Abstract
The objective of this study is to evaluate the efficacy and safety of non-suture dural closure using a novel dural substitute (GM111) consisting of polyglycolic acid felt with a fibrin-glue-coated area commensurate in size with the dural defect. This was a non-controlled, open-label, multicenter clinical trial. The efficacy evaluation endpoints were (1) GM111’s intra-operative capability to close dural defects and (2) prevention of cerebrospinal fluid (CSF) leakage and subcutaneous CSF retention throughout the postoperative period (evaluated by diagnostic imaging). Patients meeting the following three preoperative and two intra-operative selection criteria were enrolled: (1) between 12 and <75 years of age; (2) the dura is surmised to be defective and in need of reconstruction; (3) informed written consent was obtained from the patient; (4) the surgical wound is class 1; and (5) the size of duraplasty is ≥0.2 cm2 to <100 cm2. Sixty patients were enrolled. The craniotomy site was supratentorial in 77.2%, infratentorial in 12.3% and sellar in 10.5%. The GM111 prosthesis size ranged from 0.24 to 42 cm2. To evaluate the efficacy, intra-operative closure was confirmed by Valsalva’s maneuver, water infusion, etc., in all patients. CSF leakage and subcutaneous CSF retention throughout the postoperative period were found in four patients. Adverse events for which a causal relationship with GM111 could not be ruled out occurred in 8.8% of the patients. There were no instances of postoperative infection due to GM111. GM111 showed good closure capability and safety when used for non-suture dural closure.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Neurosurgery sometimes results in a defect of the dura mater and/or suture failure. Dural defects occur in various sizes in surgery for meningiomas having their origin in the dura and in skull base surgery. It is not uncommon for primary suturing of the dura to be impossible in pituitary surgery, and in posterior cranial fossa surgery, especially in the elderly. Cerebrospinal fluid (CSF) leakage from a site of dural repair or suture failure with subsequent CSF retention could lead to wound infection and/or meningitis, and, although rare, even pseudoencephalocele and psudomeningocele formation [1]. Hutter et al. reported in their prospective, randomized study that CSF collection or leakage after craniotomies with dural opening occurred in 13.5% of patients [2]. Even when dural sealant solutions such as fibrin glue or polyethylene glycol hydrogel were applied, occurrence of CSF leakage remained high at around 10%. To avoid these frustrating complications, it is important to prevent CSF leakage and retention by performing appropriate dural reconstruction.
Methods for reconstructing dural defects can be roughly classified into two procedures, one using vascularized flaps, and the other using free grafts. Free grafts further fall into two categories: biological tissues, such as the temporal fascia, abdominal fat, fascia lata, etc., and synthetic materials, such as expanded polytetrafluoroethylene (Gore-Tex DM®, Japan Gore Co., Ltd., Tokyo), etc. Dural reconstruction using biological tissues has the advantage of eliciting little foreign-body reaction. But its disadvantages include that it can be difficult to harvest a tissue having a size and shape commensurate with the dural defect, and that a new surgical wound is created at a site other than the surgical site. Gore-Tex DM®, which is the most-used synthetic dural substitute in Japan, is a resin sheet that is very stable in vivo. However, since it is an artificial membrane, it is impossible to avoid the risk of cerebrospinal fluid leakage from the needle hole, or a foreign-body reaction or infection. Moreover, as we have already reported, connective tissue membrane of unknown origin always forms between the brain and Gore-Tex DM® when it is used as a dural substitute [3, 4].
CSF leakage accompanying brain surgery often occurs at deep operative field sites where suturing is difficult and the dural stump cannot be secured. For that reason, there has long been a need for a dural substitute that would enable non-suture dural closure. Although procedures for dural closure not using sutures, such as the non-penetrating titanium clip, have been tried, their closing ability is not sufficient [5]. Today, we still have no dural substitute that permits sutureless dural closure in Japan.
The ideal dural substitute for brain surgery would (1) have sufficient closure capability, (2) be replaced by biological tissue, (3) be safe and (4) enable sutureless closure. To date, we have tried a variety of materials to create dural substitutes, and we finally found that all the above requirements are satisfied by a membrane created by combining fibrin glue and polyglycolic acid felt. We have reported the results of our pressure test on non-suture closure using this membrane as well as the time-course histological changes in animal experiments [6]. Our results showed that this membrane was advantageous in that it provided excellent closing ability and was eventually replaced by biological tissues, without any incompatibility. In that report, we also proposed the potential in clinical use of this membrane as a novel dural substitute.
The objectives of our present study were to elucidate the efficacy and safety of non-suture dural closure using a novel dural substitute consisting of polyglycolic acid felt and fibrin glue (GM111) for dural defects and incomplete dural closures in clinical settings.
2 Material and methods
This was a non-controlled, open-label, multicenter clinical trial. The efficacy evaluation endpoints were the intra-operative capability of GM111 to close dural defects and its prevention of CSF leakage and subcutaneous CSF retention throughout the postoperative period (up to 24 weeks after surgery) based on the diagnostic imaging. The safety was evaluated by investigating adverse events (AEs) caused by the GM111. The protocol of this study was evaluated and approved by the Japanese Pharmaceuticals and Medical Devices Agency (PMDA). The study design was approved by the ethics committees of the participating institutions, and the study was carried out in accordance with the spirit of the Declaration of Helsinki. All participating patients granted informed consent in writing.
2.1 Patient information
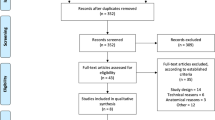
Patients who met the following three preoperative selection criteria were enrolled in the study: (1) between 12 and <75 years of age; (2) the dura is surmised to be defective and in need of reconstruction, or it is surmised that primary suturing of the dura would be impossible or incomplete with the standard procedure; and (3) informed written consent to participation in the study was obtained from the patient or his/her representative. Patients meeting the above 3 criteria were deemed eligible, for the study, and they were then enrolled if they further satisfied these 2 criteria: (1) the surgical wound is class 1 (clean); and (2) the size of the duraplasty is ≥0.2 to <10 cm2 (Figure 1).
The exclusion criteria were as follows: (1) presence of a serious systemic complication, infection or state of immunosuppression; (2) long-term treatment with adrenal cortical hormone preparations; (3) a history of craniotomy at the same site; and (4) a diagnosed life expectancy of less than 6 months.
2.2 Novel dural substitute (GM111) and non-suture dural closure
The raw material of polyglycolic acid felt is 100% polyglycolic acid. A 0.15-mm thick felt was prepared by heating fiber aggregates to cause pressure-bonding. The felt was hydrolyzed so that it would be metabolized and absorbed after about 15 weeks. As the fibrin glue used in combination with the felt, two commercial products, i.e., Beriplast® P Combi-Set Tissue Adhesion (CSL Behring, PA, USA) and Bolheal® (Kaketsuken, Kumamoto, Japan), were randomly allocated and used. These two fibrin glues contain the same concentration of fibrinogen (40 mg per 0.5 mL of solution) and show the same sealing effect in the spray method.
GM111 was prepared as described below, and then non-suture duraplasty was performed (Fig. 2). The polyglycolic acid felt was cut in a size larger than the dural defect or the incomplete dural closure site. Fibrin glue was then sprayed on the central portion of the felt to coat an area commensurate in size with the dural defect. The thickness of the coating was such that the fibers of the felt were completely covered. The felt was prepared so that a minimum 5-mm margin remained around the coated area. After applying only fibrinogen solution to the residual dural stump, the GM111 was inverted and its coated area was placed so that it would cover the dural defect site. At this time, the margin of GM111 should be soaked adequately with the fibrinogen solution on the residual dural stump. The entire GM111 was uniformly sprayed and coated with the fibrin glue, the margin was compressed, and the GM111 was firmly adhered to the residual dura mater.
The polyglycolic acid felt was cut in a size larger than the dural defect (so that a minimum 5-mm margin remained) (1a, 1b). Fibrin glue was sprayed on the central portion of the felt sheet to create a coated area (1c). The thickness of the coating was such that the fibers of the felt were completely covered. After applying only fibrinogen solution to the residual dural stump, the GM111 was inverted and its coated area was placed so that it would adhere to and cover the dural defect site (1d, 1e). The entire GM111 was uniformly sprayed and coated with the fibrin glue (1f), the margin was compressed, and the GM111 was firmly adhered to the residual dura mater
2.3 Evaluation of efficacy endpoints
For evaluation of the intra-operative dural closure capability, which was one of the efficacy assessment endpoints, five ratings, 1; excellent closure with no leakage of CSF, 2; CSF leakage occurred, but it resolved upon redoing the procedure (using only GM111), 3; CSF leakage occurred, but it resolved upon combined use of another dural substitute, 4; CSF leakage occurred, but it resolved upon removing GM111 and using another dural substitute, 5; unevaluable, were utilized. Similarly, five ratings were used to evaluate the efficacy of GM111 in preventing CSF leakage and subcutaneous CSF retention throughout the postoperative period based on postoperative CT images, 1; CT found no CSF leakage or subcutaneous CSF retention, 2; CT found CSF leakage or subcutaneous CSF retention, but it spontaneously disappeared (up to 24 weeks after surgery), 3; CT found CSF leakage or subcutaneous CSF retention, but it resolved following lumbar drainage and/or pressure bandage, not requiring an operating room, 4; CT found CSF leakage or subcutaneous CSF retention, and reoperation was required, 5; unevaluable. Ratings 1 and 2 represented efficacies in each of the evaluation. For objective assessment of the degree of subcutaneous CSF retention, CT images were obtained at 1, 4, 12 and 24 weeks postoperatively under imaging conditions of a slice thickness of ≤5 mm and a slice interval of ≤5 mm. The areas of slice images showing subcutaneous CSF retention were calculated and totaled (Fig. 3), and the volume was calculated by multiplying that total by the slice thickness. The assessment of 4 ratings was performed independently by the attending physician and a central adjudication committee composed of two experts in image diagnosis (Table 1). In the case of a disparity in the two sets of evaluation findings, the results of the central adjudication committee were fed back to the attending physician, who then decided the final evaluation.
2.4 Evaluation of safety
Patients were evaluated for AEs, including abnormal laboratory test values, at 1, 4, 12 and 24 weeks postoperatively, or at the time of discontinuation. All confirmed AEs were assessed in accordance with the Common Terminology Criteria for Adverse Events and followed up until it was verified that the symptoms/findings and laboratory abnormalities had resolved or there was no longer any clinical problem.
3 Results
Sixty patients were enrolled in the study, and 57 were included in the full analysis set (FAS) and 53 in the per protocol set (PPS). Table 2 summarizes the demographics and surgical outcomes in the 57 patients. The underlying diseases were a cerebrovascular disorder in 29.8%, a brain tumor in 59.6% and “other” in 10.5%. The mean age was 55.9 ± 14.4 years (mean ± standard deviation). Females outnumbered males, 61.4 to 38.6%. The site of the craniotomy was supratentorial in 77.2%, infratentorial in 12.3% and in the sellar region in 10.5%. The most common operative procedure was brain tumor removal, in 52.6%, followed by cerebral aneurysm clipping in 19.3%, transsphenoidal surgery in 10.5%, and “other” in 17.5%. The size of the GM111 utilized ranged from 0.24 to 42 cm2, and was ≤3 cm2 in 42.1%, 3 < ≤ 10 cm2 in 35.1%, and 10 < cm2 in 22.8%.
3.1 GM111 and non-suture dural closure
A suitable GM111 was successfully prepared for all 57 FAS patients, and the dural defect site or dural suture failure site could be closed by application of our non-suture dural closure method.
3.2 Efficacy endpoints
The status of intra-operative closure was rated as “1. Excellent closure with no leakage of CSF” in 100.0% of the patients, for an efficacy rate (95% confidence interval) of 100.0% (93.7, 100.0%). Closure was confirmed by Valsalva’s maneuver for 7.0% of the patients, water infusion for 26.3%, visually for 63.2% and other for 3.5%. The efficacy in preventing CSF leakage or subcutaneous CSF retention throughout the postoperative period was rated as “1. Image diagnosis found no CSF leakage or subcutaneous CSF retention” in 93.0% of the patients, and as “2. Image diagnosis found CSF leakage or subcutaneous CSF retention, but it spontaneously disappeared (up to 24 weeks after surgery)” in 7.0% of the patients. The efficacy rate was 100.0% (93.7, 100.0%). The results of the diagnostic image evaluations by the central adjudication committee also showed an efficacy rate of 100.0% (93.7, 100.0%).
Table 3 showed time course assessment by CT that were performed at 1, 4, 12 and 24 weeks postoperatively in 4 patients with postoperative subcutaneous CSF retention. An objective assessment was rated as “2. CT found subcutaneous CSF retention of <20 mL in volume” in all four patients at one week postoperatively. Within 4 weeks after surgery, three patients showed spontaneous resolution of subcutaneous CSF retention and at 12 weeks postoperatively, subcutaneous CSF retention spontaneously disappeared in all patients.
3.3 Evaluation of safety
Throughout the study period, AEs for which a causal relationship with GM111 was unable to be ruled out occurred in five of the 57 FAS patients (8.8%). The AEs consisted of three cases of subcutaneous CSF retention, one case of CSF leakage and one case of blepharedema. All those AEs resolved within a short time, without any treatment. There were no problematic laboratory abnormalities.
4 Discussion
The use of lyophilized human dura mater was prohibited in Japan in March 1997, and since that time there have been very few available options for dural substitutes. AlloDerm® (Life Cell, NJ, USA) and DuraGen® (Integra Life Sciences, NJ, USA) are in common use as dural substitutes in Europe and the United States, but they have not been approved in Japan [7, 8]. Gore-Tex DM® has been the most popular dural substitute in Japan, but it does not satisfy our ideal concept of a dural substitute because it is a non-absorbable membrane and requires suturing for dural closure. In 2007, approval was granted to SEAMDURA® (Gunze, Kyoto, Japan), a biocompatible dural substitute made of a copolymer of L-lactide and ε-caprolacton and polyglycolic acid in Japan [9]. However, it never became popular because the membrane lacked pliability and was difficult to manipulate during surgery. Most Japanese neurosurgeons devise workarounds by forming a dura from biological tissues, such as the temporal fascia and periosteum, and then applying a sealing solution, such as fibrin glue or DuraSeal® (Integra, NJ, USA), to the sutured line to prevent CSF leakage [10]. However, for surgeries involving a high risk of CSF leakage, such as operations on the skull base and posterior cranial fossa, there are often restrictions on suturing, and even when an onlay method is used, it is necessary to prepare a dural substitute having sufficient closure capability.
4.1 Efficacy of GM111
There are many reports of hydrodynamic complications (e.g., aseptic meningitis, hydrocephalus, cerebrospinal fluid leakage and pseudomeningocele) in infratentorial surgeries, with a reported complication rate of around 30% [11–15]. Moskowitz et al. performed dura mater reconstruction using various artificial dural substitutes for patients who had undergone infratentorial craniotomy and compared the postoperative hydrodynamic complications among the materials. The complication rates were very high, showing a range of 16.9–50% [16]. We previously reported that the rate of CSF leakage or subcutaneous CSF retention reached 45% (18 of 40 patients) in skull-base surgeries, with seven patients requiring re-exploration in spite of a fibrin sealant’s having been applied by the spray method [3]. In our present clinical study, the combined rate of infratentorial and transsphenoidal surgeries was 22.8%, but the incidence of CSF leakage and subcutaneous CSF retention throughout the postoperative period was only 7% (4 of 57 patients). In seven skull-base surgeries, only one patient showed subcutaneous CSF retention, and that disappeared spontaneously within 1 month. Those incidences were clearly lower than previously published data and the rate of 28.3% (15 of 53 patients) reported in 2002 for a clinical study of SeamDura® [9]. The closure capability of GM111 was superior to that of other dural substitutes, and GM111 is good for use with an onlay graft even in posterior cranial fossa surgery.
GM111 is a biocompatible dural substitute that is hydrolyzed and loses strength with the passage of time. We previously reported the results of our animal studies, which showed that GM111 had already been replaced by biological connective tissue at 1 month after surgery, while there were histological findings indicative of collagenous fibers extending from the dural stump, without gaps [6]. In our present clinical study, the area of the prosthesis exceeded 10 cm2 in 13 of the 57 patients, and we had worried about possible delayed CSF leakage. However, subsequent to 4 weeks after the surgery, there was not a single case of new CSF leakage or diagnostic images of subcutaneous CSF retention. Prior to this study, we repeated animal experiments in which we performed histological studies of GM111. A 5 × 5 mm dural defect was prepared on cerebral hemisphere in four male beagle dogs and the 15 × 15 mm GM111 was inverted to adhere to the hemispheric dura mater to cover the dural defect. We found that connective tissue that completely covered the GM111 had already been induced at one week after surgery when there was residual polyglycolic acid felt and fibrin glue. Based on our finding in the present clinical trial that there were no cases of delayed CSF leakage, it can be surmised that replacement of GM111 by biological tissue begins in the early postoperative period in humans, just as in our earlier animal experiments.
4.2 Safety of GM111
There are many reports of adverse reactions due to use of artificial dural substitutes. Besides the hydrodynamic complications noted earlier, wound infection, bleeding, excessive granulation, convulsions, etc., have also been reported. Throughout our present clinical trial, adverse reactions other than hydrodynamic complications were very few, and there was only one case of blepharedema. There were no cases of transient local inflammatory reaction or wound infection, indicating that GM111 is a dural substitute that has low antigenicity and can be safely used in the clinic.
5 Conclusion
The results of this study demonstrated that GM111, when used for non-suture dural closure, showed good closure capability in the early postoperative period, good biocompatibility in the long-term and also a high level of safety. Although evaluation of the economics is necessary, this surgical technique seems capable of reducing surgical complications, especially the hydrodynamic complications that are common in skull-base and posterior cranial fossa surgeries.
References
Timms C, Trost N, Wang YY, Murphy M. Brainstem distortion from postoperative cerebellar herniation through a dural and bony defect. J Clin Neurosci. 2008;15:1050–1.
Hutter G, Felten Sv, Sailer MH, Schulz M, Mariani L. Risk factors for postoperative CSF leakage after elective craniotomy and the efficacy of fleece-bound tissue sealing against dural suturing alone: a randomised controlled trial. J Neurosurg. 2014;121:735–44.
Terasaka S, Iwasaki Y, Kuroda S, Uchida T. A novel method of dural repair using polyglycolic acid non-woven fabric and fibrin glue: clinical results of 140 cases. No Shinkei Geka. 2006;34(11):1109–17.
Yamagata S, Goto K, Oda Y, Kikichi H. Clinical experience with expanded polytetrafluoroethylene sheet used as an artificial dura mater. Neurol Med Chir (Tokyo). 1993;33:582–85.
Kobayashi H, Asaoka K, Terasaka S, Murata J-i. Primary closure of cerebrospinal fluid fistula by nonpenetrating titanium clips in endoscopic endonasal transsphenoidal surgery: technical note. Skull Base. 2011;21:47–52.
Terasaka S, Iwasaki Y, Shinya N, Uchida T. Fibrin glue and polyglycolic acid nonwoven fabric as a biocompatible dural substitute. Neurosurgery. 2006;58(ONS Suppl 1):ONS134–9.
Chaplin JM, Constantino PD, Wolpoe ME, Bederson JB, Griffey ES, Zhang W. Use of an acellular dermal allograft for dural replacement: an experimental study. Neurosurgery. 1999;45(2):320–7.
Warren WL, Medary MB, Dureza CD, et al. Dural repair using acellular human dermis: experience with 200 cases: technical assessment. Neurosurgery. 2000;46(6):1391–96.
Yamada K, Miyamoto S, Takayama M, et al. Clinical application of a new bioabsorbable artificial dura mater. J Neurosurg. 2002;96:731–5.
Chauvet D, Tran V, Mutlu G, George B, Allain J-M. Study of dural suture watertightness: an vitro comparison of different sealants. Acta Neurochir. 2011;153:2465–72.
Danish SF, Samdani A, Hanna A, Storm P, Sutton L. Experience with acellular human dura and bovine collagen matrix for duraplasty after posterior fossa decompression for chiari malformation. J Neurosurg. 2006;104(Suppl Pediatrics):16–20.
Krieger MD, McComb JG, Levy ML. Toward a simpler surgical management of chiari malformation in a pediatric population. Pediatr Neurosurg. 1999;30:113–21.
Parizek J, Mericka P, Nemecek S, et al. Posterior cranial fossa surgery in 454 children. Comparison of results obtained in pre-CT and CT era and after various types of management of dura mater. Childs Nerv Syst. 1998;14(9):426–38.
Steinbok P, Singhal A, Mills J, Cochrane DD, Price AV. Cerebrospinal fluid (CSF) leak and pseudomeningocele formation after posterior fossa tumor resection in children: a retrospective analysis. Childs Nerv Syst. 2007;23:171–4.
Walcott BP, Neal JB, Sheth SA, et al. The incidence of complications in elective cranial neurosurgery associated with dural closure material. J Neurosurg. 2014;120:278–84.
Moskowitz SI, Liu J, Krishnaney AA. Postoperative complications associated with dural substitute in suboccipital craniotomies. Neurosurgery. 2009;64(ONS Suppl 1):ons28–34.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Terasaka, S., Taoka, T., Kuroda, S. et al. Efficacy and safety of non-suture dural closure using a novel dural substitute consisting of polyglycolic acid felt and fibrin glue to prevent cerebrospinal fluid leakage—A non-controlled, open-label, multicenter clinical trial—. J Mater Sci: Mater Med 28, 69 (2017). https://doi.org/10.1007/s10856-017-5877-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10856-017-5877-8