Abstract
In the present study, collagen hydrogel containing naringin was fabricated, characterized and used as the scaffold for peripheral nerve damage treatment. The collagen was dissolved in acetic acid, naringin added to the collagen solution, and cross-linked with 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide powder (EDC; 0.10 mM) to form the hydrogel. The microstructure, swelling behavior, biodegradation, and cyto/hemocompatibility of the fabricated hydrogels were assessed. Finally, the healing efficacy of the prepared collagen hydrogel loaded with naringin on the sciatic nerve crush injury was assessed in the animal model. The characterization results showed that the fabricated hydrogels have a porous structure containing interconnected pores with the average pore size of 90 µm. The degradation results demonstrated that about 70% of the primary weight of the naringin loaded hydrogel had been lost after 4 weeks of storage in PBS. The in vitro study showed that the proliferation of Schwann cells on the collagen/naringin hydrogel was higher than the control group (tissue culture plate) at both 48 and 72 h after cell seeding and even significantly higher than pure collagen 72 h after cell seeding (*p < 0.005, **p < 0.001). The animal study implied that the sciatic functional index reached to −22.13 ± 3.00 at the end of 60th days post-implantation which was statistically significant (p < 0.05) compared with the negative control (injury without the treatment) (−82.60 ± 1.06), and the pure collagen hydrogel (−59.80 ± 3.20) groups. The hot plate latency test, the compound muscle action potential, and wet weight-loss of the gastrocnemius muscle evaluation confirmed the positive effect of the prepared hydrogels on the healing process of the induced nerve injury. In the final, the histopathologic examinations depicted that the collagen/naringin hydrogel group reduced all the histological changes induced from the nerve injury and showed more resemblance to the normal sciatic nerve, with well-arranged fibers and intact myelin sheath. The overall results implied that the prepared collagen/naringin hydrogel can be utilized as a sophisticated alternative to healing peripheral nerve damages.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The proper communications between the brain and limbs are an unassailable prerequisite for human living which can be disrupted in some situations such as peripheral nerve injury (PNI). PNI can induce lifelong problems such as disability and it is estimated that approximately 23 per 100,000 persons encounter PNI per year. Various causes are related to the PNI such as stabbings, motor vehicle accidents, birth trauma, and gunshot wounds. Moreover, patients suffering from PNI-induced disabilities are also burdensome to the government and healthcare system [1, 2]. The gold standard treatment of PNI is autologous grafting technique which provides biological and physical support for axonal outgrowth. However, this method suffers from complications arises from the risks related to the harvesting surgery, the sacrifice of donor nerve function, and also the restricted supply of donor tissue [3, 4]. Therefore, various researchers and even companies are working on developing alternative solutions to ameliorate the injured peripheral nerves. In this regard, nanotechnology, tissue engineering, and functional biomaterials have attracted a great deal of attention to proposing the solutions for the drawbacks of the current treatments. Bioactive materials as the neural scaffold with proper and sophisticated design, architecture, and fabrication can trigger and promote PNI healing [5,6,7,8]. A wide range of nanostructures and functional biomaterials have been utilized as the neural tissue engineering scaffolds, for instance, nanofibers, nanostructured materials, and hydrogels [9, 10].
Hydrogels are fascinating 3D-network structures composed of physically or chemically cross-linked hydrophilic polymer chains which are able to absorb and retain a large amount of water or the other solvent (up to 100 g/g or higher) of the dried weight. Despite a large amount of retained water, hydrogels are insoluble and stable in water due to the cross-linked polymer chains. The presence of hydrophilic polymer chains in the structure of hydrogels provides the water absorption and retention properties, while network chains cross-linking preserve the hydrogel structure from dissolution in the aqueous media. The absorbed water has vital roles in the solubility, integrity, and diffusion of substances into the hydrogel, the desired properties for tissue engineering applications [11,12,13]. A wide range of synthetic and natural materials have been used to fabricate interested hydrogels for a wide range of applications including tissue engineering, wound dressing, cell encapsulation, and drug delivery. A great deal of attention has been considered toward collagen-based hydrogels as a drugs delivery vehicle and the scaffold for neural tissue construction due to their promising properties. Collagen is considered as the highest protein in the body which comprises 25% (by dry weight) of mammals [9, 14]. Collagen is widely dispersed in the peripheral nervous and has key role in the formation and maintenance of sheaths surrounding the axons and nerve fascicles [15]. It is shown that the collagen administration after PNI would enhance axonal regeneration [16,17,18]. Along with the biological functions, collagen in the form of hydrogel provides 3D structure favorable for neurite ingrowth.
Natural products with antioxidant, anti-inflammatory, and proliferative activities are helpful to improve the healing process of PNI. Naringin (4′, 5, 7-trihydroxy flavanone 7-rhamnoglucoside) is a flavanone glycoside extracts from grapefruit, tomato, and other citrus fruits. Naringin offers a wide variety of promising properties such as anti-inflammatory, anti-apoptotic, anti-osteoporotic, anti-carcinogenic, anti-ulcer, and more importantly neuroprotective properties [19,20,21]. Various researchers have been reported the potential therapeutic effects of naringin on CNS diseases. Wang et al. [22] showed that naringin improved cognitive functions in HFD-induced obese mice through AMPK activation-driven decrease in mitochondrial dysfunction and enhancement in insulin signaling. Rong et al. [20] reported that naringin improved locomotor recovery in rats suffer from induced spinal cord injury (SCI). They concluded that the observed neuroprotective effect of naringin can be attributed to the naringin-induced inhibition of neural apoptosis and upregulation of vascular endothelial growth factor (VEGF) and the brain-derived neurotrophic factor (BDNF). Accordingly, our goal in the current study was to fabricate a proper and effective scaffold containing naringin as the bioactive substance to improve and accelerate body own healing process.
2 Methods
2.1 Fabrication of collagen hydrogels
A modified protocol was used to extract type I collagen from the rat tails tendons (purchased from Pasteur Institute, Tehran, Iran) [23]. An autoclaved acetic acid (0.60%, Merck, Darmstadt, Germany) in phosphate-buffered saline was used to dissolve the sterilized extracted collagen to obtain the final concentration of 10 mg/mL. The prepared collagen solution was pH neutralized by dropwise adding an autoclaved NaOH 1 M (Sigma-Aldrich, St. Louis, USA). The naringin (Sigma-Aldrich, St. Louis, USA) was added to the prepared collagen solution with the weight ratio of 1:10 (naringin:collagen) and the obtained solution was cross-linked with 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide powder (EDC; 0.10 mM) (Sigma-Aldrich, St. Louis, USA) 10 min before implantation it the injury site. The fabricated hydrogels were thoroughly washed with double distilled water to remove unreacted EDC.
2.2 Scanning electron microscopy (SEM) imaging
Based on our previous study [24], the prepared specimens were freeze-dried, cross-sectioned, and imaged by SEM. Briefly, the fabricated hydrogels were frozen at −80 °C for 6 h, lyophilized for 24 h at −54 °C (Freeze drier, Telstar, Terrassa, Spain), sputter coated with gold for 250 s (Sputter coater, SC7620, Quorum Technologies, England), and finally, imaged using SEM (AIS2100, Seron Technology, South Korea) at the accelerating voltage of 20 kV.
2.3 In vitro hydrolytic degradation (weight loss) of hydrogels
The weight loss percentage of the prepared collagen/naringin hydrogels was evaluated in PBS (pH 7.4) at 37 °C in the predetermined time points. Briefly, the collagen/naringin hydrogels were lyophilized (frozen at – 80 °C and freeze-dried at −54 °C), accurately weighed, and immersed in 10 ml PBS for different time interval up to 28 days. PBS was refreshed every other day. At predetermined time intervals, the specimens were taken out quickly, rinsed with DI water, then frozen and lyophilized. According to Eq. 1, the initial weight and the dry weights of specimens after the incubation into PBS were used to calculate the weight loss of the collagen/naringin hydrogels [25]:
where W1 and W2 are the initial weight and the dry weights of specimens after the incubation times, respectively. Each sample was assayed in triplicate
2.4 Swelling studies
The swelling kinetics of the samples was measured in PBS (pH = 7.4) at the ambient temperature after various time interval based on Eq. 2.
where m0 and m1 are the dried mass weight and the mass swollen weight of the gel, respectively.
First of all, the prepared hydrogels were freeze dried and subsequently, a proper amount of the dried gels was weighted, submerged in PBS, and kept at ambient temperature for 3 days. At the end of the incubation period, the specimens removed from the PBS and weighted quickly. The equilibrium mass swelling percentages of the collagen/naringin hydrogels was calculated according to Eq. 2. Each sample was assayed in triplicate.
2.5 Naringin release study
The UV-visible spectroscopy was used to evaluate the in vitro release of Naringin from fabricated hydrogel under sink condition. Naringin loaded Collagen hydrogel (1 mL) incubated in simulated body fluid (SBF) (5 mL) at 37 °C d for up to 336 h. The supernatant was removed and centrifuged at the predetermined time points and the absorption intensity of released Naringin was read at 280 nm using Awareness Technology microplate-reader (Palm City, USA).
2.6 Cell culture studies
Previously described protocol was used to isolate primary rat Schwann cells (SCs) from sciatic nerves of adult male Wistar rats (weighing 200–250 g) (School of Pharmacy of Tehran University of Medical Sciences, Tehran, Iran) [26]. The isolated SCs were cultured in DMEM/F-12 (Dulbecco’s Modified Eagle Medium/Nutrient Mixture F-12; Gibco, Grand Island, USA) supplemented with fetal bovine serum (FBS; Gibco, Grand Island, USA), 100 mg/mL of streptomycin and 100 unit/mL of penicillin (Sigma-Aldrich, St. Louis, USA). The tissue culture plates were incubated in a humidified incubator at 37 °C with 5% CO2 and the culture media was changed every 48 h. The prepared hydrogels were poured in a 96-well plate, seeded with 1×104 SCs, and the proliferation of cells evaluated with by 3-(4, 5-Dimethylthiazol-2-yl)-2, 5-Diphenyltetrazolium Bromide (MTT) (Sigma-Aldrich, St. Louis, USA) assay kit based on the manufacturer’s instruction. The positive control was the cells cultured on the tissue culture plate (TCP). The experiments were repeated three times and the resulted absorbances were recorded by Awareness Technology microplate-reader (Palm City, USA).
2.7 Hemocompatibility assay
Fresh anticoagulant-treated human blood was used to measure the hemolysis degree induced by the prepared collagen/naringin hydrogels. The blood was collected from the volunteers based on the ethical guide line of the university.
2 ml of the prepared blood was diluted with 2.5 ml of normal saline and each scaffold was incubated with 0.2 ml of the diluted blood for 60 min at 37 °C. After passing the incubation time, the incubated blood was centrifuged at 1500 rpm for 10 min, the supernatant transferred to a 96-well plate where the absorbance was measured at 545 nm by using the Anthos 2020 (Biochrom, Berlin, Germany) microplate reader. The negative control and positive control were normal saline and 0.2 ml diluted blood in 10 ml deionized water, respectively. Equation 3 was used to calculate the hemolysis degree.
where Dt, Dnc, and Dpc are the absorbance of the sample, the absorbance of the negative control, and the absorbance of the positive control, respectively. Each sample was assayed in triplicate
2.8 In vivo studies
Animal experiments were approved by the ethical committee of Shahroud University of Medical Sciences and were carried out in accordance with the university guidelines.
2.8.1 Sciatic nerve defect
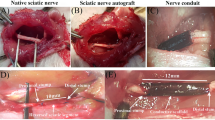
The animal study was carried out on twenty healthy adult male Wistar rats (3 months old, weighing 250–270 g) obtained from Pasteur Institute, Tehran, Iran. The animals were randomly divided into the four groups (5 rats in each group) (1) Rats with hydrogel/naringin, (2) Rats with hydrogel without naringin, (3) negative control (injury without the treatment), and (4) the autograft group (the positive control). The sciatic nerve injury was induced based on our previous study [23]. Briefly, the animals were anesthetized by intraperitoneal injection of Ketamine 100 mg/Xylazine 10 mg /kg of body weight. A skin incision was made across the left lower limb of the animals, and then a non-serrated clamp was used to exert a constant force for a period of 30 s to induce a crush injury (3-mm-long) around 10 mm above the bifurcation into the tibial and peroneal nerves (Fig. 1). The resected nerve segment was reversed and used to bridge the nerve defect in the autograft group. The hydrogel from the same bath was injected using a 16-gauge needle into the site of crush injury in the same group. Finally, polyglycolic acid and silk sutures no. 3-0(SUPA Medical Devices, Tehran, Iran) were used to close the muscle and skin at the injury site, respectively.
2.8.2 Walking-foot-print analysis
The sciatic functional index (SFI), as the indication of the functional recovery of the injured sciatic nerve, was evaluated using the rat’s footprints 4 and 8 weeks post-surgery. A millimeter-lined acrylic corridor (43 cm length, 8.7 cm width and 5.5 cm height) ended with a darkened goal box was used to analyze the footprint of the rats’ hind paws ink soaked. Equation 4 was used to calculate SFI where PL is the distance between the heel and the top of the third toe, TS is the distance from the first toe to the fifth one, and IT is the distance between the second toe and the fourth one. NPL, NTS, and NIT stand for non-operated foot and OPL, OTS, and OIT as the operated one. The recorded value from the right foot was assumed the control. An index of 0 and 100 represent the normal function and the complete loss of function, respectively.
The control was the recorded value from the right foot. An index of 0 and 100 represent the normal function and the complete loss of function, respectively.
2.8.3 Hot plate latency test
The sciatic nerve regeneration was evaluated using the hot plate latency (HPL) measurement eight weeks post-surgery. The injured limb was placed on a hot plate (56 °C) and the time they jumped or licked their paws was recorded and records from three rates in each group were averaged. The cut off time for their reaction was set at 12 s.
2.8.4 Nerve conduction test
Compound muscle action potential (CMAP) amplitude and latency were measured eight weeks post-surgery. The onset amplitude indicates the number of available motor axons, and the latency, the conduction time of the fastest fibers. The test was conducted under anesthesia induced by the I.P injection of Ketamine 100 mg/Xylazine 10 mg /kg body weight. The sciatic nerve was stimulated at the proximal to the site of the injury using a needle electrode and 3–5 mA electrical current. An electromyographic recorder (Negarandishegan, Tehran, Iran) was used to record CMAP amplitude from the needle and cap electrodes placed on the gastrocnemius muscle. The following parameters were applied during the record, filtering frequency of 10 Hz to 10 kHz, the sensitivity of 2 mV/division, and sweep speed of 1 ms/division. The measurements for five rats in each group were averaged.
2.8.5 Gastrocnemius muscle wet weight-loss
The wet weight-loss of the gastrocnemius muscles as an indication of sciatic nerve healing status was measured at the end of 8 weeks post-implantation of the scaffold. Briefly, the rats were sacrificed under anesthesia, the muscles on the damaged and healthy hind limbs were harvested and immediately weighed. Equation 5 was used to measure the wet weight-loss of the muscles [27].
2.8.6 Histological analysis
Histological staining was to demonstrate the healing efficacy of the implanted collagen/naringin hydrogel and gastrocnemius muscle appearance at the end of 8 weeks post-implantation. The harvested tissues were fixed in 10% buffered formalin, processed in paraffin, cross-sectioned, and finally stained with hematoxylin-eosin (H&E). The prepared specimens were analyzed by an independent pathologist under a light microscope (BX51; Olympus, Tokyo, Japan) equipped with a digital camera (DP72; Olympus) and photographed at × 100 magnification.
2.9 Statistical analysis
The data were statistically analyzed by Minitab 17 software (Minitab Inc., State College, PA, USA) using one-way ANOVA, with Tukey’s post hoc test. The results were expressed as the mean ± standard deviation (SD) and p 0.05 was considered statistically significant in all evaluations.
3 Results
3.1 Morphology evaluation
The microstructure of the collagen/naringin hydrogels was observed macroscopically and microscopically by SEM (Fig. 2). The prepared sol solution of collagen was cross-linked and converted to stable gel using EDC (Fig. 2a, b). The SEM images and the image processing (Image J, NIH, Bethesda, USA) depicted that the average pore sized was 90 ± 5 µm with the interconnected pores structure. As shown in Fig. 2c, the structure of the prepared collagen/naringin hydrogel consists of interconnected pores which formed due to the phase separation during the freeze-drying process. The pores structure, size, and their interconnection state are influencing factors which determine nutrient/waste exchange and cell infiltration into the deep structure of the hydrogels. According to the obtained results, the pores size and the interconnection state of the collagen/naringin hydrogel are suitable for cells infiltration and migration.
3.2 Swelling behavior and weight loss
The swelling property of collagen hydrogel was assessed based on their water uptake value and the results are depicted in Fig. 3a. The interactions between collagen chains and water molecules lead to swelling of the polymer, while the cross-linking protects the polymer from dissolution. The unique 3D structure of collagen hydrogel arises from the absorbed water and swelling of the polymer chains, providing proper microenvironment of neural cells and support cells survival, proliferation, and migration. The results exhibited that the highest swelling percent of 326 ± 10%, was achieved at 240 min after incubation and then decreased to reach 60.5 ± 10%. From the drug delivery point of view, these swelling and water retention behavior are suitable to obtain a sustained drug delivery in the nerve defect site [28]. Moreover, collagen hydrogel is able to fill the crated defect area and prevent the lesion from the collapse due to the swollen and 3D structure [29]. The biodegradation rate of a tissue engineering scaffolds is a vital property which should be matched with the healing rate of the injury. On one hand, the fast degradation rate would leave the injured site empty and subsequently abort the healing process. On the other hand, a slow degradation rate hinders the scaffold replacement with the forming tissue. Accordingly, the degradation rate assessment is an impartible test which reveals the fate of the prepared scaffolds in the body. In this regard, the weight loss as the indication of biodegradation of the prepared collagen hydrogels was assessed in PBS solution (PH: 7.4) at 37 °C for 28 days and the results are demonstrated in Fig. 3b. The results show that almost 70% of the initial weight of the collagen hydrogel has been lost after 28 days of storage in PBS which is acceptable for nerve tissue regeneration applications.
3.3 Release finding
As Fig. 4 indicated, the cumulative release profile of naringin displayed the release of 14.98 ± 1.58 and 22.5 ± 2.74% in the first 6 and 12 h, respectively, followed by a sustained release of 72.49 ± 4.39% over 14 days. These finding confirmed the sustained release of loaded naringin from the fabricated hydrogel suitable for nerve tissue engineering applications.
3.4 Hemocompatibility results
The compatibility of the scaffolds with blood cells especially erythrocytes is a prerequisite for the tissue engineering applications. The interaction of the implanted scaffold with the blood components and cells is an initial phase phenomenon which determines the subsequent body reactions such as inflammation and immune system activation [30]. The fabricated collagen/naringin hydrogel was incubated with fresh erythrocytes collected from healthy volunteers and the leaked hemoglobin was quantified with a microplate reader. As shown in Fig. 5 the hemolysis induced with the prepared collagen and collagen/naringin hydrogel are significantly lower than positive control which implied that collagen and collagen/naringin are hemocompatible. The results point out that the fabricated collagen hydrogel containing naringin did not elicit significant hemoglobin leakage from the erythrocyte.
3.5 Cytotoxicity results
Cytotoxicity of the prepared collagen/naringin hydrogel was evaluated using MTT assay kit at 48 and 72 h after cell seeding (Fig. 6). It is shown that the prepared hydrogels are not only cytocompatible but also have a proliferative effect on Schwann cells (SCs). As shown in Fig. 6, pure collagen hydrogel induced cell growth and enhanced cell proliferation at both time intervals and the proliferation was significantly higher than the control group (TCP), (p < 0.005). Moreover, this study apparently revealed the positive effect of naringin on SCs growth at both incubation times. The cell proliferation on the collagen/naringin hydrogel is statistically significant compared with TCP at 48 h and even significantly higher than pure collagen 72 h after cell seeding. (*p < 0.005, **p < 0.001). Previous studies also reported the positive effects of naringin on cell proliferation. Yin et al. reported that 10 nM to 1 μM significantly increased proliferation of hPDLSCs cells (p < 0.05) [31]. Zhang et al. [32] evaluated proliferative effect of naringin on mesenchymal stem cell (MSc) and reported the positive effect of naringin treatment on MSc growth. Our results clearly demonstrate that the incorporation of naringin into the collagen hydrogel made these engineered hydrogels more suitable for cell proliferation due to its tremendous positive effect on the cells growth.
3.6 In vivo studies results
3.6.1 Sciatic functional index results
The SFI is an informative index that widely used to evaluate peripheral nerve function. We used SFI to assess the healing efficacy of the prepared collagen/naringin hydrogels on the injured sciatic nerve. As shown in Fig. 7, the positive control (autograft) and the negative control have SFI of −5.33 ± 0.57 and −94.13 ± 0.57 after 30 days, respectively. After 60 days SFI of the positive control was unchanged, while SFI of the negative control reached to −82.60 ± 1.06. Moreover, the SFI value of pure collagen hydrogel was −76.55 ± 1.82 at 30 days post implantation which improved to −59.80 ± 3.20 after 60 days and these value for the collagen/naringin hydrogel were −61.10 ± 0.60 and −19.13 ± 3.00, respectively. As shown in Fig. 7, collagen hydrogels significantly improved SFI at both time intervals (p < 0.005) which imply the high healing efficacy of the prepared hydrogel especially the naringin loaded hydrogel.
3.6.2 The hot plate latency time results
HPL time test is based on the thermal pain sensitivity of the rats and in the current study was used to evaluate the sensory transduction of the injured nerve treated with the prepared collagen/naringin hydrogels. As shown in Fig. 8, the highest HPL time was observed in the negative control group which implied that the rates did not react against the hot plate within 12 s, while the positive control (autograft) group had the lowest hot plate latency of 3.66 ± 0.47 s. We observed that the average HPL of pure collagen was 9 s which was significantly lower than the negative control group (p < 0.005). Moreover, the collagen/naringin hydrogel group resulted in the hot plate latency average of 6.00 ± 0.81 s which was statistically significant (p < 0.005). These results clearly indicated that the prepared scaffolds improved the thermal pain sensitivity of the injured sciatic nerve.
3.6.3 The compound muscle action potential amplitude
The regeneration of motor neurons was investigated by measuring the compound muscle action potential (CMAP) amplitudes and the results are presented in Fig. 9. As shown in Fig. 9, the highest signal (22.32 ± 1.60 mV) was obtained in the treatment with the collagen/naringin which was significantly higher than the negative control (5.72 ± 1.45 mV, p < 0.005). Moreover, the pure collagen hydrogel exhibited the amplitude of 10.70 ± 2.05 mV which is also statistically significant compared with the negative control. The comparison between the pure collagen and the collagen/naringin hydrogels revealed that incorporation of naringin significantly enhanced the electrophysiological activity of the treated nerve (p < 0.005). These results clearly demonstrate that application of the hydrogels effectively enhanced healing of the injured sciatic nerve.
3.6.4 The gastrocnemius muscle wet weight-loss
The muscles weight loss is a terrible consequence of PNI and measuring the degree of the muscles weight loss can be used to interpret the efficacy of the proposed treatment. In this regard, the gastrocnemius muscle wet weight-loss measurement is a well-established method to assess the progress of PNI healing. This parameter reveals the degree of atrophy induced after sciatic nerve injury and also the efficacy of the treatment applied to the injury. The results (Fig. 10) show that the implantation of both hydrogels (pure collagen and collagen/naringin) significantly decreased the gastrocnemius muscle wet weight loss and prevented the muscle weight-loss (p < 0.005). Moreover, the collagen/naringin hydrogel group exhibited the lowest weight loss (5.7 ± 1.3%) which was statistically significant than the pure collagen hydrogel (9.1 ± 1.3%, p < 0.05). These findings revealed that the treatment with collagen hydrogels prevented the gastrocnemius muscle wet weight-loss and indicted the effectiveness of the prepared hydrogels.
3.6.5 The histopathologic examination
The healing efficacy of the prepared collagen/naringin hydrogels was further investigated using the histopathologic examination and Fig. 11 are showing the obtained results. The results indicated there were not any histopathological changes in the positive control group and the nerve fibers are intact and well-arranged myelinated. On the other hand, the negative control group showed disrupted fiber arrangement with swollen or missing axons and also various degrees of edema and vacuolation (arrowhead). Moreover, various intense damages such as degenerated nerve fibers, notable edema of the nerve fibers, a disintegration of the myelin sheath, and axonopathy were seen in this group. These observations indicated that the induced damage is not able to heal without any treatment. The histopathological observation of the test groups exhibited that the pure collagen hydrogel group showed moderate edema and vacuolation of the myelin sheet. However, the organization of fibers was similar to the normal sample. The histopathological assessment of the collagen/naringin hydrogel group revealed considerable improvements in myelin sheath regeneration and fibers condition. The signs of nerve injuries were completely disappeared except the mild vacuolation. Finally, among two treatments, the micrographs of the collagen/naringin hydrogel group alleviated all the histological changes from the nerve injury and showed more resemblance to the normal sciatic nerve, with well-arranged fibers and intact myelin sheath. Moreover, the regeneration process was significantly enhanced when naringin was added to the hydrogel.
Micrographs of the sciatic nerve and gastrocnemius muscle stained by hematoxylin-eosin (H&E) at the end of 8 weeks post-surgery. Arrowheads: vacuolation, asterisks: collagen hyperplasia, thick arrows: atrophied muscle fiber, thin arrows: edema, the magnification for muscle: ×400, the magnification of nerve:×200
3.6.6 Histopathological changes in muscle fibers
Further evaluation of the muscle fibers histological changes under influence of the applied sciatic damage were performed to confirm the healing efficacy of the prepared collagen/naringin hydrogels. The results exhibited that in the positive control (autograft) group muscle fibers were plump and red in color. On the contrary, the negative control group had shrieked, broken, and wiggly muscle fibers and also fibrotic tissues (star) have been formed between muscular cells. In the pure collagen hydrogel group, the collagen fibers were observed between the muscular fibers and some degree of muscular cell disorganization and atrophy (thick arrow), lower than the negative control group, were observed. On the other hand, regenerated muscular fibers with the lowest fibrosis and muscular shrinkage was observed in the collagen/naringin hydrogel treated group. Moreover, the healing and recovery of the gastrocnemius muscle were better than the negative control and pure collagen hydrogel group, but not as perfect as the autograft group. In overall, the muscle fibers in the collagen/naringin hydrogel group were more similar to the autograft group than others.
An image processing software (Image-Pro Plus, version 6.0, Media Cybernetics, Rockville, MD, USA) was used to quantitatively analyze the cross-sectional area (CSA) of the gastrocnemius muscle, and the results are presented in Fig. 12. The results clearly depict that the highest CSA was obtained with the collagen/naringin treatment which was statistically significant compared with negative control (p < 0.005) and pure collagen hydrogel (p < 0.05).
4 Discussion
The tissue engineering approaches utilize functional structure as the scaffold to enhance the healing capability of the body. Due to the low regeneration capacity of the neural system, the damages to any parts of this system have severe consequences such as the inability to move, sense, and in the long term the muscles atrophy. Collagen-based hydrogels are widely used to enhance the healing process of the damaged sites due to the natural source of collagen and also proper physicomechanical properties of hydrogel structures. The bioactive substance with the antioxidant, anti-inflammatory, and importantly neuroprotective activities are promising for neural damages healing along with the well-designed scaffolds. Naringin is one of the main active components of some Chinese herbal medicines with brilliant biological activates such as remarkable antioxidant property, anti-inflammatory, hypocholesterolemic, and neuroprotective effects [33,34,35,36].
The present study aimed to fabricate and develop a promising remedy based on the natural substance with well-established properties. The SEM images showed that the microstructure of the prepared collagen/naringin hydrogels with proper pore sized and the interconnected states are suited for cell infiltration into the hydrogel, growth, and migrate. The in vitro studies showed that the prepared collagen/naringin hydrogels are cytocompatible and hemocompatible which induced proliferative effects on the isolated -Schwann cells. Our previous study also showed that collagen can induce positive effects on Schwann cells growth due to the natural source and importantly bearing RGD domain (Arginyl- glycine -aspartic acid) along [23]. Moreover, the incorporation of naringin into the collagen hydrogel significantly increased SCs proliferation compared with the control group (TCP) (p < 0.005), and pure collagen hydrogel (p < 0.05). These findings are in agreement with the other researcher’s studies reported that naringin has positive effects on cells growth. Xinlong Ma et al. [37] reported that naringin can ameliorate bone loss induced by sciatic neurectomy via up-regulation of BMP-2 and activation of the PI3K-Akt signal pathway. Kim et al. [38] conducted a study in which showed that naringin protected human neuroblastoma SH-SY5Y from rotenone-induced cell death via inhibiting rotenone-induced apoptosis.
The healing efficacy of the prepared hydrogel was evaluated on the animal model and the hot plate latency, gastrocnemius muscle wet weight-loss, walking-footprint analysis, and nerve conduction were conducted to confirm the healing status. The results implied that the prepared hydrogel properly improved the healing process of the injured sciatic nerve. Previous studies confirmed that the collagen-based structures have positive effects on the healing of the injured nerve and act as a trophic factor and guide the elongation of sprouting axons [39, 40]. Eiko Goto et al. [41] conducted a study in which the migration of neural cells into the fabricated rolled sheet of collagen gel as a nerve conduit was evaluated. They reported that the cells injected into the one end of the prepared conduit migrated into the central part along the inter-layer space of the collagen gel layer. They concluded that the prepared collagen-based conduit is a promising candidate to promote neurite growth.
Along with the structural requirement, anti-oxidant and anti-inflammatory agents could be very helpful to repair the damaged nerve. It is well documented that naringin has potent antioxidant, anti-inflammatory, and neuroprotective activities [19, 42]. Our results showed that incorporation of naringin efficiently improved the healing properties of the prepared collagen hydrogel which is in agreement with the other researcher finding. Various studies have been exploited the antioxidant, anti-inflammatory, and neuroprotective properties of naringin to improve the healing process of the injured nerve. Wei Rong et al. [21] conducted a study to evaluate naringin-enhanced remyelination after spinal cord injury induced with the weight-drop method. They reported the increased thickness of myelin sheath and improved quality of myelinated nerve fibers under treatment with naringin. Moreover, they observed overexpression of 2′3′-cyclic nucleotide 3′-phosphodiesterase and NKx2.2 in the oligodendrocyte precursor cell. On the other hand, naringin treatment reduced phosphorylation of glycogen synthase kinase-3β (GSK-3β) and the expression of β-catenin. They concluded that naringin has the ability to modulate the differentiation of oligodendrocyte precursor cell and improve remyelination through the β-catenin/GSK-3β signaling pathway. In another study [43], naringin was combined with chitosan conduits and peripheral nerve defects healing evaluated in rats. They reported that the injured sciatic nerve treated and regenerated following by naringin incorporated chitosan conduits implantation. Moreover, the electrophysiological assessment showed that Conduit + Naringin groups exhibited the highest conduction velocity (CV). The protein expression assessed with western blot analysis revealed that brain-derived neurotrophic factor (BDNF) and neurotrophins nerve growth factor (NGF) were overexpressed in Conduit + Naringin group.
5 Conclusion
The objective of the present study was to fabricate and characterize a naringin-incorporated collagen hydrogel as a bioactive scaffold for peripheral nerve crush injury treatment in rats. It is shown that the fabricated collagen/naringin hydrogel is structurally proper for neural tissue engineering and moreover, it is Cyto/hemocompatible. The obtained results of animal studies clearly indicated that the collagen/naringin hydrogels could be a promising alternative to treat nerve damages. However, there is still some room for further development to completely restore the lost function after injury. This study depicted that the incorporation of an antioxidant, anti-inflammatory, and proliferative natural substance into the structurally suitable construction can result in brilliant outcomes.
References
Wang EW, Zhang J, Huang JH. Repairing peripheral nerve injury using tissue engineering techniques. Neural Regener Res. 2015;10:1393.
Özkan HS, Karatas Silistreli O, Ergur B, İrkoren S. Repairing peripheral nerve defects by vein grafts filled with adipose tissue derived stromal vascular fraction: an experimental study in rats. Ulus Travma Acids Cerrahi Derg. 2016;22:7–11.
Wang H, Wu J, Zhang X, Ding L, Zeng Q. Study of synergistic role of allogenic skin-derived precursor differentiated Schwann cells and heregulin-1β in nerve regeneration with an acellular nerve allograft. Neurochem Int. 2016;97:146–53.
Oprych KM, Whitby RL, Mikhalovsky SV, Tomlins P, Adu J. Repairing peripheral nerves: is there a role for carbon nanotubes? Adv Healthc Mater. 2016;5:1253–71.
Massoumi B, Hatamzadeh M, Firouzi N, Jaymand M. Electrically conductive nanofibrous scaffold composed of poly (ethylene glycol)-modified polypyrrole and poly (ε-caprolactone) for tissue engineering applications. Mater Sci Eng: C. 2019;98:300–10.
Mozaffari Z, Hatamzadeh M, Massoumi B, Jaymand M. Synthesis and characterization of a novel stimuli‐responsive magnetite nanohydrogel based on poly (ethylene glycol) and poly (N‐isopropylacrylamide) as drug carrier. J Appl Polym Sci. 2018;135:46657.
Massoumi B, Mozaffari Z, Jaymand M. A starch-based stimuli-responsive magnetite nanohydrogel as de novo drug delivery system. Int J Biol Macromolecules. 2018;117:418–26.
Poorgholy N, Massoumi B, Jaymand M. A novel starch-based stimuli-responsive nanosystem for theranostic applications. Int J Biol Macromolecules. 2017;97:654–61.
Abbasian M, Massoumi B, Mohammad-Rezaei R, Samadian H, Jaymand M. Scaffolding polymeric biomaterials: are naturally occurring biological macromolecules more appropriate for tissue engineering? Int J Biol Macromolecules. 2019;134:673–94.
Samadian H, Mobasheri H, Hasanpour S, Faridi-Majid R. Needleless electrospinning system, an efficient platform to fabricate carbon nanofibers. J Nano Res. 2017;50:78–89.
Casolaro M, Casolaro I. Polyelectrolyte hydrogel platforms for the delivery of antidepressant drugs. Gels. 2016;2:24.
Sgambato A, Cipolla L, Russo L. Bioresponsive hydrogels: chemical strategies and perspectives in tissue engineering. Gels. 2016;2:28.
Adibi-Motlagh B, Lotfi AS, Rezaei A, Hashemi E. Cell attachment evaluation of the immobilized bioactive peptide on a nanographene oxide composite. Mater Sci Eng: C.2018;82:323–9.
Ehterami A, Salehi M, Farzamfar S, Vaez A, Samadian H, Sahrapeyma H. In vitro and in vivo study of PCL/COLL wound dressing loaded with insulin-chitosan nanoparticles on cutaneous wound healing in rats model. Int J Biol Macromolecules. 2018;117:601–9.
Ai A, Behforouz A, Ehterami A, Sadeghvaziri N, Jalali S, Farzamfar S. Sciatic nerve regeneration with collagen type I hydrogel containing chitosan nanoparticle loaded by insulin. Int J Polym Mater Polym Biomater. 2019;68:1–10.
Yoshii S, Oka M. Collagen filaments as a scaffold for nerve regeneration. J Biomed Mater Res. 2001;56:400–5.
Phillips JB, Bunting SC, Hall SM, Brown RA. Neural tissue engineering: a self-organizing collagen guidance conduit. Tissue Eng. 2005;11:1611–7.
Kemp SW, Syed S, Walsh SK, Zochodne DW, Midha R. Collagen nerve conduits promote enhanced axonal regeneration, schwann cell association, and neovascularization compared to silicone conduits. Tissue Eng Part A. 2009;15:1975–88.
Chen R, Qi Q-L, Wang M-T, Li Q-Y. Therapeutic potential of naringin: an overview. Pharm Biol. 2016;54:3203–10.
Rong W, Wang J, Liu X, Jiang L, Wei F, Hu X. Naringin treatment improves functional recovery by increasing BDNF and VEGF expression, inhibiting neuronal apoptosis after spinal cord injury. Neurochem Res. 2012;37:1615–23.
Rong W, Pan Y-W, Cai X, Song F, Zhao Z, Xiao S-H. The mechanism of Naringin-enhanced remyelination after spinal cord injury. Neural Regen Res. 2017;12:470.
Wang D, Yan J, Chen J, Wu W, Zhu X, Wang Y. Naringin improves neuronal insulin signaling, brain mitochondrial function, and cognitive function in high-fat diet-induced obese mice. Cell Mol Neurobiol. 2015;35:1061–71.
Salehi M, Naseri-Nosar M, Ebrahimi-Barough S, Nourani M, Vaez A, Farzamfar S. Regeneration of sciatic nerve crush injury by a hydroxyapatite nanoparticle-containing collagen type I hydrogel. J Physiological Sci. 2018;68:579–87.
Ehterami A, Salehi M, Farzamfar S, Samadian H, Vaeez A, Ghorbani S. Chitosan/alginate hydrogels containing Alpha-tocopherol for wound healing in rat model. J Drug Deliv Sci Technol. 2019;51:204–213.
Salehi M, Naseri-Nosar M, Azami M, Nodooshan SJ, Arish J. Comparative study of poly (L-lactic acid) scaffolds coated with chitosan nanoparticles prepared via ultrasonication and ionic gelation techniques. Tissue Eng Regener Med. 2016;13:498–506.
Salehi M, Naseri‐Nosar M, Ebrahimi‐Barough S, Nourani M, Khojasteh A, Hamidieh AA. Sciatic nerve regeneration by transplantation of Schwann cells via erythropoietin controlled‐releasing polylactic acid/multiwalled carbon nanotubes/gelatin nanofibrils neural guidance conduit. J Biomed Mater Res Part B: Appl Biomater. 2018;106:1463–76.
alehi M, Naseri-Nosar M, Ebrahimi-Barough S, Nourani M, Khojasteh A, Farzamfar S. Polyurethane/gelatin nanofibrils neural guidance conduit containing platelet-rich plasma and melatonin for transplantation of Schwann cells. Cell Mol Neurobiol. 2018;38:703–13.
Aurand ER, Lampe KJ, Bjugstad KB. Defining and designing polymers and hydrogels for neural tissue engineering. Neurosci Res. 2012;72:199–213.
Woerly S. Porous hydrogels for neural tissue engineering. In: Materials Science Forum. Trans Tech Publ; 1997;250:53–68.
Feng G, Nguyen TD, Yi X, Lyu Y, Lan Z, Xia J. Evaluation of long-term inflammatory responses after implantation of a novel fully bioabsorbable scaffold composed of poly-l-lactic acid and amorphous calcium phosphate nanoparticles. J Nanomater. 2018;2018:1–9.
Yin L, Cheng W, Qin Z, Yu H, Yu Z, Zhong M. Effects of naringin on proliferation and osteogenic differentiation of human periodontal ligament stem cells in vitro and in vivo. Stem Cells Int. 2015;2015:47–56.
Dai K-R, Yan S-G, Yan W-Q, Chen D-Q, Xu Z-W. Effects of naringin on the proliferation and osteogenic differentiation of human bone mesenchymal stem cell. Eur J Pharmacol. 2009;607:1–5.
Avia‐Saiz M, Busto MD, Pilar‐Izquierdo MC, Ortega N, Perez‐Mateos M, Muñiz P. Antioxidant properties, radical scavenging activity and biomolecule protection capacity of flavonoid naringenin and its glycoside naringin: a comparative study. J Sci Food Agriculture. 2010;90:1238–44.
Anuja G, Latha P, Suja S, Shyamal S, Shine V, Sini S, et al. Anti-inflammatory and analgesic properties of Drynaria quercifolia (L.) J. Smith. J Ethnopharmacol. 2010;132:456–60.
Choe S-C, Kim H-S, Jeong T-S, Bok S-H, Park Y-B. Naringin has an antiatherogenic effect with the inhibition of intercellular adhesion molecule-1 in hypercholesterolemic rabbits. J Cardiovasc Pharmacol. 2001;38:947–55.
Pereira JE, Costa LM, Cabrita AM, Couto PA, Filipe VM, Magalhães LG. Methylprednisolone fails to improve functional and histological outcome following spinal cord injury in rats. Exp Neurol. 2009;220:71–81.
Ma X, Lv J, Sun X, Ma J, Xing G, Wang Y. Naringin ameliorates bone loss induced by sciatic neurectomy and increases Semaphorin 3A expression in denervated bone. Sci Rep. 2016;6:24562.
Kim HJ, Song JY, Park HJ, Park HK, Yun DH, Chung JH. Naringin protects against rotenone-induced apoptosis in human neuroblastoma SH-SY5Ycells. Korean J Physiol Pharmacol. 2009;13:281–5.
Satou T, Nishida S, Hiruma S, Tanji K, Takahashi M, Fujita S. A morphological study on the effects of collagen gel matrix on regeneration of severed rat sciatic nerve in silicone tubes. Pathol Int. 1986;36:199–208.
Chamberlain L, Yannas I, Hsu H, Strichartz G, Spector M. Collagen-GAG substrate enhances the quality of nerve regeneration through collagen tubes up to level of autograft. Exp Neurol. 1998;154:315–29.
Goto E, Mukozawa M, Mori H, Hara M. A rolled sheet of collagen gel with cultured Schwann cells: model of nerve conduit to enhance neurite growth. J Biosci Bioeng. 2010;109:512–8.
Kim HD, Jeong KH, Jung UJ, Kim SR. Naringin treatment induces neuroprotective effects in a mouse model of Parkinson’s disease in vivo, but not enough to restore the lesioned dopaminergic system. J Nutr Biochem. 2016;28:140–6.
Rong W, Cai X, Pan Y, Song F, Zhang C, Xiao S. Combination therapy of chitosan conduit and naringin facilitate regeneration of injured sciatic nerve in rats. In: BIBE 2018; International Conference on Biological Information and Biomedical Engineering. VDE 2018. pp 1–4.
Acknowledgements
The authors gratefully acknowledge the research council of Kermanshah University of Medical Sciences (grant no. 980264) for financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Samadian, H., Vaez, A., Ehterami, A. et al. Sciatic nerve regeneration by using collagen type I hydrogel containing naringin. J Mater Sci: Mater Med 30, 107 (2019). https://doi.org/10.1007/s10856-019-6309-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10856-019-6309-8