Abstract
To discover the effect of tumor necrosis factor-alpha-induced protein 8-like 2 (TIPE2) on the oral tongue squamous cell carcinoma (OTSCC) via affecting FoxP3+ regulatory T (Treg) cells. Immunohistochemistry was conducted to examine TIPE2 and FoxP3 expressions in OTSCC tumor tissues and corresponding oral mucosa. Tca8113 cells were transfected with TIPE2/control lentiviral activation particles followed by the detection with qRT-PCR, Western blotting, MTT assay, Wound healing, Transwell assay and Annexin V-FITC/PI staining. In vivo experiment was carried out on the nude mice xenografts of OTSCC with TIPE2 overexpression to observe the tumor volume and survival, and the CD4+ T cell subgroups were detected by flow cytometry. TIPE2 was lower in the OTSCC tissues than the corresponding oral mucosa, which was correlated with T stage, N stage, TNM stage, and differentiation of patients. Patients with TIPE2-positive expression had worse prognosis and lower expression of FoxP3+ Treg cells than the negative ones. Furthermore, TIPE2 overexpression curbed proliferation, invasion and migration of Tca8113 cells, while cell apoptosis was increased. Besides, TIPE2 suppressed the tumor growth and extended the survival of OTSCC mice, with the decreased proportion of FoxP3+ Treg cells in the spleen and tumor-infiltrated lymphocytes (TILs). The clinical results showed the down-regulation of TIPE2 in OTSCC tissues. Meanwhile TIPE2 overexpression affected OTSCC cells biological behavior in vitro, as well as exhibited strong tumor-growth suppressive effects in vivo, which may be a potential therapeutic target in OTSCC via regulating FoxP3+ Treg cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the whole world, there were more than 275, 000 patients dying of the oral carcinoma and 2/3 these cases are from the developing countries, including Southeast Asia (Zhou et al. 2019). Oral tongue squamous cell carcinoma (OTSCC) took up 1.5% in cancer patients and 25–40% in cases of oral squamous cell carcinoma (OSCC) (Serpa et al. 2018; Naruse et al. 2015). At present, surgery remains the primary method for the treatment of oral carcinoma, usually supplemented with the chemotherapy or radiotherapy, which, however, is not so satisfactory in efficacy due to the local recurrence and distant metastasis of tumor, with a 5-year survival rate of about 60% (Zhang et al. 2019). In the meantime, surgical treatment also tremendously affects the life quality of patients via interfering on the lingual, chewing and swallowing functions (Nicoletti et al. 2004). Thus, we should put great efforts to illustrate OTSCC pathogenesis and discover the effective therapeutic and prognostic indicators for OTSCC.
The tumor necrosis factor-alpha-induced protein 8 (TNFAIP8 or TIPE) family is a group of genes closely associating with the inflammation and tumors, and four members, including TIPE (TNFAIP8), TIPE1 (TNFAIP8L1), TIPE2 (TNFAIP8L2) and TIPE3 (TNFAIP8L3), have been identified so far (Padmavathi et al. 2018). The increasing evidences suggested TIPE2 as an oncogene or tumor suppressor in the pathogeneses of diseases. For instance, TIPE2 overexpression inhibits the malignancies of the human rectal adenocarcinoma cells, while its deficiency manifested the opposite changes (Wu et al. 2019). Moreover, Bordoloi D et al. supported that TIPE2 deficiency can curb the malignancies of lung cancer via regulating the Akt/mTOR/NF-κB signal pathway, with the arrest of cell cycle in S phase (Bordoloi et al. 2019). However, there remains little evidence illustrating the role of TIPE2 in OTSCC. Of note, the massive infiltration of immunosuppressive cells in tumor is usually correlated with the poor prognosis (Tsubaki et al. 2018), while the removal of these cells can induce and enhance the anti-tumor immune responses (Rabinovich et al. 2007). Treg cells, as a group of T cell subgroup widely spread in the peripheral blood, tissues and organs in humans with the immunosuppressive function, play key roles in maintaining the autoimmune system and immune responses, while FoxP3 is a specific marker of Treg cells (Wu et al. 2012; Wei et al. 2015), which may also play roles in OTSCC (Hanakawa et al. 2014). More importantly, TIPE2, a key element in the dynamic balance of immune responses (Li et al. 2016), is also a critical immune-regulatory molecule involved in the immunosuppressive function of CD4+CD25+Tregs (Luan et al. 2011). Besides, Ding J and his group revealed TIPE2 depletion could increase the Treg cells with suppressed Foxp3, thus abolishing the effects of Crocetin on the severity of asthma (Ding et al. 2015). Furthermore, Treg cells were demonstrated to be negatively regulated by TIPE2 in the study of Fan T et al. (Fan et al. 2018). Nevertheless, there remains no adequate evidence suggesting the involvement of TIPE2 in OTSCC by regulating Treg cells. Thus, we tried our best to find the answer to improve the treatment for of OTSCC.
Material and methods
Ethical statement
Prior patients’ consent was obtained from all subjects, and the source and use of tissue specimens were approved by the Ethical Committee of our hospital for research purposes. Besides, all animal experiments conformed to the principals for the management and use of laboratory animals, ethical requirement for animals, and the Guide for the Care and Use of Laboratory Animals (Mason and Matthews 2012).
Subjects
Between October 2012 and October 2014, a total of 53 OTSCC patients including 35 males and 18 females were prospectively enrolled with a median age of 50 years aging from 30 to 78 years, for which immunohistochemistry stainings were performed. Inclusion criteria: Patients with the diagnosis of OTSCC confirmed by clinical and pathological examinations; patients with the intact case report, pathological test results and follow-up data; patients claiming no history of anti-tumor therapy before surgery. From these patients, the adjacent normal oral mucosa was also collected. The histopathological grade and tumor-node-metastasis (TNM) stage was classified according to the WHO classification (Kosmehl 2006) and TNM classification of malignant tumor (7th edition) (Montes 2010), respectively. Following surgery, all patients were required to participate in the follow-up lasting for 60 months. During follow-up, the survival time was counted from the day of admission to the day of death, while intercept value was counted for patients dying of other diseases, or accident, or still alive at the end of follow-up.
Immunohistochemistry staining
The prospective series of 53 OTSCC tissues and the adjacent normal oral mucosa were used for TIPE2 and FoxP3 immunohistochemistry staining. Specimens were fixed in 4% formaldehyde, dehydrated in the ethanol at gradient concentrations and then embedded in the paraffin for being sliced into the sections in thickness of 5 μm. The sections, following the regular de-paraffinizing, antigen retrieval and blocking in the blocking buffer, were treated by the anti-TIPE2 antibody (1/100, ab110389, Abcam, USA) and anti-FoxP3 antibody (5 μg/ml, ab20034, Abcam, USA) at 4 °C overnight. Following washes in PBST (PBS with 0.1% Tween-20), tissues were then incubated with the HRP-conjugated IgG polyclonal (ab6721, Abcam, USA) at 37 °C for 2 h, and the results were further detected by incubating with the avidin-biotin peroxidase complex (ABC) at 37 °C for 1 h and diaminobenzidine (DAB) for the development. Then, the sections were mounted and placed under the microscope for observation of the 4 randomly selected visions. A semi-quantitative method was used to evaluate TIPE2 expression. The staining intensity: 0 point for negative, 1 point for week, 2 points for moderate, and 3 points for strong. The percentage of positive cells: 0 point for 0, 1 point for 1–25%, 2 points for 26–50%, 3 points for 51–75%, and 4 points for 76–100%. A combined staining score for each slide was combined to get a final TIPE2 expression: > 3 points (Zhang et al. 2019; Nicoletti et al. 2004; Padmavathi et al. 2018; Wu et al. 2019) were deemed as the positive expression of TIPE2, and ≤ 3 points (0–3) as the negative expression of TIPE2 (Jia et al. 2018; Zhang et al. 2016). The FoxP3+ Treg cells stained along the tumor invasive front in each field were counted, and the total count of FoxP3+ Treg cells was determined, which was used to calculate the mean number of Treg cells per case (Gonzaga et al. 2019).
Cell culture and grouping
The human OTSCC cells (Tca8113) purchased from American Type Culture Collection (ATCC, Manassas, VA, USA) were cultured in regular RPMI-1640 medium at 37 °C with 5% CO2. After reaching 80% confluence, cells were digested in 0.25% trypsin for passaging for following experiments and divided into three groups: Blank group (without transfection), Control group (cells transfected with Control lentiviral activation particles, sc-437282, Santa Cruz Biotechnology, Santa Cruz, CA, USA) and TIPE2 group (cells transfected with TIPE2 lentiviral activation particles, sc-407091-LAC, Santa Cruz Biotechnology, Santa Cruz, CA, USA). The transfection was performed by using Ultracruz® reagent (sc-395739, Santa Cruz Biotechnology, Santa Cruz, CA, USA).
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay
Cells in logarithmic phase were inoculated in a 96-well plate at density of 5.0 × 103 /well, with 100 μL in each well, followed by 24 h culture. After confluence, cells were transfected for 12, 24, 48 and 96 h respectively, and then incubated with MTT solution (Sigma-Aldrich Co., St Louis, MO, USA) at ratio of 1:10 at 37 °C for 3 to 4 h. MTT solution was then replaced by dimethylsulfoxide (DMSO) for detecting the optical density (OD) values at the wavelength of 490 nm of each well by using the microplate reader (BioTek, Vermont, USA). This experiment was repeated for three times.
Wound healing assay
Forty eight hours after transfection, a tip was used to draw 5 lines, where the cells were rinsed with PBS for three times to remove the floating cells. Thereafter, the serum-free DMEM medium was added into the cells for culture at 37 °C in 5% CO2. Following culture, cells in the fixed visions were photographed, and the percentage of the scratched zone closure at 48 h compared with time 0 was measured using ImagePro Plus software (version 6.2, Media Cybernetics, Silver Spring, USA). This experiment was conducted in triplicate.
Transwell assay
Matrigel was diluted in 1:7 by using the serum-free medium, and the chambers that were coated with the matrigel were placed at 37 °C for 4 h. Cells, following digestion in trypsin, were prepared into the single cell suspension, and after two washes in PBS, cells were resuspended in the serum-free medium, where the cell density was adjusted to 1 × 105/mL. Suspension in volume of 100 μL was slowly dropped in the matrigel-coated upper chambers, while in the lower chambers, 500 μL medium supplemented with 10% fetal bovine serum was added. Following 10 to 12 h of culture at 37 °C, chambers were taken out and the cells failing to pass through the membrane were scrubbed by using a cotton swab. The harvested cells were fixed in 95% ethanol, and stained in 0.1% crystal violet for 10 min. Following two or three washes in PBS, cells were dried in air, and the chambers were inverted on the slides that were placed under the fluorescent microscope to select 5 randomly visions (× 200) to calculate and photograph these cells. This experiment was conducted in triplicate.
Annexin V-FITC/PI staining
The cell apoptosis was detected by Alexa Fluor 488™ Annexin V and propidium iodide (PI) assay kit (Abbkine, Wuhan, China). In brief, after 48 h transfection, cells were digested and centrifuged, and the sediment was washed in icy PBS twice. Cells in each well were resuspended in 400 μL binding buffer, and filtrated into the flow cytometry tubes. Thereafter, cells were incubated in 5 μL Annexin V and 10 μL PI for flow cytometry. Annexin V+ PI+: cells in late apoptosis; Annexin V+ PI−: cells in early apoptosis. Cells apoptosis rate (%) = cells rate in early apoptosis (Annexin V+ PI−) + cells rate in late apoptosis (Annexin V+ PI+) (Yu et al. 2012).
qRT-PCR
Cells, after being transfected for 48 h, were subjected to the extraction of total RNA by using the TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and the measurement of concentration and purity of total RNA by using the ultraviolet spectrometry. As per the instruction of M-MLV reverse transcription kit (Invitrogen, Carlsbad, CA, USA), cDNA was prepared with the extracted RNA, while the amplified primers were designed in Primer Premier 5.0 software, and synthesized by Shanghai Sangon Biotech Co., Ltd. (Shanghai, China). TIPE2: 5’-ACTGAGTAAGATGGCGGGTCG-3′ (forward) and 5-TTCTGGCGAAAGCGGGTAG-3′ (reverse); GAPDH: 5’-AACGGATTTGGTCGTATTGGG-3′ (forward) and 5’-CCTGGA AGATGGTGATGGGAT-3 (reverse). PCR procedures were carried out on a Roche 480 PCR apparatus, where the mRNA expression of TIPE2 was measured using the FastStart Universal SYBR Green Master (ROX) (Roche Diagnostics, Mannheim, Germany), with GAPDH as the internal reference. The final mRNA expression of TIPE2 was quantified by the method of 2-△△Ct.
Western blotting
After being transfected for 48 h, cells in each group were washed in cold PBS twice, lysed on ice for 30 min and centrifuged to obtain the supernatant, where the protein concentration was measured by the bicinchoninic acid (BCA) kit (Sigma, St. Louis, MO, USA). Protein samples were then boiled in the 5 × protein loading buffer, cooled to the room temperature and preserved at −20 °C. Following the regular procedures of electrophoresis, membrane-transferring, blocking and washing, proteins on the membrane were then incubated with anti-TIPE2 antibody (1/500, ab110389, Abcam, USA) and anti-β-actin antibody-loading control (1/1000, ab8226, Abcam, USA) at 4 °C overnight, and then the IgG H&L (HRP) (ab205718, 1 μg/ml, Abcam, USA) at room temperature for 2 h. Then, the enhanced chemiluminescence reagent was added on the membrane to develop the protein bands, which were further detected by the ImageQuantTMLAS-4000 apparatus. Intensity of bands of TIPE2 and β-actin was quantified in Image J software, and the relative expression of TIPE2 was expressed by the ratio of intensity of TIPE2 band to that of β-actin.
Construction of OTSCC mouse model
A total of 20 SPF, BALB/C, male mice (5-week-old, purchased from Shanghai SLAC Laboratory Animal Co., Ltd., Shanghai, China) were randomized into two groups, with 10 mice in each group. Initially, mice were anesthetized by intraperitoneal injection of 4% chloral hydrate for subcutaneous inoculation of Tc8113 cells (1 × 107/mouse) that were transfected by the TIPE2 lentiviral activation particles or Control lentiviral activation particles. Tumor measurement was carried out once every 5 days by using the vernier caliper. Tumor volume was calculated according to the formula L × W2 × 0.5 (L = length; W = width; all parameters in millimeters). Measurement was ended by the day of death of mouse.
T cell subgroup analysis with flow cytometry
Spleen tissues of mouse were collected to prepare the spleen cell suspension, where the Red Blood Cell Lysis Buffer (Sigma, USA) was added. Cells were then rinsed in PBS for three times, and then resuspended in FACS staining buffer (PBS containing 0.5% BSA). Tumor tissues were then collected for the preparation of single cell suspension, and mixed with the aforementioned suspension in ratio of 1:1. The mixture was dropped on the surface of Ficoll solution for centrifugation to collect cells, followed by being washed in PBS and resuspended in the RPMI-1640 medium supplemented with 10% FBS. Cells were stained by the different labeling antibodies, e.g. anti-CD4 antibody (clone RM4–5, BD Biosciences, San Jose, CA, USA), anti-CD8 antibody (clone53–6.7, BD Biosciences, San Jose, CA, USA) and anti-CD25 antibody (clone PC61, BD Biosciences, San Jose, CA, USA). For analysis of cytokines, cells in the presence of Golgi stop were cultured with byphorbol-12-myristate 13-acetate (PMA) and ionomycin to inhibit the secretion of the cytokines, followed by the washing with staining buffer, fixing and permeabilizing with BD cytofix and cytoperm™ Plus (BD Biosciences) along with 0.2% Tween 20 for 20 min. Next, cells were stained with anti-IL4 (clone 11B11, BD Biosciences, San Jose, CA, USA), anti-IFN-γ (clone 11B11, BD Biosciences, San Jose, CA, USA), anti-IL-17A (clone TC11-18H10, BD Biosciences, San Jose, CA, USA) and anti-Foxp3 (clone MF23, BD Biosciences, San Jose, CA, USA). Brilliant stain buffer and ultra-comp beads were used for the dilution of the stains and compensation respectively. All the stained cells were analyzed by flow cytometer and the data was evaluated by FlowJo software (FlowJo, Ashland, OR, USA).
Statistical methods
Data analysis was performed by using the SPSS 20.0 software (Chicago, IL, USA). Count data, expressed in form of percentage or rate, were compared by χ2 test. Survival analysis was conducted by using the Kaplan-Meier method and the Log-rank test. Measurement data were presented in form of mean ± standard deviation, compared by using the t test between either two groups, or the one-way analysis of variance (ANOVA) followed by Tukey’ s post hoc test among groups. P < 0.05 suggested the difference was statistically significant.
Results
The expression of TIPE2 and FoxP3+ Treg cells in the OTSCC tissue and its correlation with the pathological feature of patients.
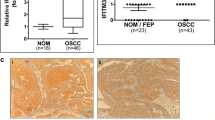
The result of immunohistochemistry revealed that TIPE2 expression was observed in tumor and stromal cells (Fig. 1a-d). A sharp decrease in the positive expression of TIPE2 was found in the in OTSCC tissues as compared with the oral mucosa (both P = 0.002, Fig. 1e). As compared to OTSCC patients with positive TIPE2 expression, those with the negative TIPE2 expression had a lower survival rate (33.3% vs. 65.4%, χ2 = 7.101, P = 0.008, Fig. 1f). Besides, in all 53 OTSCC patients, FoxP3+ Treg cells were detected along the tumor invasive front with the mean numbers ranged from 2 to 64.6. And the OTSCC patients with positive TIPE2 expression exhibited much lower expression of FoxP3+ Treg cells than those with negative expression of TIPE2 (P < 0.05, Fig. 2). Meanwhile, the expression of TIPE2 and FoxP3+ Treg cells was in an obvious correlation with the T stage, N stage, TNM stage, and differentiation degree of OTSCC patients (all P < 0.05), instead of the gender, age, smoking, primary site of involvement or treatment (all P > 0.05, Table 1 and Fig. 2).
The expression of TIPE2 in the OTSCC tissue and its correlation with the prognosis of patients (n = 53), Notes, a: TIPE2 showed a membranous defined staining pattern in OTSCC cells; b: Different intensities of TIPE2 staining in OTSCC cells (black arrow: week; red arrow: moderate); c: The red arrow indicated an area with moderate stromal staining and the black arrow indicated stromal staining at the invasive front; d: At the invasive front, OTSCC cells showed strong epithelial membranous staining (black arrow) and the stromal cells with moderate staining (red arrow); SCC: surrounding the OTSCC tissue; e: Comparison of the TIPE2 expressions in OTSCC tissues and oral mucosa; f: Correlation of TIPE2 expression in tumor tissue with the prognosis of OTSCC patients
Association of FoxP3+ Treg in the OTSCC tissue with the pathological features and with the expression of TIPE2, Notes, The histopathological grade was classified according to the WHO classification. The tumor-node-metastasis (TNM) staging was determined based on the TNM classification of malignant tumor (7th edition)
TIPE2 inhibits the migration and invasion of OTSCC cells in-vitro with the promoted apoptosis
The transfection of TIPE2 lentiviral activation particles in the Tca8113 cells resulted in an elevation in the expression of TIPE2 as detected by qRT-PCR and Western blotting (all P < 0.05, Fig. 3a-b). The result of MTT assay showed that the TIPE2 lentiviral activation particle-transfected Tce8113 cells had lower cell viability than those with control lentiviral activation particles (all P < 0.05, Fig. 3c). Moreover, TIPE2 overexpression reduced the malignancies of OTSCC cells with decreased cell abilities of invasion and migration using Transwell invasion and Wound healing assays (all P < 0.05, Fig. 3d-e). Furthermore, Annexin V-FITC/PI staining demonstrated a sharp increase in the apoptosis of Tca8113 cells in the TIPE2 group, as compared to the Control group and the Blank group (all P < 0.05, Fig. 3f).
TIPE2 inhibits the migration and invasion of OTSCC cells with the promoted apoptosis, Notes, a-b: The TIPE2 expression in Tca8113 cells detected by qRT-PCR (a) and Western blotting (b); c: The Tca8113 cells proliferation detected by MTT assay; d: The Tca8113 cells migration detected by Wound healing; e: The Tca8113 cells invasion detected by Transwell invasion assay; f: The Tca8113 cells apoptosis detected by Annexin V-FITC/PI staining; * P < 0.05 vs. the Blank group; # P < 0.05 vs. the Control group
TIPE2 decreases the in-vivo tumorigenicity of Tca8113 cells
The subcutaneous inoculation of TIPE2/control lentiviral activation particles-transfected Tca8113 cells was performed to establish the tumor models for the BALB/c mice. As the result showed in Fig. 4, the tumor volume of TIPE2-overexpressed mouse was smaller, and TIPE2 overexpression could prolong the survival of tumor-beared mice (all P < 0.05 s,). As shown in the results of FCM analysis, TIPE2 could not affect the levels of Th1, Th2, Th17 cells (all P > 0.05, Fig. 5a-b), but decrease the levels of FoxP3+ Treg cells in the spleen and TILs (P < 0.05, Fig. 5c-e).
Effect of TIPE2 on the Treg cell in the tumor microenvironment of OTSCC mice (n = 10), Note: a-b: Quantitation of CD4, Th1, Th2, Th17 cells population from spleen (a) and tumor infiltrating lymphocyte (TIL) (b) respectively; c: Representative dot plot distribution of FoxP3+ Treg cell population; d-e: Quantitation of FoxP3+ Treg cell population in spleens (d) and TIL (e)
Discussion
According to the published literature, TIPE2, as an immune negative regulator newly identified recently, was found to be reduced in various solid tumors and relevant cell lines, including gastric cancer (Liu et al. 2018), esophageal cancer (Zhu et al. 2018), breast cancer (Wang et al. 2017) or liver cancer (Wang et al. 2016; Gus-Brautbar et al. 2012). Consistent with other tumors, the expression of TIPE2 in our study was significantly reduced in the OTSCC tissues, as compared to those in the surrounding normal oral mucosa tissue, showing that TIPE2 may affect the development of OTSCC. At the same time, the down-regulated TIPE2 was also demonstrated in the peripheral blood monocytes (PBMC) in a variety of chronic inflammatory diseases (Fan et al. 2015; Jia et al. 2013; Ma et al. 2013), and its down-regulation during the course of inflammation was possibly owing to its altered epigenetic status, which in turn increased the related inflammation (Bordoloi et al. 2018). Importantly, chronic inflammation is one of the causative factors in cancer development, including OTSCC (Kheirollahi n.d.). And there was evidence supported that TIPE2 expression could be suppressed by inflammatory signals, especially in higher stage and poorer differentiation of tumor, thus contributing to inflammation-induced pathologies, suggesting the possible role of TIPE2 as a tumor suppressor in OTSCC (Gus-Brautbar et al. 2012). The expression of TIPE2 protein was noted by Hao C et al., which could be a predictor of better prognosis for diffuse large B cell lymphoma (Hao et al. 2016), and the down-regulation of TIPE2 in non-small cell lung cancer was actually demonstrated to be correlated with the TNM stage and poor prognosis in the advanced stage (Li et al. 2015). Notably, TIPE2 expression presented an association with T stage, N stage, TNM stage, and the differentiation degree of OTSCC patients in our study, and the patients with the high expression of TIPE2 had good prognosis, further exhibiting the tumor-suppressive role of TIPE2 against OTSCC progression.
Findings of the in-vitro experiments also indicated the potential role of TIPE2 in reducing the malignancies of human rectum adenocarcinoma cells (Wu et al. 2019), and the restoration of TIPE2 expression in gastric cell suppressed its cell proliferation via selectively up-regulation of N-ras and p27 (Zhao et al. 2015). As reported, TIPE2 was thought to inhibit the oncogenic Ras, and its absence enhanced the cell migration (Gus-Brautbar et al. 2012). According to the work from Cao X et al., TIPE2 can target the Rac1 to inhibit the migration and invasion of liver cancer with the down-regulation of MMP-9 and uPA (Cao et al. 2013). Depletion of TIPE2 could elevate the levels of MMP13 and the cell invasiveness of SaOS-2 osteosarcoma cells (Deng et al. 2015). Liu QQ et al. also reported that TIPE2 can alter the expressions of apoptosis-associated molecules, including Caspase-3, Caspase-9, Bcl-2 and Bax, thereby enhancing the apoptosis of lung cancer cells (Liu et al. 2015). Due to the DED domain, TIPE2 can be negatively regulated by Caspase-8, thereby being involved in the apoptosis (Oho et al. 2016). Similarly, in our work, the human Tca8113 cell line was transfected with the TIPE2 lentiviral activation particles, resulting in the decreases in the proliferation, invasion and migration of cells, and enhanced apoptosis, which was also supported by the in-vivo experiment that, TIPE2 overexpression could slow down the tumor growth rate and extend the survival of tumor-bearing mice, suggesting the possible involvement of TIPE2 as a potential therapeutic target in OTSCC.
Tumor development also shows that this is greatly associated with the human immune system, mainly the T lymphocytes (Robbins 2017) that are divided into CD4+T cells and CD8+T cells, according to the different surface markers (Borst et al. 2018). For CD4+T cells, they were further categorized into Th1, Th2, Th17 and Tregs based on the differential function and secreted cytokines (Gu-Trantien et al. 2013). Tregs can suppress the innate anti-tumor immune responses, while facilitate tumor progression (Rajendran et al. 2019). Foxp3, as a new member of Forkhead/winged helix, is widely accepted as the Treg-specific marker that can regulate the development, differentiation and maturation of Treg cells (Sadahiro et al. 2019). TIPE2, as the cytoplasmic protein, can regulate the signal transduction mediated by the T cell receptor and Toll-like receptor to maintain the immune homeostasis system (Luan et al. 2011). In our TIPE2-positive patients, the expression of FoxP3+ in Tregs was much lower than those TIPE2-negative patients, indicating that TIPE2 may negatively regulate the FoxP3+ Treg in OTSCC patients. To further demonstrate the effect of TIPE2 on Tregs, we detected the levels of CD4+ T cells in the spleen cell and TILs of OTSCC mouse, and TIPE2 overexpression could decrease the Tregs in spleen and TIL, but with no obvious alteration of Th1, Th2 and Th17 cell. Nevertheless, in TIPE2 deficient mouse, accumulation of more Treg cells was identified in the thymus, as exhibited by Fan T et al. (Fan et al. 2018), indirectly indicating that TIPE2 overexpression can suppress the Treg cells to curb its immunosuppressive function, thereby exerting the therapeutic effects on tumors.
Overall, TIPE2 was down-regulated in OTSCC and was associated with major clinical characteristics and prognosis of patients. Furthermore, TIPE2-positive OTSCC patients had the lower FoxP3+ Treg than that of TIPE2-negative patients. TIPE2 could reduce the malignant features of OTSCC cells, and enhance the cell apoptosis in vitro, which also could suppress the tumor growth in OTSCC mice with the decrease in the ratio of Treg cell in the spleen and TIL. However, there existed several limitations in our experiments: Firstly, further studies in larger population with more detailed complete clinicopathological features are needed to confirm the findings. Secondly, the association of Tregs with OTSCC cell proliferation, invasion, migration and apoptosis would be further explored in the future due to time and funding constraints.
References
Bordoloi D, Banik K, Shabnam B, Padmavathi G, Monisha J, Arfuso F, et al. (2018) TIPE Family of Proteins and Its Implications in Different Chronic Diseases. Int J Mole Sci. 19(10)
Bordoloi D, Banik K, Padmavathi G, Vikkurthi R, Harsha C, Roy NK, et al. (2019) TIPE2 Induced the Proliferation, Survival, and Migration of Lung Cancer Cells Through Modulation of Akt/mTOR/NF-kappaB Signaling Cascade. Biomolecules. 9(12)
Borst J, Ahrends T, Babala N, Melief CJM, Kastenmuller W (2018) CD4(+) T cell help in cancer immunology and immunotherapy. Nat Rev Immunol 18(10):635–647
Cao X, Zhang L, Shi Y, Sun Y, Dai S, Guo C, Zhu F, wang Q, Wang J, Wang X, Chen YH, Zhang L (2013) Human tumor necrosis factor (TNF)-alpha-induced protein 8-like 2 suppresses hepatocellular carcinoma metastasis through inhibiting Rac1. Mol Cancer 12(1):149
Deng B, Feng Y, Deng B (2015) TIPE2 mediates the suppressive effects of Shikonin on MMP13 in osteosarcoma cells. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology 37(6):2434–2443
Ding J, Su J, Zhang L, Ma J (2015) Crocetin activates Foxp3 through TIPE2 in asthma-associated Treg cells. Cellular Physiol Biochem: Int J Exp Cell Physiol, Biochem, Pharm 37(6):2425–2433
Fan YC, Wang N, Sun YY, Xiao XY, Wang K (2015) TIPE2 mRNA level in PBMCs serves as a novel biomarker for predicting short-term mortality of acute-on-chronic hepatitis B liver failure: a prospective single-center study. Medicine (Baltimore) 94(39):e1638
Fan T, Huang X, Liu C, Liu R, Wang T, Ruan Q (2018) Egress of murine regulatory T cells from the thymus requires TIPE2. Biochem Biophys Res Commun 500(2):376–383
Gonzaga AKG, Santos HBP, Crispim JCO, Souza LB, Palomino GM (2019) Immunohistochemical evaluation of HLA-G and FoxP3+ T regulatory cells in oral cavity and lower lip squamous cell carcinomas. Brazilian oral research 33:e020
Gus-Brautbar Y, Johnson D, Zhang L, Sun H, Wang P, Zhang S, Zhang L, Chen YH (2012) The anti-inflammatory TIPE2 is an inhibitor of the oncogenic Ras. Mol Cell 45(5):610–618
Gu-Trantien C, Loi S, Garaud S, Equeter C, Libin M, de Wind A, Ravoet M, le Buanec H, Sibille C, Manfouo-Foutsop G, Veys I, Haibe-Kains B, Singhal SK, Michiels S, Rothé F, Salgado R, Duvillier H, Ignatiadis M, Desmedt C, Bron D, Larsimont D, Piccart M, Sotiriou C, Willard-Gallo K (2013) CD4(+) follicular helper T cell infiltration predicts breast cancer survival. J Clin Invest 123(7):2873–2892
Hanakawa H, Orita Y, Sato Y, Takeuchi M, Ohno K, Gion Y, Tsukahara K, Tamamura R, Ito T, Nagatsuka H, Nishizaki K, Yoshino T (2014) Regulatory T-cell infiltration in tongue squamous cell carcinoma. Acta Otolaryngol 134(8):859–864
Hao C, Zhang N, Geng M, Ren Q, Li Y, Wang Y, Chen YH, Liu S (2016) Clinical significance of TIPE2 protein Upregulation in non-Hodgkin's lymphoma. J Histochem Cytochem: Off J Histochem Soc 64(9):556–564
Jia L, Gui B, Tian P, Yao G, Fu R, Wang L, Ge H, Ou Y (2013) TIPE2, a novel biomarker for clinical chronic kidney allograft rejection. Artif Organs 37(2):221–225
Jia W, Li Z, Chen J, Sun L, Liu C, Wang S, Chi J, Niu J, Lai H (2018) TIPE2 acts as a biomarker for tumor aggressiveness and suppresses cell invasiveness in papillary thyroid cancer (PTC). Cell & bioscience 8:49
Kheirollahi K Correlation between chronic inflammation and oral squamous cell carcinoma (OSCC). Oral Oncol 50(10):e52
Kosmehl H (2006) Current classification of precursor lesions of oral squamous cell carcinoma principles of the WHO classification 2005. Mund Kiefer Und Gesichtschirurgie 10(2):89–93
Li Y, Li X, Liu G, Sun R, Wang L, Wang J, Wang H (2015) Downregulated TIPE2 is associated with poor prognosis and promotes cell proliferation in non-small cell lung cancer. Biochem Biophys Res Commun 457(1):43–49
Li Z, Guo C, Liu X, Zhou C, Zhu F, Wang X, Wang Q, Shi Y, Wang J, Zhao W, Zhang L (2016) TIPE2 suppresses angiogenesis and non-small cell lung cancer (NSCLC) invasiveness via inhibiting Rac1 activation and VEGF expression. Oncotarget. 7(38):62224–62239
Liu QQ, Zhang FF, Wang F, Qiu JH, Luo CH, Zhu GY, Liu YF (2015) TIPE2 inhibits lung Cancer growth attributing to promotion of apoptosis by regulating some apoptotic molecules expression. PLoS One 10(5):e0126176
Liu W, Fan Y, Shi Y, Lin Z, Huang X, Huang W, Shen D, Qi Z (2018) Knockdown of TIPE2 increases the proliferation in lipopolysaccharide-stimulated gastric cancer cells. BMC Cancer 18(1):857
Luan YY, Yao YM, Zhang L, Dong N, Zhang QH, Yu Y, Sheng ZY (2011) Expression of tumor necrosis factor-alpha induced protein 8 like-2 contributes to the immunosuppressive property of CD4(+)CD25(+) regulatory T cells in mice. Mol Immunol 49(1–2):219–226
Ma Y, Liu X, Wei Z, Wang X, Wang Z, Zhong W, Li Y, Zhu F, Guo C, Zhang L, Wang X (2013) The expression and significance of TIPE2 in peripheral blood mononuclear cells from asthmatic children. Scand J Immunol 78(6):523–528
Mason TJ, Matthews M (2012) Aquatic environment, housing, and management in the eighth edition of the guide for the care and use of laboratory animals: additional considerations and recommendations. J Am Assoc Lab Anim Sci 51(3):329–332
Montes HZ (2010) TNM Classification of Malignant Tumors, 7th edition. Int J Rad Oncol Biol Physics 78(4):1278
Naruse T, Yanamoto S, Yamada SI, Takahashi H, Matsushita Y, Imayama N et al (2015) Immunohistochemical study of vascular endothelial growth factor-C/vascular endothelial growth factor receptor-3 expression in oral tongue squamous cell carcinoma: correlation with the induction of lymphangiogenesis. Oncol Lett 10(4):2027–2034
Nicoletti G, Soutar DS, Jackson MS, Wrench AA, Robertson G (2004) Chewing and swallowing after surgical treatment for oral cancer: functional evaluation in 196 selected cases. Plast Reconstr Surg 114(2):329–338
Oho M, Nakano R, Nakayama R, Sakurai W, Miyamoto A, Masuhiro Y, Hanazawa S (2016) TIPE2 (tumor necrosis factor alpha-induced protein 8-like 2) is a novel negative regulator of TAK1 signal. J Biol Chem 291(43):22650–22660
Padmavathi G, Banik K, Monisha J, Bordoloi D, Shabnam B, Arfuso F, Sethi G, Fan L, Kunnumakkara AB (2018) Novel tumor necrosis factor-alpha induced protein eight (TNFAIP8/TIPE) family: functions and downstream targets involved in cancer progression. Cancer Lett 432:260–271
Rabinovich GA, Gabrilovich D, Sotomayor EM (2007) Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol 25:267–296
Rajendran M, Looney S, Singh N, Elashiry M, Meghil MM, El-Awady AR et al (2019) Systemic antibiotic therapy reduces circulating inflammatory dendritic cells and Treg-Th17 plasticity in periodontitis. J Immunol 202(9):2690–2699
Robbins PF (2017) Tumor-infiltrating lymphocyte therapy and Neoantigens. Cancer J 23(2):138–143
Sadahiro S, Suzuki T, Tanaka A, Okada K, Saito G, Miyakita H et al (2019) Induction of CD3+ and FoxP3+ T cells in left-sided colorectal tumors after UFT/LV chemotherapy. Anticancer Res 39(4):1997–2005
Serpa MS, Mafra RP, Queiroz S, Silva LPD, Souza LB, Pinto LP (2018) Expression of urokinase-type plasminogen activator and its receptor in squamous cell carcinoma of the oral tongue. Brazilian oral research. 32:e93
Tsubaki T, Kadonosono T, Sakurai S, Shiozawa T, Goto T, Sakai S, Kuchimaru T, Sakamoto T, Watanabe H, Kondoh G, Kizaka-Kondoh S (2018) Novel adherent CD11b(+) gr-1(+) tumor-infiltrating cells initiate an immunosuppressive tumor microenvironment. Oncotarget. 9(13):11209–11226
Wang Y, Jiang Y, Zhou J, Song W, Li J, Wang M, Chen J, Xu R, Zhang J, Ma F, Chen YH, Ma Y (2016) Hepatitis C virus promotes hepatocellular carcinogenesis by targeting TIPE2, a new regulator of DNA damage response. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine 37(11):15265–15274
Wang K, Ren Y, Liu Y, Zhang J, He JJ (2017) Tumor necrosis factor (TNF)-alpha-induced protein 8-like-2 (TIPE2) inhibits proliferation and tumorigenesis in breast Cancer cells. Oncol Res 25(1):55–63
Wei T, Zhang J, Qin Y, Wu Y, Zhu L, Lu L, Tang G, Shen Q (2015) Increased expression of immunosuppressive molecules on intratumoral and circulating regulatory T cells in non-small-cell lung cancer patients. Am J Cancer Res 5(7):2190–2201
Wu T, Zhang L, Xu K, Sun C, Lei T, Peng J, Liu G, Wang R, Zhao Y (2012) Immunosuppressive drugs on inducing Ag-specific CD4(+)CD25(+)Foxp3(+) Treg cells during immune response in vivo. Transpl Immunol 27(1):30–38
Wu DD, Liu SY, Gao YR, Lu D, Hong Y, Chen YG, Dong PZ, Wang DY, Li T, Li HM, Ren ZG, Guo JC, He F, Ren XQ, Sun SY, Duan SF, Ji XY (2019) Tumour necrosis factor-alpha-induced protein 8-like 2 is a novel regulator of proliferation, migration, and invasion in human rectal adenocarcinoma cells. J Cell Mol Med 23(3):1698–1713
Yu T, Wu Y, Huang Y, Yan C, Liu Y, Wang Z, Wang X, Wen Y, Wang C, Li L (2012) RNAi targeting CXCR4 inhibits tumor growth through inducing cell cycle arrest and apoptosis. Molecular therapy : the journal of the American Society of Gene Therapy 20(2):398–407
Zhang Z, Liu L, Liu C, Cao S, Zhu Y, Mei Q (2016) TIPE2 suppresses the tumorigenesis, growth and metastasis of breast cancer via inhibition of the AKT and p38 signaling pathways. Oncol Rep 36(6):3311–3316
Zhang Q, Huang D, Zhang Z, Feng Y, Fu M, Wei M et al (2019) High expression of TMEM40 contributes to progressive features of tongue squamous cell carcinoma. Oncol Rep 41(1):154–164
Zhao Q, Zhao M, Dong T, Zhou C, Peng Y, Zhou X, Fan B, Ma W, Han M, Liu S (2015) Tumor necrosis factor-alpha-induced protein-8 like-2 (TIPE2) upregulates p27 to decrease gastic cancer cell proliferation. J Cell Biochem 116(6):1121–1129
Zhou WN, Du YF, Zheng Y, Zhang W, Wu YN, Song XM et al (2019) Inactivation of RUNX3 protein expression in tongue squamous cell carcinoma and its association with clinicopathological characteristics. Mol Med Rep 19(2):885–894
Zhu L, Zhang X, Fu X, Li Z, Sun Z, Wu J, Wang X, Wang F, Li X, Niu S, Ding M, Yang Z, Yang W, Yin M, Zhang L, Zhang M (2018) TIPE2 suppresses progression and tumorigenesis of esophageal carcinoma via inhibition of the Wnt/beta-catenin pathway. J Transl Med 16(1):7
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhao, LL. TIPE2 suppresses progression and tumorigenesis of the oral tongue squamous cell carcinoma by regulating FoxP3+ regulatory T cells. J Bioenerg Biomembr 52, 279–289 (2020). https://doi.org/10.1007/s10863-020-09840-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10863-020-09840-w