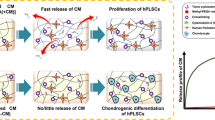
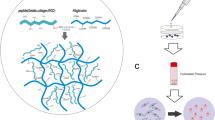
Abstract
Cartilage tissue regeneration often presents a challenging clinical situation. Recently, it has been shown that Periodontal Ligament Stem Cells (PDLSCs) possess high chondrogenic differentiation capacity. In this study, we developed a stem cell delivery system based on alginate/hyaluronic acid (HA) loaded with TGF-β1 ligand, encapsulating PDLSCs; and investigated the chondrogenic differentiation of encapsulated cells in alginate/HA hydrogel microspheres in vitro and in vivo. The results showed that PDLSCs, as well as human bone marrow mesenchymal stem cells (hBMMSCs), as the positive control, were stained positive for both toluidine blue and alcian blue staining, while exhibiting high levels of gene expression related to chondrogenesis (Col II, Aggrecan and Sox-9), as assessed via qPCR. The quantitative PCR analyses exhibited that the chondrogenic differentiation of encapsulated MSCs can be regulated by the modulus of elasticity of hydrogel delivery system, confirming the vital role of the microenvironment, and the presence of inductive signals for viability and differentiation of MSCs. In vivo, histological and immunofluorescence staining for chondrogenic specific protein markers confirmed ectopic cartilage-like tissue regeneration inside transplanted hydrogels. PDLSCs presented significantly greater capability for chondrogenic differentiation than hBMMSCs (P < 0.05). Altogether, our findings confirmed that alginate/HA hydrogels encapsulating PDLSCs are a promising candidate for cartilage regeneration.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The regeneration and repair of damaged articular cartilage remain as a challenging clinical situation due to its poor intrinsic capacity for repair [1]. Current treatment modalities including grafting of autologous osteochondral tissue have not been able to reproduce the biological composition and biomechanical properties of the original cartilage [1,2,3]. Application of mesenchymal stem cells (MSCs) can be considered as an alternative therapeutic option for regeneration of lost cartilage tissues [4, 5]. MSCs are multipotent cells that have the ability to differentiate into multiple lineages dependent on the signals that they receive from the surrounding microenvironment. Studies have shown that in the presence of the proper signaling molecules, MSCs are able to differentiate into chondrogenic tissue and deposit a cartilage-specific matrix [5,6,7]. Bone marrow MSCs (BMMSCs) have been utilized widely as the source of cell therapy for cartilage repair/regeneration studies. Recently, it has been shown that dental- and orofacial-derived MSCs, including Periodontal Ligament Stem Cells (PDLSCs) possess a strong capacity for chondrogenic differentiation [8]. Moshaverinia et al. developed an injectable and degradable delivery vehicle based on RGD-coupled alginate-hydrogel containing TGF-β1 ligands as a dental-derived MSC delivery system [8]. It was reported that the system supports the viability, and chondrogenic differentiation of PDLSCs encapsulated within RGD-coupled alginate-hydrogel scaffold in vitro and in vivo. This microencapsulation system is of particular interest for cartilage tissue engineering as periodontal ligament tissue as the source of PDLSCs is accessible via the oral environment and they can easily be found in dental clinics as discarded biological samples [9, 10].
It has been shown that the cell delivery vehicle plays a vital role in the in vivo performance of MSCs and the success of regenerative therapy [11,12,13,14]. It is well known that hydrogel-based scaffolds biomaterials are widely utilized for cartilage tissue engineering. Alginate hydrogels have been widely used for cartilage tissue engineering applications due to unique characteristics including: biodegradability and injectability. Alginate is a natural hetero-polysaccharide that is extracted from brown sea algae [15, 16]. This hydrogel biomaterial goes through an ionically crosslinking in the presence of multivalent cations such as Ca2+ and Ba2+. Additionally, alginate has the capability to facilitate the spatial distribution of the encapsulated MSCs within its 3D structure leading to the formation of a structural organization with close resemblance to native in vivo microenvironment. Furthermore, alginate hydrogel has been successfully been utilized for chondrogenic differentiation of encapsulated MSCs, due to their ability to have controlled delivery of growth factors such as TGF-β [17,18,19,20]. However, alginate is not one of the natural components of the cartilage matrix. Therefore, in the current study a cartilage extracellular matrix component, hyaluronic acid (HA), was added to alginate hydrogel to develop a novel dental MSC delivery vehicle with cartilage regeneration potential.
HA is composed of repeating disaccharides of N-acetyl-d-glucosamine and β-glucuronic acid [21]. HA hydrogel is abundantly found in synovial fluid and a main component of glycosaminoglycan superstructure complexes that are associated with other polysaccharides such as chondroitin sulfate [21, 22]. HA is able to anchor to the cell surface via cell surface receptors such as CD44 and RHAMM [23]. Studies have shown that HA is a promising delivery device for cartilage repair and regeneration [24, 25]. However, HA has some disadvantages, including its rapid degradation and participation in hydrolytic reactions [24,25,26]. In addition, HA hydrogels are difficult to handle in cell culture. The addition of alginate hydrogel can improve some of these qualities. Moreover, it has been shown that adding alginate hydrogels to HA can increase proteoglycan retention in the gel, leading to greater amounts of cartilage regeneration [19].
Furthermore, studies have shown that primarily signaling molecules from the microenvironment controls the proliferation and chondrogenic differentiation of encapsulated MSCs in hydrogel biomaterials [27, 28]. Several growth factors have been found to significantly direct the chondrogenic differentiation of MSCs. It has been shown that TGF-β1 is a key player in the chondrogenesis process [29]. It has been shown that transforming growth factor-beta (TGF-β) can stimulate chondrocyte proliferation toward chondrogenic tissues and therefore possess a vital role in chondrogenesis of MSCs [30, 31].
In light of these findings, in the current study, we designed a suitable microenvironment containing the suitable inductive signals (TGF-β1) for encapsulation and chondrogenesis of PDLSCs by regulating the physiochemical characteristics of the microenvironment to direct the fate of the encapsulated MSCs toward the chondrogenic tissue. Here, we developed a 3D alginate/ hyaluronic acid hydrogel co-delivery system containing TGF-β1 ligand for microencapsulation of PDLSCs, ensuring optimized cartilage regeneration.
2 Materials and methods
2.1 Stem cell isolation and culture
PDL tissues were obtained from the teeth of healthy fifteen patients (18–25 years old) undergoing third molar extractions. All the experiments were performed after obtaining informed consent from all subjects in accordance with the guidelines and regulations from University of Southern California with required the IRB approval. The patients who were candidates of third molar extractions or orthodontic related extractions were included in this study. An exclusion criterion was any subject with a history of periodontal disease. The extracted human PDLSCs were isolated and cultured according to published protocols in the literature [32]. Subsequently, PDLSCs as well as the positive control group, hBMMSCs (Lonza Inc. Walkersville, MD), were separately cultured in regular culture media.
2.2 Flow cytometric analysis
PDLSCs or hBMMSCs (2 × 105 cells) were incubated with specific phycoerythrin-conjugated mouse monoclonal antibodies for CD105, and CD146 (as positive MSC markers) (BD Biosciences), human CD45 (as a negative hematopoietic stem cell marker), or isotype-matched control immunoglobulin Gs (IgGs; Southern Biotechnology Associates). The specimens were subjected to flow cytometric analysis using a FACSCalibur with CellQuest software (BD Biosciences) according to previously reported methods [33].
2.3 Hydrogel biomaterial fabrication and MSC encapsulation
High glucuronic acid containing alginate hydrogel (NovaMatrix FMC Biopolymer, Norway) was purified and oxidized (2%) according to previously reported methods [22, 23]. Then, hyaluronic acid (Sigma-Aldrich) was dissolved in PBS to make a 5% w/v solution. The HA solution was filtered using a 0.22 µm sterile syringe filter (Millipore) and was added to alginate powder to make a hydrogel mixture with alginate to HA 1:1 w/w and 2:1 w/w ratios. Finally, TGF-β1 (Abcam, Cambridge, MA) (50 μg/mL) the HA/Alginate solution was sterile filtered through 0.22 µm syringe filter. Next, TGF-β1 (Abcam, Cambridge, MA) (50 μg/mL) was added to the HA/alginate hydrogel, the mixture was concentrated, and freeze-dried subsequently.
2 × 106 PDLSCs or hBMMSCs were encapsulated in 1 mL of either HA/Alginate solution (alginate/HA 1/1 or 2/1 w/w ratios) containing TGF-β1 ligand (50 μg/mL) [24]. Microsphere formation was accomplished according to previously described methods [33]. Passage four cells were used in all the experiments. Alginate/HA hydrogel droplets were added dropwise into a 100 mM CaCl2 solution to form hydrogel microbeads. In order to complete the ionic cross-linking, the fabricated microspheres were incubated at 37 °C for 45 min followed by washing with non-supplemented DMEM for three times. The effects of the presence of HA on cell viability, adhesion, and chondrogenesis were analyzed on PDLSCs and hBMMSCs encapsulated in alginate hydrogels. In the current study, as a negative control, cell-free HA/alginate hydrogel was utilized. Passage four cells were utilized in all experiments.
2.4 Characterization of the fabricated scaffolds
In order to characterize the release profile of TGF-β1 ligands from the developed HA/alginate hydrogel, microspheres loaded with TGF-β1 (50 μg/mL) were incubated in high-glucose DMEM (500 mL) in 48-well plates for two weeks at 37 °C. At different time intervals, the amounts of released TGF-β1 were measured using an anti-human recombinant TGF-β1 ELISA kit (Abcam). Additionally, retained TGF-β1 ligands were detected with antibodies against TGF-β1 (Abcam) and measured using immunofluorescence staining. Additionally, the cumulative released TGF-β1 was evaluated.
The morphological characteristics of fabricated scaffolds were studied using scanning electron microscopy (SEM) (JEOL 5300, Peabody, MA). After two weeks of culturing in the regular culture medium, the MSC – hydrogel scaffold constructs were conditioned and prepared according to previously published protocols and observed using SEM [9]. Pore size ranges and pore diameters were determined using SEM-associated image analysis software [34].
2.5 Cell encapsulation, live/dead staining, and cell proliferation analysis
PDLSCs and hBMMSCs were encapsulated separately in HA/alginate hydrogels (1:1 and 2:1 w/w ratios) loaded with TGF-β1 (50 μg/mL). MSCs were encapsulated in HA/alginate mixture (2 × 106 cells/mL) according to the abovementioned protocol. Cell free HA/alginate hydrogels (and no TGF-β1) were utilized as the negative control. After 14 days of culturing in regular culture medium, the viability of the encapsulated MSCs was assessed using Calcein AM/ethidium bromide homodimer-1 (Invitrogen) live/dead staining according to methods in the literature [10]. The percentage of live cells was evaluated using NIH ImageJ software (NIH, Bethesda, MD).
To analyze the metabolic activity of encapsulated MSCs in the HA/alginate microspheres containing TGF-β1, a 3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyl- tetrazolium bromide (MTT) assay was used according to methods already in the literature [33]. At each time interval, six independent specimens were evaluated for each tested group.
2.6 In vitro chondrogenic assay
To induce chondrogenic differentiation, PDLSCs and hBMMSCs (2 × 106 cells) encapsulated in 1 mL of HA/alginate microspheres were cultured in a chondrogenic media. As the control group, HA/alginate hydrogel without any cell or growth factor were utilized. The specimens were fixed with 4% PFA four weeks after culturing in chondrogenic inductive media. The fixed specimens were paraffin embedded and sections were made. Chondrogenic differentiation was evaluated by staining with 0.1% toluidine blue (Sigma) or Alcian blue (Sigma) solution.
Anti -Sox 9 and Collagen II (Abcam) antibodies were utilized to immunolabel the specimens at 4 °C overnight and were detected using Alexa fluor conjugated secondary antibody (1:200 dilution; Invitrogen). The slides were then counterstained with DAPI. Six independent specimens were analyzed for each group. NIH Image-J software (NIH, Bethesda, MD) was utilized to measure the positively stained areas.
The proliferation of the encapsulated MSCs during chondrogenic differentiation was also determined by DNA quantification. At different time intervals post-chondro-differentiation, the specimens were retrieved to quantify the total amount of DNA. A DNeasy tissue kit (Qiagen, Chatsworth, CA) was utilized to isolate the DNA content of each specimen according to the manufacturer’s instructions. Total DNA content was measured by a microplate reader (Thermofisher) at a wavelength of 280 nm.
Additionally, the effects of the modulus of the elasticity (E) of the alginate/HA hydrogels on the chondrogenic differentiation capacity of encapsulated MSCs were analyzed. An Instron mechanical testing machine (Norwood, PA) was used to measure the elasticity of each prepared hydrogel (with different alginate-to-HA ratios (1:1 and 2:1 w/w)) according to the methods already in the literature [35].
2.7 Real time PCR analysis
After 4 weeks of chondrogenic differentiation of encapsulated MSCs, as mentioned above, RNA was extracted from the encapsulated cells. Briefly, RNA was extracted using Trizol reagent (Invitrogen). Single-stranded cDNA was synthesized with 100 ng total RNA using a Superscript III cDNA synthesis kit (InvitrogenData were analyzed by the 2-∆∆Ct method and normalized to the Ct of GAPDH as the housekeeping gene. Table 1 is presenting the sequences of the primers that were used. Furthermore, to examine the effects of the matrix modulus of elasticity on the chondrogenesis of encapsulated MSCs, PDLSCs or hBMMSCs were encapsulated in HA/alginate (2:1) hydrogels with different elasticity and after 4 weeks of chondro-induction, qPCR was used to analyze the expression levels of chondrogenic related genes.
2.8 Western blot analysis
In order to run Western blot analysis, after 2 weeks of chondro-induction, MSCs were recovered from hydrogels and lysed using protein extraction buffer (Bio-Rad, Irvine, CA). The extracted proteins were fractionated in 10% sodium dodecyl sulfate-polyacrylamide gels (PAGE), and electrophoretically transferred to a nitrocellulose membrane (Bio-Rad). Next, the membranes were incubated with antibodies against rabbit Sox9 antibodies (Abcam). The membranes were re-probed with an antibody against the housekeeping gene b-actin (Abcam). Subsequently, chemiluminescent reagent (Amersham Life Science, Pittsburgh, PA) was used and the membranes were exposed to X-ray film (Thermo Scientific, Rockford, IL). The experiments were repeated three times to confirm the reproducibility of the data.
2.9 MSC-mediated chondrogenic-like tissue regeneration in vivo
5-month-old Beige nude XID III (NU/NU) mice (Harlan, Livermore, CA) were used in the current study. PDLSCs or hBMMSCs (4 × 106 cells) were encapsulated in HA/alginate microspheres containing TGF-β1 and approximately 10 microspheres (500 μl) were subcutaneously transplanted into the dorsal surface of the mice (N = 5). Eight weeks post-implantation, the animals were sacrificed, and tissues were surgically retrieved. The harvested tissue samples were examined using histochemical, histological, and immunofluorescence staining. For the negative control group hydrogels without cells were used. All investigations in this study conform to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health and approved by the Institutional Animal Care and Use Committee at the University of Southern California.
2.10 Histochemical, Histological, and Immunofluorescence analysis
The retrieved specimens were prepared (fixed, paraffin embedded, and sectioned) according to the methods published earlier in the literature. The prepared glass slide specimens were stained with Hematoxylin & Eosin (H&E) (N = 5 for each group). Moreover, the prepared sections were stained with toluidine blue to detect newly synthesized cartilage ECM glycosaminoglycans (GAGs). Image-J software was utilized to evaluate and quantify the percentage of positively stained area (N = 6).
For immunofluorescence staining, the prepared specimens were treated with 3% H2O2 and then with a blocking buffer containing 1% BSA and 0.25% Triton X-100 in PBS. Rabbit anti-mouse collagen II (Calbiochem, San Diego, CA) and anti-Ki67 (proliferative activity marker) antibodies (1:100 dilution) were used to stain the specimens. Detection of antibodies were performed using Alexa Fluor conjugated secondary antibody (1:200 dilution). Subsequently, the specimens were counterstained with DAPI (Vector Laboratories, Burlingame, CA). The proliferative activity of the encapsulated MSCs three weeks post-implantation was evaluated via immunofluorescence staining using antibodies against ki67 (Abcam) and counterstained with DAPI.
2.11 Statistical analysis
Quantitative data were expressed as mean and standard deviation (SD). One-way and two-way analyses of variance, followed by Tukey’s test at a significance level of α = 0.05, were used for comparison among multiple sample means. For animal studies, the data were analyzed using Kruskal-Wallis rank sum test at a significance level of α = 0.05. Whenever needed, Student’s t-tests were utilized for comparisons.
3 Results
3.1 HA/alginate hydrogel fabrication and release profile characterization
In the current study, PDLSCs were isolated from periodontal tissues of fifteen patients with healthy periodontium. The isolated MSCs were expanded in vitro, encapsulated in the HA/alginate hydrogel delivery system and utilized for chondrogenic differentiation assays in vitro and in vivo. Human BMMSCs were used as the positive control, while cell-free HA/alginate hydrogels were utilized as the negative control. Next, the capability of the HA/alginate hydrogel encapsulation system to contribute to MSC viability and chondrogenic differentiation was examined in vitro.
Flow cytometric analysis was performed to evaluate the stemness of the isolated cells. Specific MSC markers including CD73, CD105, and CD146 (Supplementary Fig. s1) were expressed both by PDLSCs as and hBMMSCs. Additionally, none of the tested MSCs expressed hematopoietic lineage markers (e.g., CD34 or CD45) supporting the MSC properties of PDLSCs.
Live/dead staining showed high in vitro viability for PDLSCs and hBMMSCs encapsulated in fabricated hydrogels after 14 days of culturing in regular culture media (Figs. 1a, b). Moreover, our MTT assay confirmed high metabolic activity of the encapsulated MSCs in HA/alginate hydrogels loaded with TGF-β1 after 21 days of culturing in regular media in vitro (Fig. 1c). The measured amounts of DNA content were consistent with the MTT assay results (Fig. 1d). DNA content (cell proliferation) in Alginate/HA hydrogels was higher (P > 0.05) in the group with alginate scaffold alone after 1 week of culturing. No significant difference in the DNA content was found between the groups with alginate/HA at 2:1 w/w and 1:1 w/w ratios (P > 0.05) (Fig. 1d).
The viability of the encapsulated PDLSCs and hBMMSCs: a Live/dead staining of the encapsulated hBMMSCs and PDLSCs in alginate/HA hydrogel microspheres (with an average diameter of 650 nm) after one week of culturing in regular culture media. b Percentage of live cells on days up to 3 weeks exhibiting more than 75% of encapsulated MSCs remained viable. c MTT assay showing the metabolic activity of encapsulated MSCs. d DNA content of encapsulated MSCs after 7 days in different hydrogels. *P < 0.05, NS: not significant
In addition, through light microscopy, it was determined that the fabricated HA/alginate hydrogel microspheres were of uniform size with an average diameter of 950 μm, and had uniform cell distribution (Fig. 2a). SEM examination showed a porous morphology (110 μm average pore diameter with a rage from 58 to 153 μm) for fabricated HA/alginate hydrogel with encapsulated MSCs spread within the matrix (Figs. 2b, c). Figure 2d showed the elastic modulus of hydrogels of different alginate /HA ratio and composition, confirming that presence of higher concentration of HA decreased the elasticity of hydrogel.
Characterization of fabricated hydrogel microspheres and release profile analysis: a Light microscopy analysis showing that the hydrogel microspheres (Alg/HA 1:1 here) retain their spherical shapes with an average diameter of 650 μm and MSCs evenly distributed within their matrix. b, c SEM image of fabricated alginate/HA hydrogels showing their micro-porous structure with 150 μm average pore diameter and presence of MSCs encapsulated within the hydrogel matrix. d Influence of alginate/HA ratios and hydrogel composition on the elastic modulus. e In vitro release profile characterization of TGF-β1 for fabricated alginate/HA and alginate hydrogels confirming sustained release of TGF-β1. A slight increase in the initial release of the growth factor was observed from the hydrogel mixture with the higher concentration of HA; however, no significant difference was observed in releasing rates of growth factors from the scaffolds (P > 0.05), while both exhibited first-order kinetics. f Immunofluorescence staining against anti-TGF-β1 antibodies showing the retained TGF-β1 ligands remained within alginate/HA 2:1, alginate/HA 1:1, and alginate hydrogels after 14 days in vitro. g Semi-quantitative analysis of retained TGF-β1 ligands in panel e. *P < 0.05, NS: not significant
Subsequently, the alginate/HA hydrogel was utilized as a growth factor delivery vehicle. Briefly, alginate/HA microspheres were loaded with TGF-β1 and the TGF-β1 release from the alginate/HA (1:1 and 2:1 w/w ratio) scaffolds was analyzed for two weeks. Figure 2e shows the cumulative release of TGF-β1 from the HA/alginate hydrogel scaffolds. The results confirmed continued release of TGF-β1 for up to 14 days. A slight increase in the initial release of growth factor was observed from hydrogel mixture with the higher concentration of HA; however, no significant difference was observed in release of the growth factor from the two tested scaffolds for up to two weeks (p value > 0.05). Moreover, immunofluorescent staining with the anti-TGF-β1 antibody showed less retained TGF-β1 in alginate/HA hydrogels than alginate hydrogel without HA after 14 days (Figs. 2f and 2g).
3.2 Chondrogenic differentiation of MSCs in vitro and the role of the microenvironment
After four weeks of differentiation of encapsulated PDLSCs and hBMMSCs in vitro, positive toluidine blue (Fig. 3a) and Alcian blue (Fig. 3b) staining confirmed chondrogenic differentiation. (Figs. 3a–c). Additionally, histochemical and immunofluorescent staining results correlated well with each other, confirming that PDLSCs and hBMMSCs expressed type II collagen (Col II) gene (Fig. 3c). Our quantitative evaluation of the specimens, showed that PDLSCs higher expression level for chondrogenesis-related genes than hBMMSCs (P < 0.05) (Fig. 3d). MSCs encapsulated in alginate/HA at a 2:1 w/w ratio showed greater (P > 0.05) amounts of expression levels of Col II compared to MSCs encapsulated in HA/alginate at a 1:1 w/w ratio. Both alginate/HA groups showed statistically greater amounts (P < 0.05) of Col II staining in comparison to alginate hydrogel alone as the encapsulating matrix (Fig. 3d). Additionally, encapsulated PDLSCs and hBMMSCs showed a significant increase in the proliferation rate (cell number) after three week of culturing (Fig. 3e).
In vitro chondrogenic differentiation of encapsulated MSCs. The histochemical analysis confirmed spread-out cell morphology for both alginate/HA and alginate hydrogel with positive toluidine blue a and alcian blue b staining showing the formation of chondrogenic tissue-related extracellular matrix. c Positive staining in the immunofluorescence labeling confirming the production of type-II collagen by encapsulated PDLSCs. d The percentage of cells positive for antibodies against Col II was quantified and greater Col II expression levels in PDLSCs compared to hBMMSCs (P < 0.05) was observed. Both alginate/HA groups showed statistically greater amounts (P < 0.05) of Col II staining than the group with alginate hydrogel alone as the encapsulating matrix. (e) DNA content for MSCs encapsulated in different hydrogels after three weeks of chondrogenic induction. *P < 0.05, **P < 0.01, NS: not significant
Gene expression analysis was performed to evaluate the molecular mechanism underlying the chondrogenic differentiation of encapsulated MSCs. Several chondrogenic differentiation markers (e.g., Col II, Sox9, and ACAN) were analyzed (Fig. 4a). Quantitative PCR was utilized to analyze the expression levels of chondrogenesis-related genes. Our data showed that that PDLSCs and hBMMSCs both abundantly expressed Col II, Sox 9, and Aggrecan 2 weeks after in vitro chondrogenic differentiation (Fig. 4a).
Molecular analysis of chondro-differentiation of encapsulated MSCs. a The expression levels of Collagen type II (Col II), Aggrecan (Agg) and Sox9 genes after 14 days of chondrogenic induction in vitro, analyzed using RT-PCR. b Western blot analysis exhibiting the expression levels of chondrogenesis regulator gene Sox9. The level of Sox9 expression is higher in the encapsulated MSCs in alginate/HA hydrogels containing TGF-β1. Two-way ANOVA was used to compare the results. *P < 0.05, NS: not significant
Quantitative gene expression analysis revealed that PDLSCs showed higher amounts of chondrogenic differentiation compared to hBMMSCs (P < 0.05) (Fig. 4a). For all the analyzed chondrogenic genes, the alginate/HA groups showed statistically higher (P < 0.05) levels of chondrogenesis-related gene expression than the group with alginate hydrogel alone as the encapsulating matrix.
Additionally, Western Blot analysis showed that MSCs encapsulated in alginate/HA at a 2:1 w/w ratio had a higher expression level of Sox9 when compared to MSCs encapsulated in HA/alginate at a 1:1 w/w ratio (Fig. 4b) suggesting that the hydrogel elasticity can contribute to chondrogenic differentiation of MSCs, where an increased elasticity of hydrogel biomaterial reduced Sox9 expression levels.
In the next step, Alg/HA 2:1 hydrogels with three different concentrations of Calcium ions (and therefore different elasticity) were fabricated and used for PDLSCs encapsulation. After four weeks of in vitro chondrogenesis, both immunofluorescence (Figs. 5a and b) and PCR analyses (Fig. 5c) showed that the chondrogenic differentiation of encapsulated MSCs is regulated by the elasticity of the HA/alginate hydrogel. Quantitative PCR analysis showed that MSCs encapsulated in hydrogel with higher elasticity (more rigid) (>30 kPa, no HA), showed lowest amounts of chondro-differentiation compared to the hydrogel with low to intermediate elasticity (10–18 kPa, lower concentrations of HA). Soft hydrogels with elasticity of less than 9 kPa (higher concentration of HA) showed lower levels of expression levels for chondrogenic differentiation related genes (Fig. 5c). Generally, MSCs encapsulated in HA/alginate at 2:1 w/w ratio showed statistically greater amounts of Col II expression compared to MSCs encapsulated in HA/alginate at a 1:1 w/w ratio or alginate alone.
Matrix elasticity regulates the differentiation of encapsulated PDLSCs toward chondrogenesis. Alg/HA 2:1 hydrogels with three different concentrations of calcium ions (50, 100, and, 200 mM), and therefore different moduli of elasticity, were fabricated and used for PDLSCs encapsulation. After four weeks of in vitro chondrogenesis, a Immunofluorescence labeling with antibodies against Col II (counterstained with DAPI) for encapsulated MSCs in alginate/HA hydrogel with different elasticity. b Percentage of positively stained cells against anti-Col II antibodies in panel a. c RT-PCR analysis of Col II gene expression of encapsulated PDLSCs in alginate/HA with 2:1 w/w ratio hydrogel with different elasticity following 4 weeks of chondrogenic induction assay. *P < 0.05, NS: not significant
3.3 PDLSC contribution to chondrogenesis in vivo
In order to assess the in vivo chondrogenic tissue formation capability of encapsulated PDLSCs in HA/alginate hydrogel scaffold containing TGF-β1, encapsulated PDLSCs were subcutaneously implanted in immunocompromised mice. In this animal model, hBMMSCs encapsulated in HA/alginate hydrogel were used as the positive control, and cell free HA/alginate hydrogel was used as the negative one. To evaluate chondrogenic tissue formation, histological, histochemical, and immunofluorescence analyses were utilized. Results of H&E staining showed that encapsulated MSCs possess morphological structure similar to human chondrocytes with large islands of rounded cells in the alginate/HA scaffolds. This type of morphology was not found in the cell-free implants (Fig. 6a). Chondrogenic differentiation of the encapsulated MSCs was confirmed by positive toluidine blue staining of the matrices. The round morphology of committed chondrocytes was observed in the PDLSC or hBMMSC groups. In contracts, our control group (HA/alginate hydrogel alone) showed no positive staining (Fig. 6b).
Subcutaneous transplantation of encapsulated MSCs: a H&E analysis of the harvested specimens showing the presence of islands of rounded cells with a similar morphology to human chondrocytes. b Toluidine blue staining and Immunofluorescence staining c, with anti- collagen type II antibodies, of retrieved. d The percentage of positive cells in each specimen according to immunostaining results of panel c. (#: unresorbed hydrogel scaffold). e Proliferative activity of encapsulated PDLSCs was evaluated using immunofluorescence staining with an anti-ki67 antibody were positive cells were stained red. f Percentage of proliferating PDLSCs encapsulated in different hydrogels 21 days after transplantation. *P < 0.05, NS: not significant
Immunofluorescent staining was used to characterize the chondrogenic differentiation of MSCs in vivo and to identify neo cartiage-like tissue formation gene expression analysis was utilized. Extensive staining with Col II antibodies within the regenerated chondrogenic tissues was observed 8 weeks after subcutaneous implantation in nude mice (Fig. 6c). Our semi-quantitative analysis showed that engrafted PDLSCs had significantly higher expression levels of chondrogenic related genes compared to hBMMSCs (P < 0.05) (Fig. 6d). However, both alginate/HA groups showed statistically greater amounts (P < 0.05) of Col II expression than the group with alginate hydrogel alone as the encapsulating matrix. Additionally, the proliferative activity of encapsulated MSCs 21 days after implantation was evaluated via immunostaining against anti-ki67 antibody. Our results showed significantly greater percentages of ki67-positive cells encapsulated in the alginate/HA groups in comparison to the group without HA (Figs. 6e and f).
4 Discussion
It has been reported that, subsequent to trauma or disease the articular cartilage possesses very limited intrinsic healing capacity, due to the presence of a limited number of chondrocytes and absence of suitable vascular supply [1]. Currently available treatment modalities for cartilage regeneration/repair include tissue debridement, arthroscopic lavage, microfracture of the subchondral bone and abrasion arthroplasty. However, most of these approaches are not effective at generating high-quality tissues or restoring functional cartilage [1,2,3]. To that end, cell-based tissue engineering therapies provide an attractive, potentially advantageous alternative to currently available approaches for cartilage repair and regeneration. The identification of an optimal source of cells with suitable chondro-differentiation capacity is of the utmost importance for cartilage tissue regeneration.
Transplantation of chondrocytes or MSCs has shown promise as a treatment strategy. However, chondrocytes are unstable in monolayer culture conditions, which lead to the loss of their phenotypic characteristics. Besides, the limited proliferation capability of chondrocytes may lead to considerable difficulty in producing a sufficient number of cells for the cartilage repair processes [36, 37]. Alternatively, MSCs, as the source of cell for cartilage tissue engineering, can be easily isolated, retain high proliferation capability, and show multilineage plasticity (e.g., chondrogenesis) [38, 39]. In our previous studies, we showed that dental-derived MSCs (e.g., PDLSCs) are promising candidates for cartilage tissue engineering. These types of MSCs are a source of superior MSCs for this purpose, and importantly, they are also readily accessible in the oral cavity and can be found in discarded biological tissues in dental clinics. These unique characteristics make periodontal ligament tissues the ideal sources for stem cell banking purposes [8, 9]. Hence, human PDLSC can be considered as a favorable cell for cartilage tissue regeneration and repair.
It is well known that the encapsulating biomaterial has a fundamental role in the successful in vivo performance of stem cells in tissue engineering [11, 12]. Studies have confirmed that the porous structure of the scaffold can be advantageous for cell attachment, proliferation and differentiation leading to appropriate cell phenotype [11,12,13,14]. An alginate/HA hydrogel system has an ideal porous structure, which supports cell adhesion and migration and permits transportation of oxygen, nutrients, waste molecules, and growth factors, influencing cell behavior and phenotype [8, 33, 40, 41]. In our previous studies, alginate hydrogel was utilized as a 3D scaffold for the encapsulation of MSCs and their chondro-differentiation. However, alginate is not a natural component of the cartilage matrix. Therefore, in the current study, hyaluronic acid (HA), a cartilage extracellular matrix component, was added to the hydrogel to develop a dental MSC delivery vehicle with cartilage regeneration potential. Our results demonstrate that the addition of HA to the alginate hydrogel can enhance cell proliferation and retention, probably due to the ability of HA to anchor to the cell surface via CD44 cell surface receptors [23, 24]. It has been reported that CD 44 receptor is expressed in PDLSCs, and that can bind HA [42, 43]. Our findings correlated well with previous reports that an alginate/HA scaffold seems to be better for both proliferation and chondrogenic differentiation of encapsulated MSCs than alginate alone [21]. The interaction of the biomaterial matrix and the encapsulated MSCs can be facilitated by the porous structure of HA and the presence of RGD tripeptide enhancing cell adhesion and viability via increased access of cells to oxygen, nutrients, and growth factors [44]. In the current study, we observed more ki67-positive MSCs encapsulated in alginate/HA hydrogels than in alginate hydrogel, showing that MSCs had higher proliferative activity inside alginate/HA microspheres. This confirms the vital role of the MSC delivery vehicle in fate determination and successful tissue regeneration/repair.
Furthermore, we demonstrated here that modulus of elasticity of the matrix partially determines the fate of encapsulated MSCs. While the addition of HA hydrogel to alginate increased the chondrogenic differentiation of encapsulated PDLSCs in comparison to alginate alone, a higher concentration of HA led to a decrease in the modulus of the elasticity of the alginate/HA matrix and reduced the capacity of encapsulated PDLSCs to be differentiated toward chondrogenic tissues.
To our knowledge, alginate/HA microencapsulation system has not been developed and utilized for the chondrogenic differentiation of orofacial-derived MSCs. In the injectable system developed in this study, encapsulated MSCs showed high degrees of viability, proliferation, and chondrogenic differentiation potential. Considering the source of the MSCs utilized in this study, it can be concluded that the periodontal ligament provides a great source of stem cells in light of their accessibility in the oral environment and autologous transplantation capacity. Our laboratory and others have confirmed the presence of populations of multipotent postnatal stem cells in the periodontal ligament tissues. These stem cells can be easily isolated and expanded in vitro [8, 9, 32, 45, 46]. Hence, human PDLSCs can be considered unique MSCs for MSC-mediated therapies for cartilage tissue repair/regeneration.
In addition, we confirmed substantial chondrogenic differentiation capacity for PDLSCs in the presence of TGF-β1 via our in vitro and in vivo studies. In this study, encapsulated PDLSCs in alginate/HA hydrogels promoted cartilage regeneration in situ 8 weeks after subcutaneous implantation in immunocompromised mice. Furthermore, we confirmed that the encapsulating biomaterials’ physiochemical properties (e.g., elasticity and the presence of cell binding motifs) and the presentation of signaling ligands (e.g., TGF-β1) regulate the viability, function, and differentiation and therefore the fate of the implanted MSCs, leading to enhanced tissue regeneration. Our current findings suggest that besides the elastic modulus of the encapsulating biomaterial, the biochemical microenvironment also affects the fate of the engrafted MSCs. The other important parameter that affects the fate of the encapsulated MSCs is the molecular mesh size, which governs transport diffusion of nutrients and cytokines. This study confirmed that PDLSCs have significantly greater chondrogenic differentiation capacity in the presence of TGF-β1 in comparison to hBMMSCs. One of the limitations of the current study is the source of stem cells as periodontal tissues are not always easily available/accessible. As an alternative cell source gingival mesenchymal stem cells will be utilized in our future work for chondrogenic tissue regeneration. In our future work, this system will be optimized and used in more clinically relevant animal models for cartilage tissue regeneration. The results of these endeavors will be reported in due course.
5 Conclusions
Here, we demonstrate that PDLSCs when encapsulated in an appropriately designed alginate/hyaluronic acid-based delivery system containing suitable growth factors possess potential to be used in repair/regeneration of chondrogenic tissues. Taken together, our findings confirmed that our 3D injectable cell delivery scaffold based on alginate/hyaluronic acid comprises a promising modality of treatment for cartilage tissue regeneration and repair.
References
Buckwalter JA, Mankin HJ. Articular cartilage repair and transplantation. Arthr Rheum. 1998;41:1331–42.
Clouet J, et al. From osteoarthritis treatments to future regenerative therapies for cartilage. Drug Discov Today. 2009;14:913–25.
Hunziker EB. Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthr Cartil. 2002;10:432–63.
Kurth T, et al. Chondrogenic potential of human synovial mesenchymal stem cells in alginate. Osteoarthr Cartil. 2007;15:1178–89.
Redman SN, Oldfield SF, Archer CW. Current strategies for articular cartilage repair. Eur Cell Mater. 2005;9:23–32.
Salinas CN, Anseth KS. The enhancement of chondrogenic differentiation of human mesenchymal stem cells by enzymatically regulated RGD functional- ities. Biomaterials. 2008;29:2370–7.
Re’em T, Tsur-Gang O, Cohen S. The effect of immobilized RGD peptide in macroporous alginate scaffolds on TGFb1-induced chondrogenesis of human mesenchymal stem cells. Biomaterials. 2010;31:6746–55.
Moshaverinia A, et al. Dental mesenchymal stem cells encapsulated in an alginate hydrogel co-delivery microencapsulation system for cartilage regeneration. Acta Biomater. 2013;9:9343–50.
Moshaverinia A, et al. Application of stem cells derived from the periodontal ligament or gingival tissue sources for tendon tissue regeneration. Biomaterials. 2014;35:2642–50.
Moshaverinia A, et al. Encapsulated dental-derived mesenchymal stem cells in an injectable and biodegradable scaffold for applications in bone tissue engineering. J Biomed Mater Res A. 2013;101:3285–94.
Liu J, Sato C, Cerletti M, Wagers A. Notch signaling in the regulation of stem cell self-renewal and differentiation. Curr Top Dev Biol. 2010;92:367e409.
Kratchmarova I, Blagoev B, Haack-Sorensen M, Kassem M, Mann M. Mechanism of divergent growth factor effects in mesenchymal stem cell differentiation. Science. 2005;308:1472–7.
Cantu DA, Hematti P, Kao WJ. Cell encapsulating biomaterial regulates mesenchymal stromal/stem cell differentiation and macrophage immunophenotype. Stem Cells Transl Med. 2012;1:740–9.
Czyz J, Wobus AM. Embryonic stem cell differentiation: the role of extracellular factors. Differentiation. 2001;68:167–74.
Alsberg A, Anderson KW, Albeiruti A, Franceschi RT, Mooney DJ. Cell- interactive alginate hydrogels for bone tissue engineering. J Dent Res. 2001;80:2025–9.
Drury JL, Dennis RG, Mooney DJ. The tensile properties of alginate hydrogels. Biomaterials. 2004;25:3187–99.
Mehlhorn AT, et al. Mesenchymal stem cells maintain TGF-beta-mediated chondrogenic phenotype in alginate bead culture. Tissue Eng. 2006;12:1393–403.
Kurth T, et al. Chondrogenic potential of human synovial mesenchymal stem cells in alginate. Osteoarthritis Cartilage. 2007;15:1178–89.
Little CJ, Kulyk WM, Chen X. The effect of chondroitin sulphate and hyaluronic acid on chondrocytes cultured within a fibrin-alginate hydrogel. J Funct Biomater. 2014;18:197–210.
Liao YH, Jones SA, Forbes B, Martin GP, Brown MB. Hyaluronan: pharmaceutical characterization and drug delivery. Drug Deliv. 2005;12:327–42.
Son YJ, et al. Porous hyaluronic acid/sodium alginate composite scaffolds for human adipose-derived stem cells delivery. Int J Biol Macromol. 2013;61:175–81.
Bulpitt P, Aeschlimann D. New strategy for chemical modification of hyaluronic acid: Preparation of functionalized derivatives and their use in the formation of novel biocompatible hydrogels. J Biomed Mater Res. 1999;47:152–69.
Kang JY, et al. Novel porous matrix of hyaluronic acid for the three- dimensional culture of chondrocytes. Int J Pharm. 2009;18:114–20.
Chung C, Burdick JA. Influence of 3D hyaluronic acid microenvironments on mesenchymal stem cell chondrogenesis. Tissue Eng Part A. 2009;15:243–54.
Knudson CB. Hyaluronan and CD44: strategic players for cell- matrix interactions during chondrogenesis and matrix assembly. Birth Defect Res C Embryo Today. 2003;69:174–96.
Patterson J, Siew R, Herring SW, Lin AS, Guldberg R, Stayton PS. Hyaluronic acid hydrogels with controlled degradation properties for oriented bone regeneration. Biomaterials. 2010;31:6772–81.
Burdick JA, Chung C, Jia X, Randolph MA, Langer R. Controlled degradation and mechanical behavior of photopolymerized hyaluronic acid networks. Biomacromolecules. 2005;6:386–91.
Chang JC, Hsu SH, Chen DC. The promotion of chondrogenesis in adipose- derived adult stem cells by an RGD-chimeric protein in 3D alginate culture. Biomaterials. 2009;30:6265–75.
Tchetina E, Mwale F, Poole AR. Distinct phases of coordinated early and late gene expression in growth plate chondrocytes in relationship to cell proliferation, matrix assembly, remodeling, and cell differentiation. J Bone Miner Res. 2003;18:844–51.
Bian L, et al. Enhanced MSC chondrogenesis following delivery of TGF-b3 from alginate microspheres within hyaluronic acid hydrogels in vitro and in vivo. Biomaterials. 2011;32:6425–34.
Guo JF, Jourdian GW, MacCallum DK. Culture and growth characteristics of chondrocytes encapsulated in alginate beads. Connect Tissue Res 1. 1989;9:277–97. 28
Seo BM, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149–1155.
Moshaverinia A, et al. Bone regeneration potential of stem cells derived from periodontal ligament or gingival tissue sources encapsulated in RGD-modified alginate scaffold. Tissue Eng Part A. 2014;20:611–21.
Wang CC, Yang KC, Lin KH, Liu HC, Lin FH. A highly organized three- dimensional alginate scaffold for cartilage tissue engineering prepared by microfl uidic technology. Biomaterials. 2011;32:7118–26.
Huebsch N, et al. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat Mater. 2010;9:518–26.
Peterson L. Technique of autologous chondrocyte transplantation. Tech Knee Surg. 2002;1:2–12.
Homicz MR, Schumacher BL, Sah RL, Watson D. Effects of Serial Expansion of Septal Chondrocytes on Tissue-Engineered Neocartilage Composition. Otolaryngol Head Neck Surg. 2002;127:398–408.
Ma HL, Hung S, Lin S, Chen Y, Lo W. Chondrogenesis of human mesenchymal stem cells encapsulated in alginate beads. J Biomed Mater Res. 2003;64A:273–81.
Stevens MM, Qanadilo HF, Langer R, Prasad Shastri V. A rapid-curing alginate gel system: utility in periosteum-derived cartilage tissue engineering. Biomaterials. 2004;25:887–94.
Allemann F, et al. Effects of hyaluronan on engineered articular cartilage extracellular matrix gene expression in 3-dimensionalcollagen scaffolds. J Biomed Mater Res. 2001;55:13–9.
O’Brien FJ, Harley BA, Yannas IV, Gibson LJ. The effect of pore size on cell adhesion in collagen-GAG scaffolds. Biomaterials. 2005;26:433–41.
Chen SC, Marino V, Gronthos S, Bartold PM. Location of putative stem cells in human periodontal ligament. J Periodontal Res. 2006;41:547–53.
Mitrano TI, Grob MS, Carrión F, Nova-Lamperti E, Luz PA, Fierro FS, Quintero A, Chaparro A, Sanz A. Culture and characterization of mesenchymal stem cells from human gingival tissue. J Periodontol. 2010;81:917–25.
Moshaverinia A, Chen C, Xu X, Ansari S, Zadeh HH, Schricker SR, et al. Regulation of the stem cell-host immune system interplay using hydrogel coencapsulation system with an antiinflammatory drug. Adv Func Mater. 2015;15:2296–307.
Zhang Q, et al. Mesenchymal stem cells derived from human gingiva are capable of immunomodulatory functions and ameliorate inflammation-related tissue destruction in experimental colitis. J Immunol. 2009;183:7787–98.
Moshaverinia A, Chen C, Akiyama K, Ansari S, Xu X, Chee WW, Schricker SR, Shi S. Alginate hydrogel as a promising scaffold for dental-derived stem cells: an in vitro study. J Mater Sci Mater Med. 2012;23:3041–51.
Acknowledgements
This work was supported by grants from the National Institute of Dental, Craniofacial Research (DE023825 to A.M.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Sahar Ansari and Ivana M. Diniz are contributed equally to this work.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Ansari, S., Diniz, I.M., Chen, C. et al. Alginate/hyaluronic acid hydrogel delivery system characteristics regulate the differentiation of periodontal ligament stem cells toward chondrogenic lineage. J Mater Sci: Mater Med 28, 162 (2017). https://doi.org/10.1007/s10856-017-5974-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10856-017-5974-8