Abstract
A simple method for the synthesis of water-soluble carbon quantum dots (CQDs) has been developed by chemical oxidation treatment of the flour. The as-synthesized CQDs were monodispersed sphere particles with the approximate diameter of 5–8 nm, and exhibited strong fluorescence, excitation-dependent photoluminescence behavior and high photostability in a wide range of pH values. We investigated the cytotoxicity of as-prepared CQDs using rat mesangial cells (RMC). Compared with CdTe quantum dots, CQDs show no apparent cytotoxicity and much better biosafety property. The as-synthesized CQDs were also tested to label and image RMC in vitro and demonstrated to be highly promising biological fluorescent probes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Nanomaterials have attracted great interest due to excellent optical properties as fluorescence probes [1, 2]. To date, lots of nanomaterials (quantum dots, gold/silver nanoparticles, iron oxide nanoparticles, etc.) have been studied and utilized in the field of biomedicine and biotechnology [3, 4]. However, the biotoxicity of the heavy metal, the main ingredient of fluorescence semiconductor nanocrystals, have raised concerns for their bio-applications and environmental harm [5–7]. Therefore, it is very desirable to search for fluorescence nanoparticles with superior photoluminescence (PL) and biosafety property.
In recent years, carbon quantum dots (CQDs) superior to conventional semiconductor quantum dots in terms of environmentally friendly and biocompatibility have drawn much attention [8–11]. Routes to prepare CQDs can be generally summarized as two groups: top-down and bottom-up approaches. Top-down methods, including arc discharge [12], laser ablation [13, 14], electrochemical oxidation [15, 16], and chemical oxidation [17] are currently formed through post-treating carbon particles broken from a larger carbon structure. Bottom-up approaches, by which CQDs are transformed from suitable molecular precursors, consist of thermal carbonization [18], combustion [19], acid dehydration [20] and ultrasonic treatment [21]. The methods mentioned above always involve intricate and strict synthesis conditions, expensive original materials or great energy-consuming apparatus. Typically, nitric acid is always used as the oxidant to synthesize CQDs and has certain environmental hazards. Thus, it is still urgent to develop a facile and environmentally benign method for the synthesis CQDs with low-cost raw materials, high photostability and low toxicity.
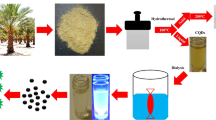
Carbon is hardly considered as an intrinsically toxic element. Currently there have been some literatures [22, 23] on biomedical applications of CQDs synthesized from rice husk or molasses, and cell labeling was achieved through cellular uptake of as-prepared CQDs requirement with surface functionalization. Herein, we proposed one novel synthetic route to prepare CQDs without further surface modification in two step. The resource-rich flour is employed as carbon source, and CQDs is obtained through soft chemical oxidation with mixed oxidant (V(HAc):V(H2O2) = 1:2). The approach is green, economic and sustainable in which no extra strong acid, alkali, or highly toxic organic solvents are used. Compare with semiconductor quantum dots, the biotoxicity of as-synthesized CQDs for cellular imaging in vitro is investigated and the results showed the CQDs pose lower toxicity effects.
2 Experimental
2.1 Materials and instruments
The flour was purchased from the local supermarket (Nanjing, China). Potassium periodate (KIO4), hydrochloric acid (36–38 %), sodium borohydride (NaBH4) and acetic acid (HAc) were all purchased from Nanjing chemical reagent corporation (Nanjing, China). 30 % hydrogen peroxide (H2O2) was purchased from Sinopharm Chemical Reagent limited corporation (Shanghai, China). Thioglycolic acid (TGA), tellurium powder, and CdCl2·2.5H2O were purchased from Guangfu Fine Chemical Research Institute (Tianjin, China). The rat mesangial cells (RMC) line was obtained from China Center for Type Culture Collection in Wuhan University (Wuhan, China). Dulbecco minimum essential media (DMEM, with d-glucose at 5.56 mmol/L, lot nos. H10517) was purchased from Gibico Company (USA). Fetal bovine serum (FBS) was supplied by PAA Cell Culture Company (Pasching, Australia). 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) was obtained from Sigma (St. Louis, MO, USA). All other chemicals were of analytical grade or the best grade commercially available. Doubly distilled water (DDW) was used throughout the study.
Fluorescence spectra and intensities were performed on a RF-5301PC spectrofluorometer (Hitachi, Japan). UV absorption spectra were obtained using a UV-2100 UV/Vis spectrophotometer (Shimadzu, Japan). Fourier Transform Infrared Spectroscopy (FT-IR) spectra were conducted on a Tensor spectrometer (Bruker, German). X-ray diffraction (XRD) analysis (Co Kα X-ray, 30 kV, 30 mA, scanning are 5°–80°) was carried from ARL X’TRA (Thermo Electron Corporation, USA). TEM images were acquired by using an H-7650 transmission electron microscope (Hitachi, Japan) operating at an acceleration voltage of 80 kv. The cell imaging was operated by an IX71 inverted fluorescence microscope (Olympus, Japan). All optical measurements were performed at room temperature under ambient conditions.
2.2 Synthesis of CQDs
2.0 g of flour and 0.4 g of potassium periodate were added into 10 ml of DDW and 4 mL of hydrochloric acid, which was dispersed uniformly by the sonication for 5 min at room temperature. Mixed solution was poured into a high pressure digestion tank and reacted for 4 h at 200 °C. The obtained black carbon source was washed with alkali liquor, DDW and ethanol orderly. 0.2 g of obtained carbon source and 60 mL of mixed oxidant (V(HAc):V(H2O2) = 1:2) were placed in a three-necked bottle, and refluxed for 8 h at 120 °C. Bright yellow solution was obtained and displayed bright blue fluorescence under ultraviolet lamp. The prepared CDs solution was dialyzed with dialysis bag for 24 h and evaporated. Finally, light yellow CDs powder was obtained by vacuum drying.
2.3 Synthesis of CdTe QDs
Following a method described previously [24], the CdTe quantum dots (QDs) was synthesized using the reaction between Cd2+ and NaHTe solution with TGA as a stabilizing agent. Briefly, tellurium powder was reacted with sodium borohydride (1:2 molar ratio) in water to produce NaHTe. The CdTe QDs (1:0.1:1.4 molar ratio of Cd2+:NaHTe:TGA) were obtained through hydrothermally heating at 180 °C for 1 h. The as-synthesized CdTe QDs after purification were used for the comparison with CQDs in the following cell cytotoxicity study (Sect. 2.4).
2.4 The cytotoxicity study of CQDs and CdTe QDs
The RMC was used to study the cytotoxicity of as-prepared CQDs and CdTe QDs. RMC line was cultured in DMEM and 5.5 mM d-glucose, and supplemented with 10 % FBS, 100 unites/mL penicillin and 100 μg/mL streptomycin. The cell viability assay was assessed by using the MTT assay, determining absorbance at 490 nm to explain the cells viability. Initially, the 5th–8th generations of RMC were seeded into two 96-well plates and incubated in culture medium for 24 h at 37 °C in 5 % CO2. Subsequently, the culture medium was removed and 200 μL of 0.8 mg/mL CQDs and 0.15 mg/mL CdTe QDs in PBS were added to every well and co-incubated with the cells for 2, 4, 6 and 8 h, respectively, which was comparatively investigated the cytotoxicity of CQDs and CdTe QDs with different incubation time. Furthermore, we also investigate the cellular cytotoxicity associated with different concentration of CQDs and CdTe QDs. The RMC seeded into two 96-well plates at the same density as above was added with 200 μL of CQDs of 0.6, 0.8, 1.0, and 1.2 mg/mL and 200 μL of CdTe QDs of 0.1, 0.125, 0.15 and 0.175 mg/mL, and incubated for 8 h respectively.
2.5 Cellular imaging
The RMC was also used to study the application of CQDs and CdTe QDs for cellular imaging. As described in Sect. 2.4, the RMC was maintained in culture medium mentioned above. Two days before imaging, the RMC were plated on tissue culture plates and incubated in fresh media at 37 °C in 5 % CO2. After cells confluence reached at 60–80 %, the RMC were incubated with 200 μL of 0.8 mg/mL CQDs and 0.15 mg/mL CdTe QDs in the DMEM medium for 3, 6 and 12 h, respectively. Then, the medium was removed and the RMC washed 3 times with PBS. Lastly, 1 mL of PBS was added to the plate and the cells were observed under Olympus IX71 inverted fluorescence microscopy.
3 Results and discussion
3.1 Characterization of the prepared CQDs
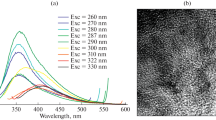
A prominent and wide absorption peak was observed in the ultraviolet range and the characteristic absorption peak was observed around 220 nm (Fig. 1a) [25, 26]. The well-dispersed CQDs solution exhibits bright blue fluorescence under ultraviolet lamp, which could be easily observed with the naked eye (inset in Fig. 1a). The emission peaks of as-prepared CQDs under varied excitation wavelengths did not obviously shift, and remained at approximate 450 nm. The as-obtained CQDs exhibit some property of excitation-independent emissions, which can avoid some autofluorescence during their applications. The emission band with highest-level fluorescence intensity was observed around 450 nm when excited at 380 nm (Fig. 1b). Therefore, we selected 380 nm as the excitation wavelength for the following experiments. The TEM image of as-prepared CQDs showed that the CQDs were uniform and monodispersed sphere particles with the approximate diameter of 5–8 nm (Fig. 1c). A sharp Bragg’s reflection at 2θ values of 27.8° in XRD spectrum is from as-synthesized CQDs (Fig. 1d).
FT-IR spectra were recorded to identify the surface groups of as-prepared CQDs (Fig. 2). The broad peak centered at 3443 cm−1 corresponds to the stretching vibrations of O-H. The sharp peaks at 1722 and 1217 cm−1 are the stretching vibration bands of C=O and C–O respectively. Thus, the above characteristic absorption bands indicate the presence of carboxylic acid and other oxygen-containing functional groups. In addition, several peaks at 2932, 1404 and 773 cm−1 reveal the existence of C=C, CH2 and CH3 on the CQDs [27, 28]. Moreover, the fluorescence quantum yield was calculated according to the method recorded in the literature [29], and was about 16.2 %.
3.2 Optimization of synthesis conditions
To get the best CQDs, we did many experiments to optimize the synthesis conditions of CQDs. The absorbance at 380 nm of CQDs was used to estimate the concentration. During the procedure of experiments, the mass of carbon source was 0.2 g and the volume of mixed oxidant (VHAc:VH2O2 = 1:2) was 60 mL. We first studied CQDs synthesized for different reaction time (1–12 h) at 100 °C, and we found that CQDs synthesized at 8 h had the strongest fluorescence (Fig. 3a). We thus chose 8 h as the optimum reaction time. We then investigated the effect of the reaction temperature (60–140 °C) on CQDs for 8 h. The fluorescence intensity of CQDs synthesized at 120 °C for 8 h was the highest. The reaction temperature of 120 °C was selected for the next experiments (Fig. 3b). Therefore, the optimum synthesis conditions of CQDs were obtained: the mass of carbon source was 0.2 g for 60 mL of mixed oxidant (VHAc:VH2O2 = 1:2), the reaction time was 8 h and the reaction temperature was 120 °C. In addition, the fluorescence emission peak is slight blue shift with time or temperature (Fig. 3a, b). Thus, there may be small change of the CQDs size even the CQDs structure.
Dialysis and extraction methods were chosen to purify the obtained CQDs. Ether, ethyl acetate and n-butyl alcohol were used as the extracting agent. The results showed that the effect extracted with the extracting agent was bad, and dialysis was higher efficient to enhance fluorescence intensity (Fig. 3c). Thus, we choose dialysis to purify C-dots. The pH-dependent PL behavior was also investigated in different pH PBS solutions. Our experimental results showed that PL intensities decrease in solutions with higher or lower pH and CQDs have a wider pH stability range for optimal fluorescence emission (Fig. 3d). Therefore, it is significant for as-prepared CQDs to apply in the field of biomedical such as cells and tissue markers in vitro or vivo.
3.3 Cytotoxicity
Biocompatibility of CQDs is one of the most important properties, which is considered importantly for their application in cell-labeling and imaging [4, 30]. Herein, we investigate the cytotoxicity of CQDs and CdTe QDs at the diverse time and concentrations. As shown in Fig. 4a, the results suggested that the cytotoxicity of both the as-prepared CQDs and CdTe QDs was incubation-time dependent. As shown in Fig. 4b, there is a mild reduction in the viability of RMC after 8 h incubation with diverse concentrations of CQDs, but in comparison, RMC incubated with CdTe QDs had a sharp reduction in cell viability with increasing concentrations. Our studies reveal that comparing with CdTe QDs, the CQDs have less biotoxicity and may warrant further evaluation to be a promising functional nanomaterials utilized in biology and biomedicine.
3.4 Imaging of RMC
Due to the excellent fluorescent characteristics, the CQDs could be used to target specific cells. Here, the as-prepared CQDs were used as a probe to label the RMCs. The images of RMC labeled with CQDs and CdTe QDs were shown in Fig. 5. After incubation with the CQDs at 37 °C for 12 h, the living RMC showed brightly illuminated under green background when imaged under a fluorescence microscope equipped with UV excitation; The red of cell shape co-incubated with CdTe QDs was apparent but the cell condition was poor. In addition, the obtained bright field image of RMC for different time also visualize clearly that the CQDs has less biotoxicity while inversely the CdTe QDs apparently made RMC depletion. Both results highly demonstrated that the as-obtained CQDs can be used as excellent and promissing probes for bioimaging and cell labeling.
4 Conclusions
In this study, a new chemical oxidation method for the synthesis of water-soluble CQDs has been developed using the flour as carbon source. The as-prepared CQDs with good dispersion and approximate diameter of 5–8 nm exhibited high fluorescence intensity, excitation-dependent PL behavior, and excellent photostability in a wide range of pH values. Compared with as-synthesized CdTe QDs, the cytotoxicity experiment of as-prepared CQDs only showed minor effect on RMC viability and significantly better biocompatibility. Moreover, the as-obtaind CQDs and CdTe QDs using as the probes successfully imaged the RMCs. Taking into account of the advantages of CQDs, they could serve as promising candidates for biological and biomedical applications.
References
Whitesides GM. Nanoscience, nanotechnology, and chemistry. Small. 2005;1:172–9.
Michalet X, Pinaud FF, Bentolila LA, Tsay JM, Doose S, Li JJ, et al. Quantum dots for live cells, in vivo imaging, and diagnostics. Science. 2005;307:538–44.
Li H, Kang Z, Liu Y, Lee ST. Carbon nanodots: synthesis, properties and applications. J Mater Chem. 2012;22:24230–53.
Barreto JA, O’Malley W, Kubeil M, Graham B, Stephan H, Spiccia L. Nanomaterials: applications in cancer imaging and therapy. Adv Mater. 2011;23:H18–40.
Derfus AM, Chan WCW, Bhatia SN. Probing the cytotoxicity of semiconductor quantum dots. Nano Lett. 2004;4:11–8.
Lewinski N, Colvin V, Drezek R. Cytotoxicity of nanoparticles. Small. 2008;4:26–49.
Hardman R. A toxicologic review of quantum dots: toxicity depends on physicochemical and environmental factors. Environ Health Perspect. 2006;114:165–72.
Baker SN, Baker GA. Luminescent carbon nanodots: emergent nanolights. Angew Chem Int Ed Engl. 2010;49:6726–44.
Yang ST, Cao L, Luo PG, Lu F, Wang X, Wang H, et al. Carbon dots for optical imaging in vivo. J Am Chem Soc. 2009;131:11308–9.
Esteves da Silva JCG, Goncalves HMR. Analytical and bioanalytical applications of carbon dots. TrAC Trends Anal Chem. 2011;30:1327–36.
Li Q, Ohulchanskyy TY, Liu RL, Koynov K, Wu DQ, Best A, et al. Photoluminescent carbon dots as biocompatible nanoprobes for targeting cancer cells in vitro. J Phys Chem C. 2010;114:12062–8.
Xu XY, Ray R, Gu YL, Ploehn HJ, Gearheart L, Raker K, et al. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J Am Chem Soc. 2004;126:12736–7.
Wang X, Cao L, Lu FS, Meziani MJ, Li H, Qi G, et al. Photoinduced electron transfers with carbon dots. Chem Commun. 2009;25:3774–6.
Wang J, Wang CF, Chen S. Amphiphilic egg-derived carbon dots: rapid plasma fabrication, pyrolysis process, and multicolor printing patterns. Angew Chem Int Ed. 2012;51:9297–301.
Zhao QL, Zhang ZL, Huang BH, Peng J, Zhang M, Pang DW. Facile preparation of low cytotoxicity fluorescent carbon nanocrystals by electrooxidation of graphite. Chem Commun. 2008;41:5116–8.
Zheng LY, Chi YW, Dong YQ, Lin JP, Wang BB. Electrochemiluminescence of water-soluble carbon nanocrystals released electrochemically from graphite. J Am Chem Soc. 2009;131:4564–5.
Krysmann MJ, Kelarakis A, Giannelis EP. Photoluminescent carbogenic nanoparticles directly derived from crude biomass. Green Chem. 2012;14:3141–5.
Yang ZC, Wang M, Yong AM, Wong SY, Zhang XH, Tan H, et al. Intrinsically fluorescent carbon dots with tunable emission derived from hydrothermal treatment of glucose in the presence of monopotassium phosphate. Chem Commun. 2011;47:11615–7.
Tian L, Ghosh D, Chen W, Pradhan S, Chang X, Chen S. Nanosized carbon particles from natural gas soot. Chem Mater. 2009;21:2803–9.
Peng H, Travas-Sejdic J. Simple aqueous solution route to luminescent carbogenic dots from carbohydrates. Chem Mater. 2009;21:5563–5.
Li H, He X, Liu Y, Huang H, Lian S, Lee ST, et al. One-step ultrasonic synthesis of water-soluble carbon nanoparticles with excellent photoluminescent properties. Carbon. 2011;49:605–9.
Pandey S, Mewada A, Thakur M, Pillai S, Dharmattii R, Phadke C, et al. Synthesis of mesoporous silica oxide/C-dot complex (meso-SiO2/C-dots) using pyrolysed rice husk and its application in bioimaging. RSC Adv. 2014;4:1174–9.
Das B, Dadhich P, Pal P, Srivas PK, Bankoti K, Dhara S. Carbon nanodots from date molasses: new nanolights for the in vitro scavenging of reactive oxygen species. J Mater Chem B. 2014;2:6839–47.
Li MY, Ge YX, Chen QF, Xu SK, Wang NZ, Zhang XJ. Hydrothermal synthesis of highly luminescent CdTe quantum dots by adjusting precursors’ concentration and their conjunction with BSA as biological fluorescent probes. Talanta. 2007;72:89–94.
Wang QL, Huang XX, Long YJ, Wang XL, Zhang HJ, Zhu R, et al. Hollow luminescent carbon dots for drug delivery. Carbon. 2013;59:192–9.
Xu ZH, Yu JG, Liu G. Fabrication of carbon quantum dots and their application for efficient detecting Ru(bpy) 2+3 in the solution. Sens Actuators B. 2013;181:209–14.
Chen XF, Zhan WX, Wang QJ, Fan JY. C8-structured carbon quantum dots: synthesis, blue and green double luminescence, and origins of surface defects. Carbon. 2014;79:165–73.
Liang QH, Ma WJ, Shi Y, Li Z, Yang XM. Easy synthesis of highly fluorescent carbon quantum dots from gelatin and their luminescent properties and applications. Carbon. 2013;60:421–8.
Grabolle M, Spieles M, Lesnyak V, Gaponik N, Eychmüller A, Resch-Genger U. Determination of the fluorescence quantum yield of quantum dots: suitable procedures and achievable uncertainties. Anal Chem. 2009;81:6285–94.
Kumar P, Meena R, Paulraj R, Chanchal A, Verma AK, Bohidar HB. Fluorescence behavior of non-functionalized carbon nanoparticles and their in vitro applications in imaging and cytotoxic analysis of cancer cells. Colloid Surf B. 2012;91:34–40.
Acknowledgment
This work was supported by The Research and Innovation Project for Graduate Students Academic Degree of Colleges and Universities of Jiangsu Province (KYZZ_0185) and The National Natural Science Foundation of China (NSFC 81274056).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Zhengwei Zhang and Yu Duan have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zhang, Z., Duan, Y., Yu, Y. et al. Carbon quantum dots: synthesis, characterization, and assessment of cytocompatibility. J Mater Sci: Mater Med 26, 213 (2015). https://doi.org/10.1007/s10856-015-5536-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10856-015-5536-x