Abstract
The production of wear debris particulate remains a concern due to its association with implant failure through complex biologic interactions. In the setting of unicompartmental knee arthroplasty (UKA), damage and wear of the components may introduce debris particulate into the adjacent, otherwise, healthy compartment. The purpose of this study was to investigate the in vitro effect of polymeric and metallic wear debris particles on cell proliferation, extracellular matrix regulation, and phagocytosis index of normal human articular chondrocytes (nHACs). In culture, nHACs were exposed to both cobalt-chromium-molybdenum (CoCrMo) and polymethyl-methacrylate (PMMA) wear debris particulate for 3 and 10 days. At 3 days, no significant difference in cell proliferation was found between control cells and cells exposed to both CoCrMo or PMMA particles. However, cell proliferation was significantly decreased for CoCrMo exposed nHACs at both 6 (P < 0.001) and 10 days (P < 0.001) and PMMA at 10 days (P < 0.001). Target gene expression displayed both a time- and material-dependent response to CoCrMo and PMMA particles. Significant differences in COL10A1, ACAN, VCAN, IL-1β, TNF-α, MMP3, ADAMTS1, CASP3, and CASP9 regulation were found between CoCrMo and PMMA exposed nHACs at day 3 with gene regulation returning to near baseline at 10 days. Results from our study indicate a role of wear debris induced cartilage degeneration after exposure to polymeric and metallic wear debris particulate, suggesting an additional pathway of cartilage breakdown, potentially manifesting in traditional clinical symptoms.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Unicompartmental knee arthroplasty (UKA) has been utilized as an alternative treatment for single-compartment osteoarthritis (OA) of the knee with several proposed benefits including less invasive surgery, maintenance of normal knee kinematics, reduced recovery time, increased range of motion, and improved physiologic function [1, 2]. Initial reports cited poor survivorship, largely attributed to inadequate patient selection criteria and first-generation implant designs [3, 4]. Through design improvements, increased surgeon volume, and improved patient selection, the revision risk has decreased [5], however, adjacent compartment degeneration, component loosening and polyethylene (PE) wear remain the most frequent reasons for UKA implant failure and need for revision surgery [2, 6,7,8,9,10,11]. Additionally, retrieval studies of UKA systems have previously demonstrated extensive PE wear and component damage in failed systems [12,13,14], however, these results have generally been implicated clinically with osteolysis and loss of component fixation rather than continued articular cartilage degeneration. Adjacent compartment degeneration following UKA is a topic of controversy and underscores the importance of a thorough preoperative clinical assessment of cartilage in all three compartments. It is unknown whether subclinical symptoms exist in adjacent cartilage prior to UKA implantation, predisposing the adjacent compartment to future degenerative changes in a natural progression, or if degeneration in the adjacent compartment occurs in response to mechanical or biochemical changes after UKA.
The impact of UKA on changes in the mechanical loading environment of the adjacent compartment is also not yet fully elucidated as studies have demonstrated both alterations [15,16,17] and preservation [18, 19] of knee kinematics following UKA procedures. When investigating the presence of third-body debris, Hauptmann et al. [20] reported the in vivo presence of polymethyl-methacrylate (PMMA) fragments, especially in the posterior regions, which can be easily missed during minimally-invasive UKA implantation. In addition to PMMA particle generation, evidence of damage to non-polymeric, metal articular components has also been established in the setting of joint arthroplasty, indicating that intra-articular release of metallic particles can occur due to component articulation and addition of third-body debris [21,22,23,24,25]. Malikian et al. [26] also noted significantly increased in vivo surface roughness of retrieved femoral components of unicompartmental components compared to control components indicating damage specific to UKA. The introduction of third body wear particles such as bone fragments or PMMA particles has been shown to accelerate component wear and increase wear particle production which may contribute to early implant failure [27, 28]. Schroeder et al. determined the addition of PMMA particles to a UKA wear simulation study increased the wear rates of polyethylene in vitro. UKA implant retrieval studies of failed, explanted UKAs have established that wear resulting in mean volumetric changes of UKA liners occurs via several damage modes, including abrasion, starching and pitting, which may play a role in wear debris generation [29,30,31].
This presence of third body particulate and component wear may expose the adjacent femoral compartment to foreign debris particulate. Both in vitro and in vivo wear debris studies have investigated the potential chondrocyte response after exposure to wear debris particulate. Chang et al. [29] investigated the effect of PE exposure on chondrocytes isolated from porcine knees, identifying elevated secretion of precursor mediators of osteoarthritis (nitrogen oxide and prostaglandin E2) suggesting a detrimental effect of debris particulate on cartilage viability. Lorber et al. [30] and Utzchneider et al. [31] both exposed murine knee joints to varying types of PE and PEEK wear particles and observed elevated levels of pro-inflammatory cytokines in the articular cartilage. Park et al. [32] also demonstrated that exposure of rat chondrocytes to polymeric wear debris resulted in increased pro-inflammatory cytokine expression in an in vitro 2D culture, and exacerbated cartilage degeneration in vivo, providing evidence that polymeric wear debris induces articular cartilage degeneration. The results of these studies elucidated a role of PE wear debris in the osteoarthritic cascade, but little research exists investigating the effect of non-polymeric wear particulate on chondrocytes and cartilage degeneration.
The purpose of this study was to evaluate the effect of third body wear debris (PMMA and CoCrMo wear particulate) on normal human articular chondrocytes (nHACs) in an in vitro cell culture experiment evaluating particle-cell phagocytosis, cellular proliferation, and target gene expression profiles. We hypothesized that both materials would have a detrimental impact on cell proliferation and altered target gene expression profiles compared to control cells. It was also hypothesized that CoCrMo would have a greater effect on cell proliferation compared to PMMA particulate.
2 Materials and methods
2.1 Cell culture and particle exposure
Commercially obtained PMMA and CoCrMo wear particles (Bioengineering Solutions Inc., Oak Park, IL, USA) were produced under class 100 sterile conditions via proprietary cryo-milling/cryo-pulverization techniques from prepared bone cement (Palacos, Zimmer, Warsaw IN, USA) and a total hip arthroplasty femoral head component (Zimmer, Warsaw, IN), respectively. Particle size was confirmed via low angle laser light scattering (LALLS) to be within the phagocytosable range [33] with average particle sizes for PMMA and CoCrMo of 1.82 µm and 0.83 µm, respectively. Particles were also verified to be endotoxin free.
Normal human articular chondrocytes (nHACs; Lonza, Basel, Switzerland) were cultured in Dulbecco’s Modified Eagle Medium with Hams Nutrient Mixture F-12 (DMEM-F12) supplemented with 5% fetal bovine serum, 1% penicillin streptomycin, and 0.05 mg/mL ascorbic acid. Experimental and control wells of a 12-well plate were seeded at densities of 2 × 104 and 1 × 104 cells/cm2, respectively, and allowed to attach for 24 h at 37 °C and 5% CO2. Lower densities were used in control wells to minimize overcrowding at later time points, however, all data were normalized to account for this difference for subsequent analyses. Particles were suspended in culture medium using sonication and added to wells at a concentration of 10:1 particles to cells. This particle-to-cell concentration has been shown to elicit phagocytosis of UHMWPE particles by primary human chondrocytes [32]. To avoid wear particle loss at the time of medium exchange (every 72 h), supernatant was removed and centrifuged to pellet any wear debris particles present, which were then re-suspended in fresh medium and added back into respective wells.
2.2 Cell proliferation
At time zero, 3-day, 6-day, and 10-day time points, cell proliferation of control and experimental samples (n = 6 per group) was measured using an Alamar Blue assay (Life Technologies, Carlsbad, CA, USA) [34,35,36]. For each measurement, supernatant was removed and replaced with 500 µL of a 10:1 dilution of Alamar blue stock solution in culture medium and incubated 1.5 h, as established during preliminary work. Duplicate 100 µL aliquots of each sample were transferred to a 96-well plate and the fluorescence intensity evaluated at an excitation wavelength of 540–570 nm and emission wavelength of 580–610 nm, using a microplate reader (Gemini EM Fluorescence Microplate Reader, Molecular Devices, Sunnyvale, CA, USA). Fluorescence was subtracted from negative control samples that included medium alone to mitigate the effect of cell culture medium on the fluorescence intensity. Fold change over the initial intensity (time zero measurement) fluorescence intensity in each group was calculated for each subsequent time point. Data was represented as change in initial fluorescent intensity, which is linearly related to cell number. After completion of the assay, cells were fixed in 4% glutaraldehyde at room temperature for 30 min, and imaged via low-vacuum scanning electron microscopy (Quanta FEG 400, FEI, Hillsboro, OR, USA) to qualitatively evaluate cell and particle morphology, as well as the proximity of particles to cells.
2.3 PCR analysis
At 3- and 10-day time points, cells were lysed (n = 6 samples per group, per time point), and RNA collected for quantitative reverse transcription polymerase chain reaction (qRT-PCR) analysis of 14 target genes along with 18 s ribosomal RNA, which served as the endogenous control (Table 1). Wells were rinsed with 500 µL PBS, and cells lysed using 100 µL RLT buffer containing 2 M Dithiothreitol (DTT), and lysate collected using a cell scraper.
RNA was isolated using a commercially-available RNA isolation kit (RNeasy Micro Kit, Qiagen, Valencia, CA, USA), SuperScript Vilo Master Mix (Life Technologies, Carlsbard, CA, USA) was used for the synthesis of cDNA from 100 ng RNA, and qRT-PCR was performed with custom TaqMan array 96-well plates (Life Technologies, Carlsbard, CA, USA). Samples were run in duplicate and each plate contained six assays of one manufacturer control, one endogenous control, and 14 target genes. qRT-PCR reaction mixture was prepared containing 2.5 ng cDNA, 1x TaqMan Gene Expression Master Mix (Life Technologies, Carlsbard, CA, USA), and 1x Gene Expression Assay (Life Technologies, Carlsbard, CA, USA). Gene expression levels were quantified using the ViiA™ 7 Real-Time PCR System (Life Technologies, Carlsbard, CA, USA), with the following thermocycling condition: 50 °C for 2 min, 95 °C for 10 min, and 40 amplification cycles of 95 °C for 15 s/60 °C for 1 min.
2.4 Phagocytosis analysis
To determine whether the chondrocytes endocytosed particles, a flow cytometry experiment was undertaken based on previously published methodology [32, 37]. Cells were stained in suspension using Vybrant CFDA SE Cell Tracer Kit (Life Technologies, Carlsbad, CA, USA) per the manufacturer’s protocol, and seeded in 24-well plates as described above. Particles were added after 24 h, and wells imaged daily via light microscopy at 10× and 20× magnifications. At 3-day and 10- day time points, samples were detached using colorless trypsin with EDTA (n = 6 samples per experimental group, n = 3 cell-only control samples), diluted with culture medium containing FBS to inactivate the trypsin, filtered through falcon flow cytometry tubes to eliminate cell clumping, and analyzed via flow cytometry (FACSCanto II, BD Biosciences, San Jose, CA, USA) to detect changes in cell granularity and size between control and experimental samples. The phagocytosis index, which represents the degree of granularity and is an indicator of the number of phagocytic chondrocytes, was calculated as previously described [32, 37]. Furthermore, the particle ingestion index, an indicator of the number of particles ingested per phagocytic chondrocyte, was calculated using median side scatter [37]. Both indices were normalized to control, unexposed chondrocytes.
2.5 Data analysis and statistical methods
All statistical comparisons were performed in SPSS (v20, IBM, Armonk, NY, USA). Alamar Blue data was represented as fluorescent intensity, which is linearly related to cell number [35]. A relative change in cell number was calculated at each time point as fold-change over initial fluorescence intensity for each group. Alamar Blue data was normally-distributed and compared as “fold-change over time zero” fluorescence signal using Two-Way Analysis of Variance (ANOVA; Factor A: Material; Factor B: Time Point). Post-hoc comparisons were performed using the modified Bonferroni test. Raw qRT-PCR data was normalized as fold-change over the control/housekeeping gene (18 s) using the standard delta-delta-CT method [38]. The normality and equal variance assumptions were tested using the Kolmorogov-Smirnov test and the F-test, respectively. Fold-change over control data was not originally normally distributed and was transformed using a Log10 transform, which, was confirmed to be meet normality and equal variance assumptions. Transformed data was then compared using Two-way ANOVA (Factor A: Materials; Factor B: Time Point). Post-hoc comparisons were performed using the modified Bonferroni test. Significance was set at P < 0.05.
3 Results
3.1 Cell proliferation
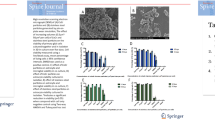
Cell proliferation determined by fold-change over initial fluorescence intensity (cell number at time-zero) (Fig. 1). At day 3, there was no significant difference between control cells and cells treated with CoCrMo (P = 0.908) or PMMA (P = 0.659). At 6 and 10 day time points, both PMMA and CoCrMo particles negatively impacted the proliferation of articular chondrocytes. Exposure to CoCrMo particles at 6 days, and CoCrMo or PMMA at 10 days, significantly decreased the fold change in cell number compared to control (P < 0.001). Cell proliferation was impacted significantly more by CoCrMo than PMMA at both 6 days (P = 0.001) and 10 days (P < 0.001). The Control group exhibited sustained cellular proliferation throughout length of the experiment, with significant increases in fold change in cell number at each time point (P < 0.001). Cells exposed to PMMA particles exhibited a significant increase in fold-change from 3 to 6 days (P < 0.001) and 6 to 10 days (P = 0.040). No significant increase in fold-change between 3 and 6 days (P = 0.166) and between 6 and 10 days (P = 0.361) was noted in the CoCrMo group.
Cellular proliferation as analyzed by Alamar Blue Assay. Cells treated with CoCrMo exhibited significantly decreased cell proliferation, as measured by fold change over initial intensity compared to control cells at both 6 days (P < 0.001) and 10 days (P < 0.001). Cells treated with PMMA exhibited significantly decreased cell proliferation at 10 days (P < 0.001). There was a significant difference in fold change between CoCrMo-treated and PMMA-treated cells at both 6 days (P = 0.001) and 10 days (P < 0.001)
3.2 PCR analysis
qRT-PCR results demonstrate a differential gene expression profile of chondrocytes exposed to CoCrMo and PMMA particulate which was both time-dependent and material-dependent. At Day 3 (Fig. 2a), Col10a1, ACAN, VCAN, IL-1β, TNF-α, MMP3, ADAMTS1, CASP3, and CASP9 were expressed at a significantly higher level in the CoCrMo group compared to the PMMA group. However, at Day 10 (Fig. 2b), only COL2A1 expression was significantly higher in the CoCrMo group compared to the PMMA group. Within the CoCrMo group, Col10a1, MMP3, and MMP13 were expressed at a significantly higher level at Day 3 compared to Day 10. Col2a1 expression was significantly greater at Day 10 than at Day 3 in the CoCrMo group.
3.3 Phagocytosis analysis
Light microscopy demonstrated direct cell-particle contact in both CoCrMo (Fig. 3a) and PMMA (Fig. 3b) material groups throughout the experiment. Similarly, scanning electron microscopy also displayed interaction between nHACs and particles for both CoCrMo (Fig. 3c) and PMMA (Fig. 3d) groups. Numerous cells with large particle clusters within the cell border were observed, and by Day 10, nearly all particles were observed to be in immediate proximity to a cell rather than evenly-dispersed throughout the culture well. Both materials exhibited a positive phagocytosis index, indicating chondrocyte phagocytosis of particles (Fig. 4a). There was no significant difference in phagocytosis index in the CoCrMo group between Day 3 and Day 10 (3.94 % ± 2.6; day 10: 8.87 % ± 5.64, P = 0.093), but PMMA exhibited a significant increase in phagocytosis index between the two time points (Day 3: 1.73 % ± 1.49; Day 10: 7.68 % ± 4.21, P = 0.004). There was no difference in phagocytosis index between CoCrMo and PMMA at either Day 3 (P = 0.132) or Day 10 (P = 0.818). Both material groups exhibited increases in particle ingestion index over control cells (Fig. 4b). There was no significant difference in particle ingestion index in the CoCrMo group between Day 3 and Day 10 (Day 3: 14.22 % ± 6.8; Day 10: 19.06 % ± 12.21, P = 0.589), but PMMA exhibited a significant increase between Day 3 and Day 10 (Day 3: 5.68 % ± 1.61; Day 10: 15.89 % ± 8.52, P = 0.041). At Day 3, CoCrMo had significantly higher particle ingestion index compared to PMMA (P = 0.002), but there was no difference between the two groups at Day 10 (P = 0.818).
4 Discussion
Adjacent compartment degeneration following UKA remains a topic of extensive discussion, and its etiology remains largely unknown. While polyethylene wear in UKA has been implicated as a reason for revision in retrieval analyses [12,13,14], a paucity of data exists regarding the biological interaction between wear particles and adjacent compartment tissues, specifically, the response of human articular chondrocytes to wear particles from UKA systems. This investigation assessed the effect of CoCrMo and PMMA particles on cell proliferation, gene expression, and particle phagocytosis by articular chondrocytes. Our results demonstrate that chondrocytes phagocytose both materials, inducing inhibited cellular proliferation, and a material-dependent extracellular matrix, pro-inflammatory cytokine, protease, and apoptosis gene expression profile. Furthermore, our data indicates that CoCrMo particles have a more detrimental effect on chondrocyte proliferation compared to PMMA particles, and that CoCrMo particles induce an acute overexpression of numerous genes.
The ability of chondrocytes to phagocytose latex particles and cartilage debris was first established by Castillo, et al. [39]. Subsequent studies by Chang, et al. [29] and Park, et al. [32] have shown that chondrocytes are also capable of engulfing UHMWPE particulate, which result in increased expression of pro-inflammatory cytokines and mediators, as well as alterations in cell viability. While UHMWPE is the primary sacrificial bearing in UKA systems, there also exists the potential for the mechanical or electrochemical generation of particulate from the CoCrMo condylar component, as well as debris from the PMMA cement mantle used for fixation of the tibial and/or condylar component. In the present study, flow cytometric analyses confirmed the ability of chondrocytes to phagocytose both CoCrMo and PMMA debris. Further, CoCrMo particles were more readily phagocytosed by the chondrocytes, especially at the Day 3 time point, as evidenced by a significant difference in the particle ingestion index. Considering the similarity in size and shape of CoCrMo and PMMA debris used in this study, it is hypothesized that differences in surface phenomena, such as protein adsorption, may contribute to the differential uptake observed. Elfick, et al. demonstrated that macrophages responded to polyethylene wear debris as a function of proteins adsorbed to the particle surface, which is related to the zeta potential of the particle–fluid system [40].
Phagocytosis of the particulate debris by chondrocytes led to significant decreases in chondrocyte proliferation at both 6 and 10 days, again with CoCrMo yielding the largest effect. A significant increase in mRNA-level expression of CASP3 and CASP9 at the 3-day time point for cells exposed to CoCrMo suggests an apoptotic mechanism for the decreased cell proliferation. Cells exposed to PMMA particulate displayed decreased mRNA expression of CASP3 and CASP9 at the 3-day time point, and expression returned to baseline at the 10-day time point for both PMMA- and CoCrMo-exposed cells. In addition to playing a prominent role in osteoarthritis pathology, Park, et al. showed that intraarticular injection of UHMWPE particles led to chondrocyte death in a dose-dependent manner. Results from our in vitro study would indicate a similar effect of CoCrMo and PMMA debris, though confirmatory in vivo studies should be undertaken to confirm this hypothesis.
Wear debris exposure is also well-documented to induce the expression of pro-inflammatory cytokines, inflammatory mediators, and proteases, which are implicated in the development and progression of wear-induced periprosthetic osteolysis in joint replacement [41]. Park, et al. found that primary human chondrocytes exposed to UHMWPE debris expressed IL-1□, IL-6, TNF-□, PGE2 and NO in a dose-dependent fashion. Chang, et al. also showed that exposure of chondrocytes to UHMWPE led to increases in PGE2 and NO synthesis. Similarly, we demonstrated that in vitro exposure of nHACs to CoCrMo and PMMA wear debris led to significant material-dependent alterations in the mRNA-level expression of IL-1β, TNFa, and MMP-3 at the Day 3 time point, with CoCrMo-exposed cells expressing higher levels compared to control cells and PMMA-exposed cells expressing lower levels. By the 10 day time point, gene expression had largely returned to baseline. Aside from MMP13, PMMA debris did not elicit a substantive increase over baseline gene expression, which is similar to a finding by Lohmann et al. demonstrating limited reaction of MG63 cells to PMMA [42].
Exposure of chondrocytes to CoCrMo and PMMA debris also resulted in alterations in mRNA-level expression of extracellular matrix genes at the early time point. CoCrMo exposure led to increased expression of Col1a1, Col10a1, ACAN, and VCAN, while PMMA exposure resulted in decreased Col1a1, Col2a1, ACAN, and VCAN expression. Park et al. demonstrated that intraarticular administration of UHMWPE particulate led to worse OARSI-modified Mankin scores, particularly when osteoarthritic pathology was surgically induced. They observed matrix loss and extensive disruption of the superficial layer of articular cartilage, hypocellularity, and collagen disorganization in osteoarthritic animals exposed to high-dose UHMWPE particulate. While an in vivo study was not performed, results from our in vitro study suggest that both CoCrMo and PMMA particulate may lead to disruption of articular cartilage matrix metabolism.
This investigation had several limitations. Chondrocytes have been shown to de-differentiate in long-term 2D cultures. These effects were mitigated by normalizing all results to control wells and selecting short-term endpoints for analysis. Additionally, our study only investigated one concentration, varying particle:cell concentrations may yield differing results. Future studies are currently underway investigating wear particulate debris interactions in a novel three-dimensional culture system which has been shown to limit de-differentiation in culture. Furthermore, additional investigations may look at varying particle concentrations. Due to the limited number of genes examined, a potential selection bias may also have played a role in our results. However, this effect was minimized by selecting genes that have been documented to play a role in cartilage degeneration pathways and comparing results to a documented control gene.
5 Conclusion
In conclusion, this study demonstrates the material-dependent and time-dependent response of articular chondrocytes to wear debris from PMMA and CoCrMo, two common materials found in UKA devices that have not been investigated previously. We found that both materials inhibit cellular proliferation, with the greatest reduction in proliferation observed in cells exposed to CoCrMo. A differential gene expression response was observed between the two materials at our Day 3 time point, with CoCrMo inducing a greater expression of inflammatory mediators, catabolic proteases, and markers of apoptosis. Lastly, flow cytometric analysis demonstrated an increase in chondrocyte granularity, indicative of endocytosed particles within the cells. This study suggests the potential role of wear debris in biologic degeneration of articular cartilage in a compartment adjacent to UKA, and future studies are required to elucidate the biological interaction between chondrocytes and wear debris.
References
Borus T, Thornhill T. Unicompartmental knee arthroplasty. J Am Acad Orthop Surg. 2008;16(1):9–18.
Deshmukh RV, Scott RD. Unicompartmental knee arthroplasty: long-term results. Clin Orthop Relat Res. 2001;392:272–8.
Insall J, Walker P. Unicondylar knee replacement. Clin Orthop Relat Res. 1976;120(1):83–5.
Laskin RS. Unicompartmental tibiofemoral resurfacing arthroplasty. J Bone Joint Surg Am. 1978;60(2):182–5.
Bini S, Khatod M, Cafri G, Chen Y, Paxton EW. Surgeon, implant, and patient variables may explain variability in early revision rates reported for unicompartmental arthroplasty. J Bone Joint Surg Am. 2013;95(24):2195–202. doi:10.2106/JBJS.L.01006.
Weale AE, Newman JH. Unicompartmental arthroplasty and high tibial osteotomy for osteoarthrosis of the knee. A comparative study with a 12- to 17-year follow-up period. Clin Orthop Relat Res. 1994;302(1):134–7.
Kerens B, Boonen B, Schotanus MG, Lacroix H, Emans PJ, Kort NP. Revision from unicompartmental to total knee replacement: the clinical outcome depends on reason for revision. Bone Joint J. 2013;95-B(9):1204–8. doi:10.1302/0301-620X.95B9.31085.
Citak M, Dersch K, Kamath AF, Haasper C, Gehrke T, Kendoff D. Common causes of failed unicompartmental knee arthroplasty: a single-centre analysis of four hundred and seventy one cases. Int Orthop. 2014. doi:10.1007/s00264-013-2263-0.
Cartier P, Sanouiller JL, Grelsamer RP. Unicompartmental knee arthroplasty surgery. 10-year minimum follow-up period. J Arthroplasty. 1996;11(7):782–8.
Berger RA, Meneghini RM, Sheinkop MB, Della Valle CJ, Jacobs JJ, Rosenberg AG et al. The progression of patellofemoral arthrosis after medial unicompartmental replacement: results at 11 to 15 years. Clin Orthop Relat Res. 2004;428(1):92–9.
Epinette JA, Brunschweiler B, Mertl P, Mole D, Cazenave A. French Society for H et al. Unicompartmental knee arthroplasty modes of failure: wear is not the main reason for failure: a multicentre study of 418 failed knees. Orthop Traumatol Surg Res. 2012;98(6 Suppl):S124–30. doi:10.1016/j.otsr.2012.07.002.
Bartley RE, Stulberg SD, Robb WJ, 3rd, Sweeney HJ. Polyethylene wear in unicompartmental knee arthroplasty. Clin Orthop Relat Res. 1994;299(1):18–24.
Engh GA, Dwyer KA, Hanes CK. Polyethylene wear of metal-backed tibial components in total and unicompartmental knee prostheses. J Bone Joint Surg Br. 1992;74(1):9–17.
Kendrick BJ, Simpson DJ, Kaptein BL, Valstar ER, Gill HS, Murray DW, et al. Polyethylene wear of mobile-bearing unicompartmental knee replacement at 20 years. J Bone Joint Surg Br. 2011;93(4):470–5. doi:10.1302/0301-620X.93B4.25605.
Mochizuki T, Sato T, Blaha JD, Tanifuji O, Kobayashi K, Yamagiwa H, et al. Kinematics of the knee after unicompartmental arthroplasty is not the same as normal and is similar to the kinematics of the knee with osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2014;22(8):1911–7. doi:10.1007/s00167-013-2767-6.
Mochizuki T, Sato T, Tanifuji O, Kobayashi K, Yamagiwa H, Watanabe S, et al. Unicompartmental knee arthroplasty cannot restore the functional flexion axis of a living knee to normal. Knee Surg Sports Traumatol Arthrosc. 2015;23(12):3736–42. doi:10.1007/s00167-014-3296-7.
Mochizuki T, Sato T, Tanifuji O, Kobayashi K, Koga Y, Yamagiwa H, et al. In vivo pre- and postoperative three-dimensional knee kinematics in unicompartmental knee arthroplasty. J Orthop Sci. 2013;18(1):54–60. doi:10.1007/s00776-012-0322-9.
Argenson JN, Komistek RD, Aubaniac JM, Dennis DA, Northcut EJ, Anderson DT, et al. In vivo determination of knee kinematics for subjects implanted with a unicompartmental arthroplasty. J Arthroplasty. 2002;17(8):1049–54. doi:10.1054/arth.2002.34527.
Heyse TJ, El-Zayat BF, De Corte R, Chevalier Y, Scheys L, Innocenti B, et al. UKA closely preserves natural knee kinematics in vitro. Knee Surg Sports Traumatol Arthrosc. 2014;22(8):1902–10. doi:10.1007/s00167-013-2752-0.
Hauptmann SM, Weber P, Glaser C, Birkenmaier C, Jansson V, Muller PE. Free bone cement fragments after minimally invasive unicompartmental knee arthroplasty: an underappreciated problem. Knee Surg Sports Traumatol Arthrosc. 2008;16(8):770–5. doi:10.1007/s00167-008-0563-5.
Jasty M, Bragdon CR, Lee K, Hanson A, Harris WH. Surface damage to cobalt-chrome femoral head prostheses. J Bone Joint Surg Br. 1994;76(1):73–7.
Heyse TJ, Chen DX, Kelly N, Boettner F, Wright TM, Haas SB. Matched-pair total knee arthroplasty retrieval analysis: oxidized zirconium vs. CoCrMo. Knee. 2011;18(6):448–52. doi:10.1016/j.knee.2010.08.011.
Tipper JL, Ingham E, Hailey JL, Besong AA, Fisher J, Wroblewski BM, et al. Quantitative analysis of polyethylene wear debris, wear rate and head damage in retrieved Charnley hip prostheses. J Mater Sci Mater Med. 2000;11(2):117–24. doi:10.1023/A:1008901302646.
Davidson JA. Characteristics of metal and ceramic total hip bearing surfaces and their effect on long-term ultra high molecular weight polyethylene wear. Clin Orthop Relat Res. 1993;294:361–78.
Case C, Langkamer V, James C, Palmer M, Kemp A, Heap P, et al. Widespread dissemination of metal debris from implants. Bone & Joint Journal. 1994;76(5):701–12.
Malikian R, Maruthainar K, Stammers J, Cannon SR, Carrington R, Skinner JA, et al. In vivo roughening of retrieved total knee arthroplasty femoral components. The Knee. 2014;21(1):278–82.
Schroeder C, Grupp TM, Fritz B, Schilling C, Chevalier Y, Utzschneider S, et al. The influence of third-body particles on wear rate in unicondylar knee arthroplasty: a wear simulator study with bone and cement debris. J Mater Sci Mater Med. 2013;24(5):1319–25. doi:10.1007/s10856-013-4883-8.
Paulus AC, Franke M, Kraxenberger M, Schroder C, Jansson V, Utzschneider S. PMMA third-body wear after unicondylar knee arthroplasty decuples the UHMWPE wear particle generation in vitro. Biomed Res Int. 2015;2015:575849 doi:10.1155/2015/575849.
Chang C-H, Fang H-W, Ho Y-C, Huang H-T. Chondrocyte acting as phagocyte to internalize polyethylene wear particles and leads to the elevations of osteoarthritis associated NO and PGE 2. Biochem Biophys Res Commun. 2008;369(3):884–8.
Lorber V, Paulus AC, Buschmann A, Schmitt B, Grupp TM, Jansson V, et al. Elevated cytokine expression of different PEEK wear particles compared to UHMWPE in vivo. J Mater Sci Mater Med. 2014;25(1):141–9. doi:10.1007/s10856-013-5037-8.
Utzschneider S, Lorber V, Dedic M, Paulus AC, Schroder C, Gottschalk O, et al. Biological activity and migration of wear particles in the knee joint: an in vivo comparison of six different polyethylene materials. J Mater Sci Mater Med. 2014;25(6):1599–612. doi:10.1007/s10856-014-5176-6.
Park DY, Min BH, Kim DW, Song BR, Kim M, Kim YJ. Polyethylene wear particles play a role in development of osteoarthritis via detrimental effects on cartilage, meniscus, and synovium. Osteoarthritis Cartilage. 2013;21(12):2021–9. doi:10.1016/j.joca.2013.09.013.
Hallab NJ, McAllister K, Brady M, Jarman‐Smith M. Macrophage reactivity to different polymers demonstrates particle size‐and material‐specific reactivity: PEEK‐OPTIMA® particles versus UHMWPE particles in the submicron, micron, and 10 micron size ranges. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2012;100(2):480–92.
Wang Y, Blasioli DJ, Kim HJ, Kim HS, Kaplan DL. Cartilage tissue engineering with silk scaffolds and human articular chondrocytes. Biomaterials. 2006;27(25):4434–42. doi:10.1016/j.biomaterials.2006.03.050.
Nakayama GR, Caton MC, Nova MP, Parandoosh Z. Assessment of the Alamar Blue assay for cellular growth and viability in vitro. J Immunol Methods. 1997;204(2):205–8.
Li Z, Ramay HR, Hauch KD, Xiao D, Zhang M. Chitosan-alginate hybrid scaffolds for bone tissue engineering. Biomaterials. 2005;26(18):3919–28. doi:10.1016/j.biomaterials.2004.09.062.
Catelas I, Huk OL, Petit A, Zukor DJ, Marchand R, Yahia L. Flow cytometric analysis of macrophage response to ceramic and polyethylene particles: effects of size, concentration, and composition. J Biomed Mater Res. 1998;41(4):600–7.
Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3(6):1101–8.
Castillo ECG, Kourí JB. A new role for chondrocytes as non‐professional phagocytes. An in vitro study. Microsc Res Tech. 2004;64(3):269–78.
Elfick AP, Green SM, McCaskie AW, Birch MA. Opsonization of polyethylene wear particles regulates macrophage and osteoblast responses in vitro. J Biomed Mater Res B Appl Biomater. 2004;71(2):244–51. doi:10.1002/jbm.b.30095.
Jiang Y, Jia T, Wooley PH, Yang SY. Current research in the pathogenesis of aseptic implant loosening associated with particulate wear debris. Acta Orthop Belg. 2013;79(1):1–9.
Lohmann CH, Dean DD, Koster G, Casasola D, Buchhorn GH, Fink U, et al. Ceramic and PMMA particles differentially affect osteoblast phenotype. Biomaterials. 2002;23(8):1855–63.
Acknowledgements
The authors would also like to acknowledge the Clinician Development Program grant from the Orthopaedic Research and Education Fund (OREF Grant #12-062), which supported the education and research component of our Institution’s Adult Reconstruction Fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Kurdziel, M.D., Salisbury, M., Kaplan, L. et al. Exposure of articular chondrocytes to wear particles induces phagocytosis, differential inflammatory gene expression, and reduced proliferation. J Mater Sci: Mater Med 28, 106 (2017). https://doi.org/10.1007/s10856-017-5917-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10856-017-5917-4