with 0.1% diethyl pyrocarbonate/phosphate-buffered saline (PBS). The cells were incubated with 0.3% Triton-X100 for 5 min at 4 °C, followed by incubation with the miR-203a-3p probe (5′-CUAGUGGUCCUAAACAUUUCAC-3′ conjugated with CY5 at the 5′ end) overnight at 37 °C in the dark. Next, the cells were incubated with VEGFA antibody (cat# ab1316; 1:500; Abcam, Cambridge, USA) in 3% bovine serum albumin/PBS at 4 °C overnight in the dark. After washing with PBS, the cells were probed against Goat Anti-Mouse IgG H&L (Alexa Fluor® 488; cat# ab150113, 1:200, Abcam) for 1 h at room temperature in the dark. After washing with PBS, the cells were counterstained with 4,6-diamidino-2-phenylindole at room temperature for 10 min in the dark. The fluorescence signal was examined under a confocal fluorescence microscope.
BPD animal model and treatments
Mature Sprague-Dawley (SD) rats (220–250 g) were purchased from the Department of Animals, Experimental Center, Southern Medical University (Guangzhou, China). All animal experiments were approved and supervised by the Animal Ethics Committee of Shenzhen Peoples’ Hospital. The newborn SD rats were randomly divided into the model (exposed to hyperoxia [85% O2] from the day of birth) and the control (exposed to normoxia [21% O2]) groups. To avoid O2 toxicity, maternal rats within the model and control groups were switched once every 24 h. For miRNA treatment, the rats exposed to hyperoxia were intranasally administered with miR-203a-3p inhibitor (BPD + miR-203a-3p antagomir group) or the inhibitor NC (BDP + antagomir NC group) at a dosage of 5 nmol for consecutive days from the day of birth. Rats were given ad libitum access to water and food. At 14 days after the start of exposure to hyperoxia or normoxia, eight newborn rats from each model or control group were anesthetized with 5% chloral hydrate through intraperitoneal injection, and the whole lungs were collected aseptically through chest opening. The lungs were snap-frozen in liquid nitrogen for qRT-PCR analysis or fixed in PFA for subsequent hematoxylin and eosin (H&E) and immunohistochemical staining.
Morphometric and immunohistochemical analysis
Lungs were inflation-fixed with 4% methanol for 24 h and embedded in paraffin. To assess alveolarization, lung sections were subjected to H&E staining. Five non-overlapping, representative microphotographs were taken at 100X magnification by an investigator blinded to the assignment of the groups.
In a separate study, lung sections were immunohistochemically stained. The sections were incubated with primary mouse monoclonal antibodies including anti-VEGFA (cat# ab1316; 1:500; Abcam), anti-ERK (cat# 9102; 1:100; Cell Signaling Technology, Danvers, USA), anti-PI3K (cat# AP0152; 1:200, Bioworld Technology), and anti-p38 (cat#4511S; 1:200, Cell Signaling Technology) followed by incubation with rabbit anti-mouse secondary antibody (# cat# ab6728; 1:1000; Abcam, USA). The deposition of brown particles in the cytoplasm/nucleus indicates a positive result. Cells displaying brown particles in the cytoplasm/nucleus were counted under the microscope, within the area of the set viewing window. Scoring was comprehensively conducted depending on the staining intensity (0 for no staining, 1 for weak staining, 2 for moderate staining and 3 for strong staining) and percentage of positively stained cells (0 for 0–5% of cells, 1 for 6–25% of cells, 2 for 26–50% of cells, 3 for 51–75% of cells and 4 for 76–100% of cells). The product of both grades was calculated as the final expression score.
Statistical analysis
All the statistical analysis was performed using the SPSS 15.0 software (SPSS, Inc., Chicago, USA). Data are presented as mean ± standard deviation. Unpaired t-test was used to determine the significant differences between two treatment groups. One-way ANOVA was used to determine the significant differences among multiple treatment groups. A p value <0.05 indicated a statistically significant difference.
Results
Expression of miR-203a-3p, VEGFA, and HIF-1α in the serum of BDP patients
We performed qRT-PCR assay to examine the expression of miR-203a-3p, VEGFA mRNA, and HIF-1α mRNA in the serum of BPD patients and healthy controls. As shown in Fig. 1a, the expression of miR-203a-3p in the serum of the BDP patients was significantly higher than that in the healthy controls (Fig. 1a). It has been suggested that VEGFA and HIF-1α are involved in the pathogenesis of BPD, and hence, the mRNA expression levels of the two genes were further evaluated by qRT-PCR. The results showed that mRNA expression levels of VEGFA and HIF-1α were significantly lower in the BDP group than that in the healthy control group (Fig. 1b and c).
VEGFA is a direct target of miR-203a-3p in RLE-6TN cells
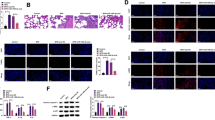
In another experiment, we tested the expression of miR-203a-3p in the RLE-6TN cells after being transfected with miR-203a-3p mimics, and we found that miR-203a-3p overexpression significantly repressed the mRNA expression level of VEGFA in RLE-6TN cells (Fig. 2a). Furthermore, we inhibited the expression of miR-203a-3p in RLE-6TN cells by transfecting miR-203a-3p inhibitors, and we found that miR-203a-3p knockdown markedly up-regulated the expression of VEGFA (Fig. 2b). These results suggest that miR-203a-3p negatively regulated the expression of VEGFA in RLE-6TN cells. In order to explore the mechanism through which miR-203-3p regulated VEGFA expression, we performed bioinformatics analysis and found that miR-203-3p could potentially bind to the 3′ UTR of VEGFA (Fig. 2c). The luciferase reporter vectors with wild type or mutant VEGFA 3′ UTR were constructed, and miR-203a-3p overexpression significantly reduced the luciferase activity of the wild type VEGFA 3′ UTR reporter vector but not that of the mutant VEGFA 3′ UTR (Fig. 2d), suggesting that miR-203a-3p directly acted on the VEGFA 3′ UTR. The immunofluorescence staining results showed that the RLE-6TN cells transfected with miR-203a-3p mimics exhibited significantly enhanced immunofluorescence staining for miR-203a-3p and markedly reduced immunofluorescence staining for VEGFA (Fig. 2e). Moreover, miR-203a-3p transfection demonstrated opposite effects on the immunofluorescence staining intensity of miR-203a and VEGFA in RLE-6TN cells (Fig. 2f).
MiR-203a-3p targets VEGFA and suppresses its expression. a and b qRT-PCR analysis of miR-203a-3p and VEGFA expression in RLE-6TN cells after being transfected with mimics-NC, miR-203a-3p mimics, inhibitor NC or miR-203a-3p inhibitor. c TargetScan analysis of the predicted binding sites between miR-203a-3p and VEGFA 3’UTR. d Dual-luciferase reporter assay determined the luciferase activities of different reporter constructs in RLE-6TN cells with mimics-NC or miR-203a-3p mimics transfection. e and f Immunofluorescent staining of VEGFA and miR-203a-3p in RLE-6TN cells after being transfected with mimics-NC, miR-203a-3p mimics, inhibitor NC or miR-203a-3p inhibitor. Scale bar = 500 μm. N = 3. **P < 0.01 and ***P < 0.001
Based on bioinformatics prediction, miR-203a-3p could target the 3’UTR of HIF-1α (Supplemental Fig. S1A). Further qRT-PCR results showed that HIF-1α mRNA expression level in RLE-6TN cells was not affected by miR-203a-3p mimics or miR-203a-3p inhibitor transfection (Supplemental Fig. S1B and S1C). On the other hand, we found that activation of HIF-1α signaling by treating RLE-6TN cells with DMOG significantly repressed the miR-203a-3p expression (Supplemental Fig. S1C).
Effects of miR-203a-3p on the viability and apoptosis of RLE-6TN cells
Gain-of-function and loss-of-function studies were performed to determine the effects of miR-203a-3p on the viability and apoptosis of RLE-6TN cells. As shown in Fig. 3a, miR-203a-3p overexpression significantly reduced the viability of RLE-6TN when compared to that of the mimics-NC-transfected cells (Fig. 3a), whereas transfection with miR-203a-3p inhibitor enhanced the viability of RLE-6TN cells when compared to the inhibitor-NC-transfected cells (Fig. 3b). Consistently, the flow cytometry results showed that miR-203a-3p overexpression induced cell apoptosis in RLE-6TN cells (Fig. 3c), whereas miR-203a-3p inhibition exerted inhibitory effects on the apoptosis of RLE-6TN cells (Fig. 3d). Taken together, these results implied that miR-203a-3p inhibited cell viability and induced apoptosis in RLE-6TN cells. To further confirm the interaction between miR-203a-3p and VEGFA in regulating RLE-6TN cell apoptosis, we performed the rescue experiments. As shown in Fig. 3e, VEGFA treatment significantly attenuated the increase in the RLE-6TN cell apoptotic rates induced by miR-203a-3p overexpression (Fig. 3e). On the other hand, VEGFA knockdown significantly increased the cell apoptotic rates of RLE-6TN cells, which was partially reversed by the treatment with miR-203a-3p inhibitor (Fig. 3f).
Effects of miR-203a-3p overexpression/knockdown on the cell viability and apoptosis of RLE-6TN cells. a and b MTT analysis of cell viability of RLE-6TN cells after being transfected with mimics-NC, miR-203a-3p mimics, inhibitor NC or miR-203a-3p inhibitor. c and d Flow cytometry analysis of cell apoptosis of RLE-6TN cells after being transfected with mimics-NC, miR-203a-3p mimics, inhibitor NC or miR-203a-3p inhibitor. e Flow cytometry analysis of cell apoptosis of RLE-6TN cells after being treated with mimics NC, rno-miR-203a-3p mimics, or rno-miR-203a-3p mimics + VEGFA. f Flow cytometry analysis of cell apoptosis of RLE-6TN cells after being co-transfected with siNC + inhibitor NC, VEGFA siRNA + inhibitor NC, or VEGFA siRNA + rno-miR-203a-3p inhibitor. N = 3. *P < 0.05 and **P < 0.01
MiR-203a-3p is up-regulated and VEGFA is down-regulated in the lung tissues of BPD rats
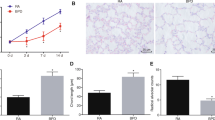
In order to confirm the role of miR-203a-3p in BPD in vivo, we established hyperoxia-induced BPD rats. As shown in Fig. 4a, the lung tissues of hyperoxia-treated rats were significantly damaged when compared to those of normoxia-treated animals as determined by H&E staining (Fig. 4a). Additionally, we examined the expression of miR-203a-3p and VEGFA in the lung tissues of the hyperoxia-treated rats using qRT-PCR. We found that miR-203a-3p was significantly up-regulated and VEGFA mRNA was down-regulated in the lung tissues of the hyperoxia-treated rats when compared to those in the normoxia-treated ones (Fig. 4b and c). Furthermore, we examined VEGFA protein using IHC staining, and we consistently found that the VEGFA protein level was reduced in the lung tissues of hyperoxia-treated rats when compared to that in the normoxia group (Fig. 4d).
Expression of miR-203a-3p and VEGFA in the serum and lung tissues of the DBP rats. a IHC analysis of the lung morphology of the rats exposed to normoxia or hyperoxia. Scale bar = 500 μm. b qRT-PCR analysis of VEGFA and miR-203a-3p expression in the serum from the rats exposed to normoxia or hyperoxia. c qRT-PCR analysis of VEGFA and miR-203a-3p expression in the lung tissues from the rats exposed to normoxia or hyperoxia. d IHC analysis of VEGFA protein expression in the lung tissues from the rats exposed to normoxia or hyperoxia. Scale bar = 1000 μm. N = 6. *P < 0.05 and **P < 0.01
Inhibition of miR-203a-3p attenuates hyperoxia-induced BPD in rats
As miR-203a-3p was up-regulated in the hyperoxia-induced BPD rats, we further examined if sequestration of the expression of miR-203a-3p could mitigate the progression of hyperoxia-induced BPD in the rats. The H&E staining results showed that the knockdown of miR-203a-3p remarkably prevented hyperoxia-induced lung damage in the BPD rats, compared to the rats treated with miR-203a-3p inhibitor NC (Fig. 5a). The qRT-PCR results showed that treatment with miR-203a-3p inhibitor down-regulated the expression of miR-203a-3p and up-regulated mRNA expression of VEGFA in the lung tissues of the BPD rats (Fig. 5b and c). Consistently, the results of the IHC staining showed that the knockdown of miR-203a-3p enhanced the protein expression of VEGFA in the lung tissues of BPD rats (Fig. 5d). VEGFA can regulate various down-stream signaling pathways including MAPK and PI3K signaling, and hence, we further examined if miR-203a-3p could influence the protein expression of these mediators. As shown in Fig. 5e, the protein levels of ERK, PI3K, and p38 were significantly up-regulated in the BPD rats treated with miR-203a-3p inhibitor (Fig. 5e), suggesting that the up-regulation of VEGFA by miR-203a-3p inhibitor might regulate the downstream signaling pathways such as MAPK and PI3K signaling.
Expression of miR-203a-3p and VEGFA in the serum and lung tissues of the DBP rats treated with miR-203a-3p inhibitor. a IHC analysis of the lung morphology of the DBP rats treated with antagomir NC or miR-203a antagomir. Scale bar = 1000 μm. b qRT-PCR analysis of VEGFA and miR-203a-3p expression in the serum from the DBP rats treated with antagomir NC or miR-203a antagomir. c qRT-PCR analysis of VEGFA and miR-203a-3p expression in the lung tissues from the DBP rats treated with antagomir NC or miR-203a antagomir. d IHC analysis of VEGFA protein expression in the lung tissues from the DBP rats treated with antagomir NC or miR-203a antagomir. Scale bar = 1000 μm. e IHC analysis of ERK, PI3K and p38 protein expression in the lung tissues from the DBP rats treated with antagomir NC or miR-203a antagomir. Scale bar = 1000 μm. N = 6. *P < 0.05 and **P < 0.01
Discussion
BPD is characterized by impaired vascular and alveolar development, and the underlying molecular mechanisms have remained elusive. MiRNAs act as important players in various biological functions including the pathogenesis of BPD (Ameis et al. 2017; Arora et al. 2018; Yang et al. 2013). In this study, we examined the expression of miR-203a-3p in the peripheral blood of BPD patients, and we found that miR-203a-3p was up-regulated in BPD patients. The mRNA expression levels of VEGFA and HIF-1α were down-regulated in the BPD patients. Further in vitro studies showed that miR-203a-3p suppressed the expression of VEGFA in RLE-6TN cells by targeting the VEGFA 3′UTR. In vitro functional studies revealed that the overexpression of miR-203a-3p inhibited cell viability and induced cell apoptosis of RLE-6TN cells, whereas the knockdown of miR-203a-3p exerted opposite effects. VEGFA could attenuate the effects of miR-203a-3p on the apoptosis of RLE-6TN cells. Furthermore, the role of miR-203a-3p in the pathogenesis of BPD was evaluated in the hyperoxia-induced BPD rat model. MiR-203a-3p was up-regulated and VEGFA was down-regulated in the lung tissues of BPD rats, and sequestration of the expression of miR-203a-3p prevented hyperoxia-induced lung damage, increased VEGFA mRNA and protein expression, and promoted the protein expression of ERK, PI3K, and p38 in the lung tissues of BDP rats. Taken together, the present study underscores the important role of miR-203a-3p in the pathogenesis of BPD.
The diverse biological functions of miR-203a-3p have been reported by several studies. miR-203a-3p can act as oncogenes in colorectal cancer (Chen et al. 2018) and breast cancer (Xu et al. 2019), and as tumor suppressor in gastric cancer (Wang et al. 2018) and ovarian cancer (Liu et al. 2019). Recently, miR-203a-3p expression in the lung has been reported to be suppressed by high-altitude hypoxia (Cai et al. 2020), which implies that miR-203a-3p expression can be affected by oxygen levels under physiological conditions. It has been reported that miR-203a-3p can suppress cell proliferation and induce cell apoptosis in lung cancer cells. In our study, we found that miR-203a-3p was up-regulated in the clinical blood samples of BDP patients as well as in hyperoxia-induced BPD rats. Furthermore, miR-203a-3p suppressed lung cell proliferation and induced apoptosis. Collectively, these results imply that miR-203-3p contributes to the development of BPD through the suppression of alveolar cell proliferation and the induction of apoptosis in the lung tissues.
In order to further decipher the role of miR-203a-3p in the pathogenesis of BPD, we performed further mechanistic experiments and found that miR-203a-3p could target the 3′ UTR of VEGFA and repress its expression in the RLE-6TN cells. This suggests that VEGFA can be a downstream mediator of miR-203a-3p. Based on the literature, it was found that the mRNA and protein levels of VEGFA were both decreased in a baboon model of BPD (Maniscalco et al. 2002). Recombinant human VEGF-A therapy and VEGFA gene therapy restored vascular growth and lung structure in rat BPD model (Kunig et al. 2005; Thebaud et al. 2005). Similarly, in human studies, the mRNA expression of VEGFA was down-regulated in BPD infants (Bhatt et al. 2001). Moreover, decreased VEGFA level in the urine was associated with BPD (Levesque et al. 2013). Consistently, our results showed that VEGFA mRNA was down-regulated in the serum of the BPD patients. Further in vivo studies confirmed that, compared to those in the control rats, mRNA and protein expression of VEGFA were lower in the lung tissues of the hyperoxia-induced BPD rats, indicating the important role of VEGFA in the pathophysiology of BPD. Furthermore, we found that the inhibition of miR-203a-3p significantly alleviated hyperoxia-induced lung damage and increased the mRNA and protein expression of VEGFA. These results implied that miR-203a-3p regulated hyperoxia-induced lung damage in the BPD rats by targeting VEGFA. As VEGFA could interact with different signaling pathways including MAPK and PI3K signaling pathways (Claesson-Welsh and Welsh 2013; Samadi et al. 2018), we further attempted to investigate if the inhibition of miR-203a-3p could affect the protein levels of these mediators in the signaling pathways. Our results showed that the inhibition of miR-203a-3p increased the protein expression of ERK, PI3K, and p38 in the lung tissues of BPD rats, suggesting that these signaling pathways might also be involved in miR-203a-3p-mediated activities. However, the interaction between miR-203a-3p and the signaling pathways requires further investigation.
In the study, we found that miR-203a-3p had no effect on the HIF-1α expression in the RLE-6TN cells, although we detected the down-regulation of HIF-1α in the blood samples from BPD patients. On the other hand, we found that activation of HIF-1α signaling was effective to down-regulate miR-203a-3p expression, which may imply that HIF-1α may act as an upstream mediator of miR-203a-3p. Studies by Cai et al., demonstrated hypoxia could down-regulate the expression of miR-203a-3p, which subsequently leads to the up-regulation of VEGFA in pulmonary microvascular endothelial cells (Cai et al. 2020). However, the interaction between HIF-1α and miR-203a-3p in the pathophysiology of BPD still requires further studies. The present study only examined the VEGFA as the target of miR-203a-3p; however, based on the bioinformatics prediction, other targets may be also regulated miR-203a-3p, which should be investigated in further studies. Though miR-203a-3p inhibitor could attenuate the development of BPD, the effects of miR-203a-3p on the development of BPD in the rats under normoxic condition have not been determined yet in our studies, and future studies should consider this option to confirm our findings. As VEGFA involves many downstream signaling pathways, the future studies investigating other signaling pathways are warranted. The clinical sample size is relatively small in our study, and the expression profiles of these targets may be confirmed in large clinical samples in the future.
In summary, our study, for the first time, demonstrated that that miR-203a-3p was down-regulated in the serum of BPD patients and in hyperoxia-induced BPD rat model. The in vitro studies showed that miR-203a-3p regulated the RLE-6TN cell apoptosis via targeting VEGFA. Further in vivo experiments indicated that miR-203a-3p knockdown alleviated hyperoxia-induced lung tissue damage in the BPD rat model, and its effect may be associated with the up-regulation of VEGF. The present study provides some preliminary insights into the novel role of miR-203a-3p in the pathophysiology of BPD. Further studies are warranted to determine the detailed molecular mechanisms associated with the role of miR-203a-3p in BPD.
Data availability
All the data generated in this study are available upon reasonable request from the corresponding author.
References
Ameis D, Khoshgoo N, Iwasiow BM, Snarr P, Keijzer R (2017) MicroRNAs in lung development and disease. Paediatr Respir Rev 22:38–43. https://doi.org/10.1016/j.prrv.2016.12.002
Arora S, Dev K, Agarwal B, Das P, Syed MA (2018) Macrophages: their role, activation and polarization in pulmonary diseases. Immunobiology 223(4–5):383–396. https://doi.org/10.1016/j.imbio.2017.11.001
Asikainen TM, Ahmad A, Schneider BK, Ho WB, Arend M, Brenner M, Günzler V, White CW (2005) Stimulation of HIF-1alpha, HIF-2alpha, and VEGF by prolyl 4-hydroxylase inhibition in human lung endothelial and epithelial cells. Free Radic Biol Med 38(8):1002–1013. https://doi.org/10.1016/j.freeradbiomed.2004.12.004
Bancalari E, Jain D (2018) Bronchopulmonary dysplasia: can we agree on a definition? Am J Perinatol 35(6):537–540. https://doi.org/10.1055/s-0038-1637761
Bhatt AJ, Pryhuber GS, Huyck H, Watkins RH, Metlay LA, Maniscalco WM (2001) Disrupted pulmonary vasculature and decreased vascular endothelial growth factor, Flt-1, and TIE-2 in human infants dying with bronchopulmonary dysplasia. Am J Respir Crit Care Med 164(10 Pt 1):1971–1980. https://doi.org/10.1164/ajrccm.164.10.2101140
Brener Dik PH, Nino Gualdron YM, Galletti MF, Cribioli CM, Mariani GL (2017) Bronchopulmonary dysplasia: incidence and risk factors. Arch Argent Pediatr 115(5):476–482. https://doi.org/10.5546/aap.2017.eng.476
Cai W, Liu S, Liu Z, Hou S, Lv Q, Cui H et al (2020) Downregulation of lung miR-203a-3p expression by high-altitude hypoxia enhances VEGF/notch signaling. Aging (Albany NY) 12(5):4247–4267. https://doi.org/10.18632/aging.102878
Chen L, Gao H, Liang J, Qiao J, Duan J, Shi H, Zhen T, Li H, Zhang F, Zhu Z, Han A (2018) miR-203a-3p promotes colorectal cancer proliferation and migration by targeting PDE4D. Am J Cancer Res 8(12):2387–2401
Cheng H, Wu B, Wang L, Hu T, Deng Z, Li D (2020) Insights into the expression profiles and functions of circRNAs in a newborn hyperoxia-induced rat bronchopulmonary dysplasia model. J Gene Med 22:e3163. https://doi.org/10.1002/jgm.3163
Claesson-Welsh L, Welsh M (2013) VEGFA and tumour angiogenesis. J Intern Med 273(2):114–127. https://doi.org/10.1111/joim.12019
Day CL, Ryan RM (2017) Bronchopulmonary dysplasia: new becomes old again! Pediatr Res 81(1–2):210–213. https://doi.org/10.1038/pr.2016.201
Durrani-Kolarik S, Pool CA, Gray A, Heyob KM, Cismowski MJ, Pryhuber G, Lee LJ, Yang Z, Tipple TE, Rogers LK (2017) miR-29b supplementation decreases expression of matrix proteins and improves alveolarization in mice exposed to maternal inflammation and neonatal hyperoxia. Am J Physiol Lung Cell Mol Physiol 313(2):L339–l349. https://doi.org/10.1152/ajplung.00273.2016
Gentle SJ, Lal CV (2019) Predicting BPD: lessons learned from the airway microbiome of preterm infants. Front Pediatr 7:564. https://doi.org/10.3389/fped.2019.00564
Kunig AM, Balasubramaniam V, Markham NE, Morgan D, Montgomery G, Grover TR, Abman SH (2005) Recombinant human VEGF treatment enhances alveolarization after hyperoxic lung injury in neonatal rats. Am J Physiol Lung Cell Mol Physiol 289(4):L529–L535. https://doi.org/10.1152/ajplung.00336.2004
Lal CV, Olave N, Travers C, Rezonzew G, Dolma K, Simpson A, Halloran B, Aghai Z, Das P, Sharma N, Xu X, Genschmer K, Russell D, Szul T, Yi N, Blalock JE, Gaggar A, Bhandari V, Ambalavanan N (2018) Exosomal microRNA predicts and protects against severe bronchopulmonary dysplasia in extremely premature infants. JCI Insight 3(5):e93994. https://doi.org/10.1172/jci.insight.93994
Levesque BM, Kalish LA, Winston AB, Parad RB, Hernandez-Diaz S, Phillips M, Zolit A, Morey JA, Gupta M, Mammoto A, Ingber DE, van Marter LJ (2013) Low urine vascular endothelial growth factor levels are associated with mechanical ventilation, bronchopulmonary dysplasia and retinopathy of prematurity. Neonatology 104(1):56–64. https://doi.org/10.1159/000351040
Liu HY, Zhang YY, Zhu BL, Feng FZ, Zhang HT, Yan H, Zhou B (2019) MiR-203a-3p regulates the biological behaviors of ovarian cancer cells through mediating the Akt/GSK-3beta/snail signaling pathway by targeting ATM. J Ovarian Res 12(1):60. https://doi.org/10.1186/s13048-019-0532-2
Liu X, Li K, Zhang F, Zhang Y, Deng C, Guo C (2020) Ablation of glutaredoxin 1 promotes pulmonary angiogenesis and alveolar formation in hyperoxia-injured lungs by modifying HIF-1alpha stability and inhibiting the NF-kappaB pathway. Biochem Biophys Res Commun 525:528–535. https://doi.org/10.1016/j.bbrc.2020.02.129
Maniscalco WM, Watkins RH, Pryhuber GS, Bhatt A, Shea C, Huyck H (2002) Angiogenic factors and alveolar vasculature: development and alterations by injury in very premature baboons. Am J Physiol Lung Cell Mol Physiol 282(4):L811–L823. https://doi.org/10.1152/ajplung.00325.2001
Principi N, Di Pietro GM, Esposito S (2018) Bronchopulmonary dysplasia: clinical aspects and preventive and therapeutic strategies. J Transl Med 16(1):36. https://doi.org/10.1186/s12967-018-1417-7
Samadi P, Saki S, Dermani FK, Pourjafar M, Saidijam M (2018) Emerging ways to treat breast cancer: will promises be met? Cell Oncol (Dordr) 41(6):605–621. https://doi.org/10.1007/s13402-018-0409-1
Sudhadevi T, Ha AW, Ebenezer DL, Fu P, Putherickal V, Natarajan V, Harijith A (2020) Advancements in understanding the role of lysophospholipids and their receptors in lung disorders including bronchopulmonary dysplasia. Biochim Biophys Acta Mol Cell Biol Lipids 1865(7):158685. https://doi.org/10.1016/j.bbalip.2020.158685
Syed M, Das P, Pawar A, Aghai ZH, Kaskinen A, Zhuang ZW, Ambalavanan N, Pryhuber G, Andersson S, Bhandari V (2017) Hyperoxia causes miR-34a-mediated injury via angiopoietin-1 in neonatal lungs. Nat Commun 8(1):1173. https://doi.org/10.1038/s41467-017-01349-y
Thebaud B, Ladha F, Michelakis ED, Sawicka M, Thurston G, Eaton F et al (2005) Vascular endothelial growth factor gene therapy increases survival, promotes lung angiogenesis, and prevents alveolar damage in hyperoxia-induced lung injury: evidence that angiogenesis participates in alveolarization. Circulation 112(16):2477–2486. https://doi.org/10.1161/circulationaha.105.541524
Wang Z, Zhao Z, Yang Y, Luo M, Zhang M, Wang X, Liu L, Hou N, Guo Q, Song T, Guo B, Huang C (2018) MiR-99b-5p and miR-203a-3p function as tumor suppressors by targeting IGF-1R in gastric cancer. Sci Rep 8(1):10119. https://doi.org/10.1038/s41598-018-27583-y
Xu JZ, Shao CC, Wang XJ, Zhao X, Chen JQ, Ouyang YX, Feng J, Zhang F, Huang WH, Ying Q, Chen CF, Wei XL, Dong HY, Zhang GJ, Chen M (2019) circTADA2As suppress breast cancer progression and metastasis via targeting miR-203a-3p/SOCS3 axis. Cell Death Dis 10(3):175. https://doi.org/10.1038/s41419-019-1382-y
Yang Y, Qiu J, Kan Q, Zhou XG, Zhou XY (2013) MicroRNA expression profiling studies on bronchopulmonary dysplasia: a systematic review and meta-analysis. Genet Mol Res 12(4):5195–5206. https://doi.org/10.4238/2013.October.30.4
Yin R, Yuan L, Ping L, Hu L (2016) Neonatal bronchopulmonary dysplasia increases neuronal apoptosis in the hippocampus through the HIF-1alpha and p53 pathways. Respir Physiol Neurobiol 220:81–87. https://doi.org/10.1016/j.resp.2015.09.011
Zhang Y, Dong X, Lingappan K (2019) Role of HIF-1alpha-miR30a-Snai1 Axis in neonatal Hyperoxic lung injury. Oxidative Med Cell Longev 2019:8327486–8327489. https://doi.org/10.1155/2019/8327486
Author information
Authors and Affiliations
Contributions
BW and DL conceived the study; LC and YW performed the data analysis and the experiments; TH edited the manuscript. All the authors approved the manuscript for submission.
Corresponding authors
Ethics declarations
Ethics approval
The study was approved by the Ethics Committee of Shenzhen People’s Hospital.
Statement of human and animal rights
The study was approved by the Ethics Committee of Shenzhen People’s Hospital. Human blood samples were obtained from these patients and written informed consent was obtained from the guardians of the patients.
Statement of informed consent
Not applicable.
Conflict of interest
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplemental Figure S1
MiR-203a-3p was regulated by HIF-1α signaling in RLE-6TN cells. (A) TargetScan analysis of the predicted binding sites between miR-203a-3p and HIF-1α 3’UTR. (B and C) qRT-PCR analysis of HIF-1α expression in RLE-6TN cells after being transfected with mimics-NC, miR-203a-3p mimics, inhibitor NC or miR-203a-3p inhibitor. (D) qRT-PCR analysis of miR-203a-3p expression in RLE-6TN cells after being treated with HIF-1α activator (DMOG). N = 3. *P < 0.05. (PNG 5473 kb)
Rights and permissions
About this article
Cite this article
Cheng, H., Chen, L., Wei, Y. et al. Knockdown of miR-203a-3p alleviates the development of bronchopulmonary dysplasia partly via the up-regulation of vascular endothelial growth factor A. J Bioenerg Biomembr 53, 13–23 (2021). https://doi.org/10.1007/s10863-020-09863-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10863-020-09863-3