Abstract
Hypoxic conditions, which large or infiltrative hypovascular tumors may encounter, also produce acidic environments. Carbonic anhydrase-IX (CA-IX), an enzyme involved in lowering pH, is overexpressed in hepatocellular carcinoma (HCC). In the present study, whether inhibition of CA-IX enhances the efficacy of a hexokinase II inhibitor in an in vivo murine model was examined and its prognostic implication in HCC patients was investigated. CA-IX expression was evaluated using quantitative real-time PCR and western blot analysis using human HCC cell lines. 3-bromopyruvate (3-BP), a hexokinase II inhibitor, and acetazolamide, a carbonic anhydrase inhibitor, were used to target hexokinase II and CA-IX in vitro and in vivo, respectively. A human HCC cell line (Huh-7) was tested as a subcutaneous tumor model in BALB/c nu/nu mice. The prognostic role of CA-IX was evaluated in the TCGA database. Quantitative real-time PCR and western blot analysis revealed that CA-IX expression was activated in the presence of 3-BP. Further analysis showed that introducing an additional stress by treating the orally active CA-IX inhibitor (acetazolamide) can synergistically increase the efficacy of 3-BP in vivo, which was confirmed using a mouse model. We also found that HCC patients with high CA-IX expression show poor overall survival in TCGA database. These results indicate CA-IX is a promising therapeutic target for enhancing the efficacy of 3-BP and can be a prognostic factor for HCC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is a characteristically hypervascular tumor and therefore, transarterial chemoembolization (TACE) is considered as a favorable treatment options for unresectable HCC (Lee and Khan 2017). However, HCC cells survived after TACE-induced hypoxic insult can grow more swiftly (Tezuka et al. 2007). Moreover, HCCs occasionally exhibit an infiltrating rather than a mass-forming growth pattern (Weinstein-Oppenheimer et al. 2001), and these infiltrative HCCs usually show hypovascular, aggressive tumor phenotypes, and have a poorer prognosis than mass-forming hypervascular types. Therefore, hypoxia appears to promote survival and proliferation of HCC cells in a hypoxic milieu (Gwak et al. 2005). We previously demonstrated that hypoxia stimulates HCC cell growth by inducing hexokinase (HK) II expression which is essential for the glycolytic phenotype, and inhibition of HK II by 3-bromopyruvate (3-BP) in vivo exhibits anti-tumor effects by inducing apoptosis (Gwak et al. 2005; Kim et al. 2007).
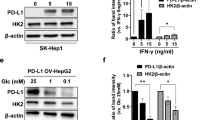
Carbonic anhydrase-IX (CA-IX) is a hypoxia inducible protein involving maintenance of intracellular pH by expediting the pericellular metabolism of CO2. Tumor-specific, hypoxia-induced overexpression of CA-IX plays important roles in the cell proliferation, metastasis, and the acquisition of chemo and radio-resistance (Swinson et al. 2003; Korkeila et al. 2009; Supuran 2008). Therefore, inhibition of CA-IX may be a clinically relevant therapeutic strategy. Acetazolamide, a well-known CA inhibitor approved by the FDA, inhibits CA-IX activity by binding to the active site of the enzyme (Parkkila et al. 2000). According to a recent study, inhibition of CA-IX using acetazolamide enhanced 3-BP-induced HCC cell apoptosis through enhancing endoplasmic reticulum (ER) stress in hypoxic conditions (Yu et al. 2011). However, to date, the synergistic effects of acetazolamide and 3-BP combination therapy in an in vivo model of HCC have not been previously studied.
Therefore, in the present study, the synergistic effects of 3-BP and acetazolamide in a mouse HCC model were evaluated and the prognostic implication determined.
Materials and methods
Cell culture and reagents
Huh-7, HepG2 and SNU-771 HCC cell lines were used in this study. Cells were grown in DMEM supplemented with 10% fetal bovine serum, streptomycin (100 mg/L), and penicillin (100,000 U/L). Cell proliferation was performed with 3% fetal bovine serum and other experiments were performed using cells serum-starved overnight to avoid serum induced signals. Based on the experiment, cells were incubated under a standard culture condition (20% O2 and 5% CO2, at 37 °C) or hypoxic condition (1% O2, 5% CO2, and 94% N2; at 37 °C) depending on the experiment.
3-BP and acetazolamide were obtained from Sigma-Aldrich, Inc. (St. Louis, MO, USA).
Determination of CA9 mRNA expression in HCC cell lines by quantitative real time PCR
RNA was extracted from frozen tissue with QIAshredder (Qiagen) and RNeasy MiniKit (Qiagen). Complementary DNA was synthesized using iScriptTM cDNA synthesis kit (Bio-Rad). The reactions were run in triplicate using iQTM SYBR Green Supermix (Bio-Rad). The results were normalized to endogenous GAPDH expression levels. The data were shown in 2-∆∆Ct format. The sequence of primers used for quantitative real time PCR was as follows: CA9, forward primer 5′-ACCCTCTCTGAC ACCCTGTG-3′ and reverse primer 5′-GGCTGGCTTCTCAC ATTCTC-3′; GAPDH, forward primer 5′-CCTGCACCACCAACTGCTTA-3′ and reverse primer 5′- TCATGAGCCCTTCCACAATG-3′. Quantitative real time PCR was performed on the ViiATM 7 Real-Time PCR System (Life Technologies).
Immunoblot assay
Cells were lysed for 20 min on ice using lysis buffer (50 mM Tris-HCl, pH 7.4; 1% Nonidet P-40; 0.25% sodium deoxycholate; 150 mM NaCl; 1 mM EDTA; 1 mM phenylmethylsulfonylfluoride; 1 μg/mL of each of aprotinin, leupetin, and pepstatin; 1 mM Na3VO4; 1 mM NaF) and then centrifuged at 14,000 g for 10 min at 4 °C. Samples were blotted with appropriate primary antibodies and incubated with peroxidase-conjugated secondary antibodies (Biosource International, Camarillo, CA, USA). Densitometric analyses were performed using Image Gauge software (Fuji Photo Film). The arbitrary units were calculated by densitometric scanning of the intensity of CA-IX relative to actin intensity and considering 0 h as 1.
Murine HCC model
Five-week-old male BALB/c nu/nu mice were used for the animal experiments (Charles River Laboratories, Wilmington, MA, USA). All procedures performed in studies involving animals were in accordance with the ethical standards of the Institutional Animal Care and Use Committee of Seoul National University Hospital. Subcutaneous tumors were established by injection of 1 × 106 Huh-7 cells in 40 μL of matrigel in the left flank of 7-week-old male BALB/c nu/nu mice. Mice with the average tumor volume of 20mm3 were randomly assigned to 4 groups with 5 mice per groups. Tumor volume was blindly measured using calipers every 7 days and calculated with the formula:
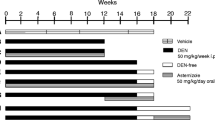
when HCC tumor volume reached 0.5–1.0 cm3, an intraperitoneal injection of 3-BP (1 mg/kg/day) and/or acetazolamide (40 mg/kg/day) was administered daily for 14 consecutive days. In the acetazolamide group, acetazolamide (40 mg/kg/d) was delivered by oral gavage every day for 14 days. In the 3-BP group, 3-BP (1 mg/kg/day) was delivered by intraperitoneal injection every day for 14 days. In the combination group, acetazolamide (40 mg/kg/d; by oral gavage) and 3-BP (1 mg/kg/d; by intraperitoneal injection) were administered every day. The control group was administered a 1.0% DMSO/sodium chloride solution without drugs by intraperitoneal injection and PBS by oral gavage. The adequate dose of medication was based on a previous in vivo study (Budak-Alpdogan et al. 2009). Two weeks after administration of 3-BP and/or acetazolamide, the mice were sacrificed under general anesthesia (isoflurane inhalation) by exsanguination via cardiac puncture. HCC tumor masses were removed, formaldehyde-fixed, and cryopreserved.
Apoptosis
TUNEL staining using ApopTag In Situ Apoptosis Detection Kits (Millipore) was performed to assess apoptosis in HCC tumor tissue. Six high-power fields (400×) with random selection were investigated, and TUNEL-positive cells were counted and averaged. Apoptosis indices refer to the percentage of apoptotic cells of the total number of cells.
Immunohistochemical staining
Antibodies against hypoxia-inducible factor-1α (HIF-1α), c-Jun N-terminal kinase (JNK), and CA-IX were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Immunohistochemical staining was performed on HCC tumor tissue using the Vectastain Elite ABC Kit (Vector Laboratories). CA-IX positivity was calculated using the Membrane Algorithm of the Aperio ImageScope (Aperio Technologies) in 6 randomly selected high-power fields (400×) and averaged. HIF-1α and JNK positivity was calculated by the Positive Pixel Count Algorithm of the Aperio ImageScope (Aperio Technologies) in 6 randomly selected high-power fields (400×) and averaged.
Role of CA-IX in HCC patient survival in the TCGA database
CA9 mRNA expression levels were retrieved from the TCGA RNA-sequence database (http://genome-cancer.ucsc.edu/). All included patients were pathologically diagnosed with HCC, received no pretreatment, and had intact overall survival (OS) information (Shen et al. 2017). This study was approved by institutional review board of Seoul National University Hospital. Patient informed consent was waived given the use of de-identified, existing public datasets.
Statistical analysis
Sample size for animal studies were based on a previous study in our laboratory in which the same BALB/c nu/nu mice strain was used. Significance of the difference between groups was calculated by Student’s unpaired t-test, one-way or two-way ANOVA (Tukey’s and Bonferroni’s multiple comparison test). Welch’s corrections were used when variances between groups were unequal. Survival analyses were performed using the Kaplan-Meier method and Cox regression model. Functional studies were performed in triplicate and repeated at least 3 times. Statistical analyses were performed using the GraphPad Prism v7.03 (GraphPad Software) and SPSS, version 19.0 (SPSS, Inc., Chicago, IL, USA). P values <0.05 were considered statistically significant.
Results
Hexokinase II inhibitor upregulated CA-IX expression in HCC
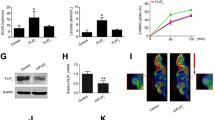
Quantitative real-time PCR was used to test the tumor cells and simultaneous increase in hexokinase II and CA-IX was observed under hypoxic culture conditions compared with normoxic culture conditions (Fig. 1a). Immunoblotting experiments also showed increased CA-IX expression in tumor cells after 3-BP-induced inhibition of hexokinase II (Fig. 1b). Based on these findings, the effects of 3-BP therapy in a murine subcutaneous HCC model were further tested using Huh-7 tumors. 3-BP therapy was started 2 weeks after injection of Huh-7 cells when tumors were palpable (average tumor volume: 20 mm3). As shown in Fig. 1c, 3-BP therapy caused only marginal inhibition of subcutaneous tumor growth (P < 0.05). Next, the tumor tissues were evaluated using immunohistochemistry and a significant upregulation of CA-IX-positive cells was observed (P < 0.001, Fig. 1d) in liver tumors after 3-BP-induced inhibition of hexokinase II.
Hexokinase II inhibitor decreased HCC tumor volume but upregulated CA-IX expression in HCC. aHK II and CA-IX expressions were evaluated by quantitative real-time PCR. n = 3, Student’s t test, *P < 0.001. Mean ± standard error of mean (SEM). b CA-IX expression was evaluated using immunoblotting and densitometer. n = 3 (triplicate data), Student’s t test, *P < 0.001. Mean ± SEM. c An in vivo HCC model was established in BALB/c nu/nu mice by subcutaneous injection of Huh-7 cells. 3-BP was injected into the mice for 14 days when the HCC volumes reached 0.2 cm3. n = 10, Student’s t test, *P < 0.05. Mean ± SEM. d Assessment of CA-IX expressing cells was performed using immunohistochemistry. Representative immunohistochemistry images of tumor tissues from control or 3-BP groups. n = 10, Student’s t test, *P < 0.001, mean ± SEM
Acetazolamide augmented antitumor efficacy of 3-BP in vivo
Because 3-BP showed only limited anti-tumor effects and the expression of CA-IX was increased after 3-BP treatment, the effects of combination therapy of 3-BP and acetazolamide in an in vivo murine HCC model were evaluated. A single therapy of 3-BP or acetazolamide caused only a mild suppression of tumor growth when compared with controls (Fig. 2a). In contrast, combined acetazolamide and 3-BP therapy led to significant tumor growth inhibition. Indeed, the mean tumor volume was significantly reduced in the 3-BP and acetazolamide combination group compared with the control, 3-BP-only, or acetazolamide-only treated group (P < 0.001 for control versus combination therapy, P < 0.01 for 3-BP only or acetazolamide-only versus combination therapy).
The augmented anti-tumor effect of 3-BP and acetazolamide in the in vivo HCC model. a An in vivo HCC model was established in BALB/c nu/nu mice by subcutaneous injection of Huh-7 cells. 3-BP and/or acetazolamide were injected into the mice for 14 days when the HCC volumes reached 0.2 cm3. n = 5, Student’s t test, * P < 0.001 for control versus combination, ** P < 0.01 for 3-BP only or acetazolamide-only versus combination, mean ± SEM. b Apoptosis in the HCC tissues was evaluated using TUNEL staining. TUNEL-positive cells were counted in 6 different randomly selected high-power fields (400×), and then averaged. n = 5, Student’s t test, * P < 0.001, mean ± SEM
The augmented antitumor effects of 3-BP and acetazolamide is mediated by increased apoptosis
To identify the mechanism underlying the augmented effects of 3-BP and acetazolamide, whether apoptotic levels were higher in 3-BP and acetazolamide-treated mice compared with the controls, 3-BP-treated, or acetazolamide-treated mice were evaluated using TUNEL staining. As shown in Fig. 2b, the percentage of TUNEL positive cells was significantly increased in mice treated with 3-BP and acetazolamide compared with the other 3 treatments (P < 0.001).
The increased apoptosis caused by 3-BP and acetazolamide may be due to JNK activation by suppression of CA-IX expression
Since hypoxia activates JNK, whether an intratumoral hypoxic condition was achieved in the mouse model was investigated by evaluating HIF-1α in tumor tissue. HIF-1α was expressed in all 4 groups, indicating the existence of a hypoxic microenvironment in all tumor tissues. As shown in Fig. 3a, HIF-1α positive cells were significantly increased in mice treated with 3-BP compared with controls (P < 0.001) and more increased in combination therapy group compared with 3-BP only treated group (P < 0.01). Consistent with previous in vitro results (Yu et al. 2011), acetazolamide combined with 3-BP significantly upregulated hypoxia-inducible JNK expression in HCC tissues compared with the 3-BP only treated group (P < 0.01, Fig. 3b). In addition, the tumor tissues were evaluated using immunohistochemistry and upregulation of CA-IX positive cells was observed in liver tumors after hexokinase II inhibition by 3-BP which was reversed by adding acetazolamide (P < 0.001 for control versus 3-BP, P < 0.05 for 3-BP only versus combination, Fig. 3c).
The increased apoptosis caused by 3-BP and acetazolamide may be due to the activation of JNK by suppression of CA-IX expression. a Immunohistochemical staining for mouse HIF-1α was performed on paraffin-embedded mouse HCC tissues. HIF-1α positivity was quantified using Aperio ImageScope (Aperio Technologies) in 6 randomly selected high-power fields (400×), and the average was calculated. n = 5, Student’s t test, * P < 0.001 for control versus 3-BP only, ** P < 0.01 for 3-BP only versus combination, mean ± SEM. b JNK positivity was quantified in 6 randomly selected high-power fields (400×), and the average was calculated. n = 5, Student’s t test, * P < 0.001 for control versus 3-BP only, ** P < 0.01 for 3-BP only versus combination, mean ± SEM. c Representative immunohistochemistry images of CA-IX expression in tumor tissues. n = 5, Student’s t test, * P < 0.001 for control versus 3-BP only, ** P < 0.05 for 3-BP only versus combination, mean ± SEM
Prognostic role of CA-IX in HCC patient survival in the TCGA database
A total of 234 eligible HCC patients were included in this study. The clinicopathological characteristics of patients are summarized in Supplementary Table 1. The median age was 62 years (range, 20–85 years). There were 166 (70.9%) patients at N0 stage, 1 (0.4%) patients at N1 stage, and 65 (27.8%) patients with unclarified stage. The majority of patients (168, 71.8%) had no distant metastases, 1 (0.4%) patients had distant metastases, and the remaining 64 (27.4%) patients had unknown metastases status. We first treated CA-IX as a continuous variable, and univariate Cox analysis indicated that CA-IX levels were significantly associated with OS of HCC patients (hazard ratio, 3.99; 95% confidence interval, 1.09–14.49; P = 0.02). When patients were stratified into 2 groups according to the CA-IX level of 0.20, which provided the maximum sum of specificity and sensitivity in predicting OS (sensitivity, 57.4%; specificity, 62.8%; area under the curve, 0.60; 95% confidence interval, 0.54–0.67; P = 0.01; Fig. 4a), 99 patients were included in the low CA-IX group, and 135 patients were included in the high CA-IX group. High CA-IX group had significantly higher proportions of male (52.3% vs 47.7%, P = 0.004), and patients with high AFP levels (≥ 200 ng/mL; 24.4% vs 12.1%, P = 0.02) compared with low CA-IX group (Supplementary Table 2). At the end of the last follow-up, 14 patients died from the disease and a significantly higher percentage of high CA-IX expression was observed in patients who died from the disease compared with those who lived. The 5-year OS of the patients with high CA-IX levels was significantly lower than that of patients with low CA-IX levels (79.5% and 90.1%, respectively; P = 0.02; Fig. 4b).
Upregulated intratumoral CA-IX predicts poor survival in HCC patients. a Receiver operating curve analysis of 5-year overall survival (OS) was performed using patients’ data in the TCGA database to determine the optimal cut-off value for CA-IX expression. The optimal cut-off value for CA-IX was 0.20 which provided the maximum sum of specificity and sensitivity in predicting OS (sensitivity, 57.4%; specificity, 62.8%; area under the curve, 0.60; 95% confidence interval, 0.54–0.67; P = 0.01). b Kaplan-Meier plots estimated OS in patients with HCC based on CA-IX expression level. The 5-year OS for CA-IX high and low group were 79.5% and 90.1%, respectively, a statistically significant difference (P = 0.02)
Discussion
To our knowledge, our study is the first to show the augmented anti-tumor effects of 3-BP and acetazolamide in a murine HCC model. This effect was attributed to the enhanced apoptosis due to JNK activation and suppression of CA-IX expression. In addition, results from the TCGA database analysis showed upregulated CA-IX expression in tumor tissue predicted poor survival of patients with HCC, confirming the prognostic role of CA-IX.
In many solid tumors, imbalance between the requirement of rapidly growing tumor cells and the supply of vascular system induces hypoxic microenvironment. In response to hypoxic milieu, tumor cells change gene expression pattern to match the requirements of the altered microenvironment such as HIF-1-dependent expression of HK II and CA-IX. Since hypoxia induces both HK II and CA-IX expression, and the HK II inhibitor (3-BP) further increased the expression of CA-IX via compensatory mechanisms (Gwak et al. 2005; Yu et al. 2011; Pastorekova et al. 2008), we postulated that simultaneous inhibition of these two enzymes may enhance the anti-tumor efficacy of 3-BP. The present study clearly showed that 3-BP-induced HCC cell apoptosis was significantly enhanced by CA-IX inhibition, suggesting that this combination strategy may be more effective for HCC, especially under hypoxic conditions such as advanced infiltrative HCC.
Next, we explored the possible mechanism of apoptosis enhancement caused by CA-IX inhibition. Pro-apoptotic JNK was more promptly and potently activated in tumor bearing mice treated with 3-BP and acetazolamide as compared to 3-BP alone-treated tumor bearing mice. Because 3-BP induces JNK activation which has a crucial role in the release of cytochrome c from the inner membrane of mitochondria and activation of mitochondrial apoptotic signaling cascades (Yu et al. 2011; Aoki et al. 2002), the enhanced JNK activation in combination therapy group may lead to more augmented apoptosis as compared to 3-BP-treated group. Furthermore, 3-BP induces ER stress by the accumulation of reactive oxygen species or free radicals and the protein misfolding in human HCC cell lines (Ganapathy-Kanniappan et al. 2010). ER stress sensors subsequently initiate unfolded protein response (UPR), resulting in the phosphorylation of eIF2α, and binding of the activating transcription factor 4 to CA9 promoter (van den Beucken et al. 2007). This suggests that 3-BP-provoked ER stress may induce CA-IX expression as one of UPRs to reduce ER stress. Thus, the simultaneous inhibition of HK II and CA-IX may enhance ER stress-induced apoptosis, as shown by our previous in vitro study (Yu et al. 2011).
Among various CA isoenzymes, CA-IX is one of the most important enzyme for tumorigenesis and prognostication in various cancers, including rectal, pancreatic, and renal cancers (Korkeila et al. 2009; Kivela et al. 2000; Benej et al. 2014). CA-IX plays an important role in cancer cell proliferation and survival by maintenance of physiologic intracellular pH (Supuran et al. 2018). Furthermore, CA-IX promotes cancer invasion and metastasis by acidification of the extracellular milieu (Martinez-Zaguilan et al. 1996). Apart from pH regulation, CA-IX also participates in epithelial-mesenchymal transition by decreasing E-cadherin-mediated intercellular adhesion through interaction with β-catenin (Svastova et al. 2003). Regarding tumor-promoting effects of CA-IX, CA-IX inhibition could therapeutically be useful in CA-IX-expressing HCCs.
In conclusion, this study shows that the combination treatment of 3-BP and acetazolamide has a synergistic anti-tumor effect in an in vivo HCC mouse model. This effect is attributed to increased sensitivity of HCC to 3-BP-induced apoptosis through JNK activation and suppression of CA-IX expression. These results indicate that acetazolamide might be an effective adjuvant therapy to 3-BP-resistant HCC cells.
References
Aoki H, Kang PM, Hampe J, Yoshimura K, Noma T, Matsuzaki M, Izumo S (2002) Direct activation of mitochondrial apoptosis machinery by c-Jun N-terminal kinase in adult cardiac myocytes. J Biol Chem 277(12):10244–10250. https://doi.org/10.1074/jbc.M112355200
Benej M, Pastorekova S, Pastorek J (2014) Carbonic anhydrase IX: regulation and role in cancer. Subcell Biochem 75:199–219. https://doi.org/10.1007/978-94-007-7359-2_11
Budak-Alpdogan T, Chen B, Warrier A, Medina DJ, Moore D, Bertino JR (2009) Retinoblastoma tumor suppressor gene expression determines the response to sequential flavopiridol and doxorubicin treatment in small-cell lung carcinoma. Clin Cancer Res 15(4):1232–1240. https://doi.org/10.1158/1078-0432.CCR-08-0810
Ganapathy-Kanniappan S, Geschwind JF, Kunjithapatham R, Buijs M, Syed LH, Rao PP, Ota S, Kwak BK, Loffroy R, Vali M (2010) 3-Bromopyruvate induces endoplasmic reticulum stress, overcomes autophagy and causes apoptosis in human HCC cell lines. Anticancer Res 30(3):923–935
Gwak GY, Yoon JH, Kim KM, Lee HS, Chung JW, Gores GJ (2005) Hypoxia stimulates proliferation of human hepatoma cells through the induction of hexokinase II expression. J Hepatol 42(3):358–364. https://doi.org/10.1016/j.jhep.2004.11.020
Kim W, Yoon JH, Jeong JM, Cheon GJ, Lee TS, Yang JI, Park SC, Lee HS (2007) Apoptosis-inducing antitumor efficacy of hexokinase II inhibitor in hepatocellular carcinoma. Mol Cancer Ther 6(9):2554–2562. https://doi.org/10.1158/1535-7163.MCT-07-0115
Kivela AJ, Parkkila S, Saarnio J, Karttunen TJ, Kivela J, Parkkila AK et al (2000) Expression of transmembrane carbonic anhydrase isoenzymes IX and XII in normal human pancreas and pancreatic tumours. Histochem Cell Biol 114(3):197–204
Korkeila E, Talvinen K, Jaakkola PM, Minn H, Syrjanen K, Sundstrom J et al (2009) Expression of carbonic anhydrase IX suggests poor outcome in rectal cancer. Br J Cancer 100(6):874–880. https://doi.org/10.1038/sj.bjc.6604949
Lee EW, Khan S (2017) Recent advances in transarterial embolotherapies in the treatment of hepatocellular carcinoma. Clin Mol Hepatol 23(4):265–272. https://doi.org/10.3350/cmh.2017.0111
Martinez-Zaguilan R, Seftor EA, Seftor RE, Chu YW, Gillies RJ, Hendrix MJ (1996) Acidic pH enhances the invasive behavior of human melanoma cells. Clin Exp Metastasis 14(2):176–186
Parkkila S, Rajaniemi H, Parkkila AK, Kivela J, Waheed A, Pastorekova S, Pastorek J, Sly WS (2000) Carbonic anhydrase inhibitor suppresses invasion of renal cancer cells in vitro. Proc Natl Acad Sci U S A 97(5):2220–2224. https://doi.org/10.1073/pnas.040554897
Pastorekova S, Ratcliffe PJ, Pastorek J (2008) Molecular mechanisms of carbonic anhydrase IX-mediated pH regulation under hypoxia. BJU Int 101(Suppl 4):8–15. https://doi.org/10.1111/j.1464-410X.2008.07642.x
Shen Z, Wang X, Yu X, Zhang Y, Qin L (2017) MMP16 promotes tumor metastasis and indicates poor prognosis in hepatocellular carcinoma. Oncotarget 8(42):72197–72204. https://doi.org/10.18632/oncotarget.20060
Supuran CT (2008) Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 7(2):168–181. https://doi.org/10.1038/nrd2467
Supuran CT, Alterio V, Di Fiore A, K DA, Carta F, Monti SM et al (2018) Inhibition of carbonic anhydrase IX targets primary tumors, metastases, and cancer stem cells: three for the price of one. Med Res Rev 38(6):1799–1836. https://doi.org/10.1002/med.21497
Svastova E, Zilka N, Zat'ovicova M, Gibadulinova A, Ciampor F, Pastorek J et al (2003) Carbonic anhydrase IX reduces E-cadherin-mediated adhesion of MDCK cells via interaction with beta-catenin. Exp Cell Res 290(2):332–345
Swinson DE, Jones JL, Richardson D, Wykoff C, Turley H, Pastorek J et al (2003) Carbonic anhydrase IX expression, a novel surrogate marker of tumor hypoxia, is associated with a poor prognosis in non-small-cell lung cancer. J Clin Oncol 21(3):473–482. https://doi.org/10.1200/JCO.2003.11.132
Tezuka M, Hayashi K, Kubota K, Sekine S, Okada Y, Ina H, Irie T (2007) Growth rate of locally recurrent hepatocellular carcinoma after transcatheter arterial chemoembolization: comparing the growth rate of locally recurrent tumor with that of primary hepatocellular carcinoma. Dig Dis Sci 52(3):783–788. https://doi.org/10.1007/s10620-006-9537-y
van den Beucken T, Magagnin MG, Savelkouls K, Lambin P, Koritzinsky M, Wouters BG (2007) Regulation of Cited2 expression provides a functional link between translational and transcriptional responses during hypoxia. Radiother Oncol 83(3):346–352. https://doi.org/10.1016/j.radonc.2007.04.026
Weinstein-Oppenheimer CR, Henriquez-Roldan CF, Davis JM, Navolanic PM, Saleh OA, Steelman LS et al (2001) Role of the Raf signal transduction cascade in the in vitro resistance to the anticancer drug doxorubicin. Clin Cancer Res 7(9):2898–2907
Yu SJ, Yoon JH, Lee JH, Myung SJ, Jang ES, Kwak MS, Cho EJ, Jang JJ, Kim YJ, Lee HS (2011) Inhibition of hypoxia-inducible carbonic anhydrase-IX enhances hexokinase II inhibitor-induced hepatocellular carcinoma cell apoptosis. Acta Pharmacol Sin 32(7):912–920. https://doi.org/10.1038/aps.2011.24
Acknowledgements
This study was funded by grants from the SNUH Research Fund (No. 04-2011-0660) and the Liver Research Foundation of Korea.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 19 kb)
Rights and permissions
About this article
Cite this article
Cho, E.J., Yu, S.J., Kim, K. et al. Carbonic anhydrase-IX inhibition enhances the efficacy of hexokinase II inhibitor for hepatocellular carcinoma in a murine model. J Bioenerg Biomembr 51, 121–129 (2019). https://doi.org/10.1007/s10863-019-09788-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10863-019-09788-6