Abstract
The biocompatibility of dental implant abutment materials depends on numerous factors including the nature of the material, its chemical composition, roughness, texture, hydrophilicity and surface charge. The aim of the present study was to compare the viability and adhesion strength of human gingival fibroblasts (HGFs) grown on several dental materials used in implant prosthodontics. Surfaces of the tested materials were assessed using an optical imaging profiler. For material toxicity and cellular adhesion evaluation, primary human gingival fibroblast cells were used. To evaluate the strength of cellular adhesion, gingival fibroblasts were cultured on the tested materials and subjected to lateral shear forces by applying 300 and 500 rpm shaking intensities. Focal adhesion kinase (FAK) expression and phosphorylation in cells grown on the specimens were registered by cell-based ELISA. There was a tendency of fibroblast adhesion strength to decrease in the following order: sandblasted titanium, polished titanium, sandblasted zirconium oxide, polished zirconium oxide, gold–alloy, chrome–cobalt alloy. Higher levels of total as well as phospho-FAK protein were registered in HGFs grown on roughened titanium. Material type and surface processing technique have an impact on gingival fibroblast interaction with dental implant abutment materials.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Dental implant abutment materials are in close contact with the surrounding soft tissues. Their biocompatibility is crucial as this influences the condition of bone and gingiva surrounding the implants. Biocompatibility depends on numerous factors including the nature of the material, its chemical composition, roughness, texture, hydrophobicity/hydrophilicity, and surface charge [1, 2]. An appropriate host cellular response to foreign body surfaces is essential for successful function of prosthetic materials.
When inserted into a tissue, a foreign material gets into contact with bodily fluids, and subsequently multiple events are triggered [3]. Fluid components such as lipids, carbohydrates and proteins adsorb to the material surface and serve as extracellular matrix (ECM) for cell adhesion [4]. Although the influence of material properties on subsequent tissue response is not yet fully understood, it is widely documented that surface features can affect the amounts and types of bound proteins, as well as their conformation, orientation and binding strength [5, 6]. The patterns in which adhesion proteins and other bioactive molecules adsorb and elicit cellular reactions are specific to the underlying physicochemical properties of the material. In vitro studies generally demonstrate favorable cell responses to charged, hydrophilic surfaces, corresponding to superior adsorption and bioactivity of adhesion proteins [7].
In the organism, cell adhesion is important for a variety of physiological and pathological processes, such as host response to implanted devices and integration of tissue-engineered constructs [8, 9]. Cell adhesive interactions are complex mechanisms integrating binding of membrane proteins to ECM, intracellular cytoskeleton reformation, and signal transduction. In vivo, cells are surrounded by ECM, which organizes cells into tissues. In vitro, ECM molecules are found naturally in some media supplements, and are secreted by cells themselves. Cell contacts with ECM are mediated by adhesion molecules, which serve as receptors sending signals into the cells. Focal adhesion kinase (FAK) is an essential non-receptor tyrosine kinase regulating cell migration, adhesion signaling, and mechanosensing [10, 11]. It is typically located at multi-protein structures, known as focal adhesions that link the ECM to the cytoskeleton [12, 13]. The autophosphorylation of FAK is critical to this regulation of adhesion strengthening [13–15]. Phosphorylated FAK becomes a docking site for mediators of multiple signaling events that regulate cell survival, proliferation and morphogenesis [16]. During the early stages of adhesion, FAK activates integrins by increasing their binding over time, which results in adhesion strengthening. These data demonstrate an important role for FAK in the time-dependent generation of cell–ECM forces [15]. Currently, the use of biomaterials for implant/abutment manufacturing is increasing. In addition, several clinical studies have shown that soft tissues may have different adaptation quality to various prosthetic materials [17–19].
The aim of the present study was to compare the viability and adhesive intensity of human gingival fibroblasts (HGFs) grown on several implant abutment materials.
2 Materials and methods
2.1 Manufacture of specimens
Material samples included commercial pure titanium (Grade 2, Everest® T-Blank, KaVo, Biberach, Germany), zirconium oxide ceramic (Everest® Z-Blank, KaVo), chrome-cobalt alloy (Ceralloy C, Eukamed, Essen, Germany) and dental gold alloy (Bio Heragold B, Heraeus Kulzer, Hanau, Germany). Cast chrome–cobalt (Cr–Co) and gold (Au) alloys were fabricated using a conventional lost wax technique. Polished specimen (titanium, zirconium oxide, chrome-cobalt alloy and gold alloy) surfaces were treated with 1200 grit silicon carbide paper (Imperial™ Wetordry™, 3 M, USA) with water cooling to eliminate surface irregularities. The samples were subsequently washed with water and dried to remove any silicon carbide particles. Next, the specimens were polished consecutively using 6 and 1 μm diamond paste and thoroughly washed in ethanol following each step. Additionally, titanium and zirconium oxide specimens were sandblasted with aluminium oxide particles sized 50 μm (Siladent, Munich, Germany). The procedure was performed at a distance of 1 cm at a pressure of 3 bars for 3 s. All specimens were equal in their diameter and height (5.2 and 2 mm, respectively). Fabricated specimens were divided into six groups (five specimens in each group): polished titanium (Ti–P), sandblasted titanium (Ti–S), polished zirconium oxide ceramic (ZrO–P), sandblasted zirconium oxide ceramic (ZrO–S), chrome-cobalt alloy (Cr–Co), gold alloy (Au). Surface roughness of the tested materials was assessed using an optical imaging profiler PLμ 2300 (Sensofar, USA) with manufacturers software. Specimen surface topographies were visualized using a TM-1000 scanning electron microscope (SEM; Hitachi High-Technologies Co., Japan).
For cell experiments, the specimens were sterilized in 96 % ethanol with subsequent exposure of both sample sides to UV light for 15 min. Later, the specimens were transferred into the wells of a 96-well plate and put in a direct contact with fibroblast suspension.
2.2 Establishment of human gingival fibroblast cell culture
The study protocol was approved by the institutional Ethics review board at Vilnius University (No. 158200-11-116-28). Gingival tissues were obtained from a healthy patient undergoing gingivectomy of the premolar region. Primary HGF culture was derived following an established procedure [20]. Briefly, the grafted tissue (6–8 mm3) was minced under sterile conditions. Fine-cut tissue suspension was diluted in cell growth medium [Iscove’s modified Dulbecco’s minimum essential medium (IDMEM, Biological Industries, Israel)] with antibiotics: 100 U/mL penicillin, 0.1 mg/mL streptomycin and 12.5 U/mL nystatin (Biological Industries, Israel) as well as 20 % of fetal calf serum (FCS, Biological Industries, Israel), and cultured at 37 °C in humidified 5 % CO2 atmosphere for 1–2 weeks. Cells started moving from the explants after 7–10 days. After a monolayer was formed, it was dispersed using a trypsin–EDTA solution (0.25 %) (Biological Industries, Israel) and subcultured in IDMEM supplemented with 10 % of FCS and antibiotics. Cells of 5–10 passages were used for the experiments.
2.3 Assessment of cytotoxicity
For the determination of cytotoxicity, HGF suspension (5 × 104 cells/mL) was prepared and 100 µL of it was placed onto tested specimens in a 96-well cell culture plate. The cells were incubated for 48, 72, 96 and 120 h. Control HGFs were maintained in polystyrene plate wells.
The effect of tested materials was evaluated on the HGFs using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, Sigma-Aldrich Inc., USA) assay in a direct-contact format according to the ISO-10993-5:1999 specifications. In test culture, MTT assay was used to register the amount of viable cells as well as to estimate the toxicity of the tested materials. After dissolving formazan (MTT metabolism product) in ethanol, optical density (OD) of the resulting solution was measured using a microtiter plate reader TECAN Infinite 200 PRO (Tecan Group Ltd., Switzerland) at 570 nm. The OD value indicated the proportion of viable cells.
The effects of surface topography and material type on cell orientation and shape were analyzed by SEM. After HGF cultivation on the specimens for 72 h, cells were fixed with 2.5 % glutaraldehyde (Sigma-Aldrich Inc.) for 30 min at room temperature, followed by fixation with 2 % OsO4 (Sigma-Aldrich Inc.) for 20 min at room temperature. The samples were subsequently dehydrated by immersion in progressively higher ethanol concentrations (25, 50, 75, 90, and 96 %, for 5 min each). Subsequently, the samples were dried using a critical point dryer (K850, Quorum Technologies, UK), then covered with a 20 nm conducting layer of gold using a sputter coater (Q150R, Quorum Technologies). Images were obtained using a SEM.
2.4 Evaluation of adhesion strength
After incubation for 24 h, specimens with adherent cells were transferred to new plate wells with fresh culture medium; the plate was immobilized on a Thermomix Comfort shaker (Eppendorf, Hamburg, Germany) used to create lateral shear forces. First part of the experiment was carried out using the shaking device in 300 rpm mode for 15 min, at 37 °C. Next, the samples were transferred to wells with trypsin/EDTA mixture; the device was used at 600 rpm at 37 °C for 10 min to detach all remaining cells from specimen surfaces. Hemocytometer and optical microscope were used to calculate the number of detached cells. Two calculations were performed. The first cell counting was done for cells that had detached from the specimen into IDMEM after the first shaking procedure. The second counting was performed for all remaining cells that were detached using trypsin–EDTA mixture. Calculated ratio (detached cell number after shaking at 300 rpm/total number of detached cells) expressed in percentages reflect the adhesion strength. Later, all specimens were cleaned and sterilized as described above and a second experiment was carried out with 500 rpm shaking mode for 15 min at 37 °C.
2.5 Cell-based ELISA
To determine FAK expression and phosphorylation, the cells were grown on the specimens in 96-well plates at a density of 1.5 × 104 cells/cm2 1 day prior to manipulation. Next day, the cells were fixed with 4 % paraformaldehyde at room temperature for 15 min, followed by washing with PBS containing 0.1 % Triton X-100. Then the cells were incubated in an antibody blocking buffer, followed by incubations with phospho- or total anti-FAK—(Cell Signaling Technology, Inc. and BD Bioscience, USA, respectively) as well as β-actin-specific primary antibodies (Millipore, USA) for 1 h at room temperature. After washing steps, HRP-conjugated antibodies were added and the cells were incubated for one hour at room temperature. Subsequently, the plates were subjected to 0.1 mg/mL TMB (3,3′,5,5′-tetramethylbenzidine, Sigma-Aldrich Inc.), according to the manufacturers protocol. The reaction was stopped with 2 M sulphuric acid and absorbance was measured at 450 nm using a Varioskan Flash plate reader (Thermo Fisher Scientific, Inc., USA). The change in FAK expression and phosphorylation status was calculated by dividing absorbance detected using phospho- or total protein-specific antibodies with that of the β-actin-specific antibody.
2.6 Statistical analysis
For all measurements, means and standard errors were calculated. Variables were checked for normal distribution by Shapiro–Wilk statistic and compared by one-way ANOVA followed by Tukey’s HSD post hoc test when normally distributed or by Kruskal–Wallis H test for non-normally distributed variables (IBM SPSS Statistics for Windows, Version 19.0. Armonk, NY: IBM Corp.). Comparison of adhesion strength between the groups was done by two-way ANOVA test. A P value < 0.05 was considered statistically significant.
3 Results
3.1 Surface analysis of tested prosthetic materials
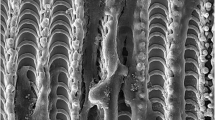
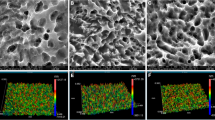
Topographical analysis of the specimens showed surface roughness ranging from 0.03 to 0.79 µm (Fig. 1). It is evident that the most irregularities were observed on sandblasted titanium and sandblasted zirconium oxide surfaces. There were a lot of small crests, valleys and some scratched areas. Particularly many irregularities with pits and spikes were observed on the sandblasted titanium. The surfaces of polished titanium, chrome-cobalt and gold alloys had some similarities. Surface of sandblasted zirconium oxide specimens contained more irregularities compared to polished ones. The polished zirconium oxide specimens were prone to scratching. Surface roughness (Ra) values are presented in Fig. 2. Ra values were highest in Ti–S and Zr–S groups (0.48–0.79 µm) while for other groups it was in the range of 0.03–0.11 µm.
Optical profiler images of disc surfaces: Surface roughness scale bars (in colour) are shown beneath the profiler images. 1 to −1 μm scale represents the Ti–P, ZrO–P, Cr–Co and Au samples, while 5 to -5 μm scale represents Ti–S and ZrO–S samples, which had a higher surface roughness. Black scale bars are 50 µm
3.2 Cytotoxic effect
Surface effect on HGFs was evaluated using MTT assay. After 48 h of culture, Cr–Co and Au samples were observed to be the most toxic with 80 and 86 % cell viability, respectively (Fig. 3). After a longer (120 h) culture period, however, the amount of viable cells reached control level on Au, but remained diminished on Cr–Co. There were no statistically significant differences in cytotoxicity between polished and sandblasted titanium as well as zirconium oxide ceramics. After 120 h, no statistically significant differences (P < 0.05) were detected between the specimen groups except for Cr–Co group (Kruskal–Wallis H test). Remarkable overrun of percentage ratio on the titanium shows favorable environment for the cells.
Viability of cells grown on the tested materials after 48, 72, 96, and 120 h. Results are presented as a ratio of the optical density measured in cell culture grown on tested surfaces for 48, 72, 96 or 120 h to the optical density measured in control cell culture grown on polystyrene, expressed in percentage
SEM analysis of fibroblast-seeded samples revealed typically mosaic-shaped confluent cell layer formed on all the specimen surfaces. The cells appeared to be intimately attached to the surfaces and aligned in rows (Fig. 4—SEM pictures of specimens without cells and Fig. 5—SEM pictures of cells grown on the specimens).
3.3 Cell adhesion
Adhesion strength evaluation showed that after shaking at 300 rpm, the least number of cells detached from Ti–P, Ti–S and ZrO–S (<20 % cells, P < 0.05) (Fig. 6). Maximum quantity of detached cells was observed on Cr–Co and Au specimens (25–30 % cells). Statistically significant (P < 0.05) changes were observed between all specimen groups except for Ti–P and ZrO–S specimens (two-way ANOVA test). A similar cell detachment tendency was noticed after shaking at 500 rpm. The least number of detached cells was observed in Ti and ZrO groups (≤35 %). Meanwhile, 43 % of cells detached in Au and 53 % in Cr–Co groups. Differences between all groups were statistically significant (P < 0.05).
Influence of material type and shaking intensity on cell adhesion strength was evaluated. Shaking intensity had the most significant effect (F-value = 3629) on cell adhesion strength whereas material type had less effect (F-value = 1639).
3.4 FAK measurement results
FAK expression and phosphorylation in HGFs grown on the surfaces was registered by cell-based ELISA (Fig. 7). Significant increase of total FAK expression was observed on Ti–S surface as compared to Ti–P (Fig. 7, A). Meanwhile, surface modifications of ZrO did not affect registered amount of FAK. A similar tendency was observed in the levels of phospho-FAK, but the difference between the groups was less expressed (Fig. 7, B). Therefore, in HGFs grown on Ti–S, higher levels of total and phospho-FAK proteins were registered.
4 Discussion
This study investigates surface impact on cell viability and adhesion strength of primary HGFs on materials widely used as implant abutments. To obtain consistent cellular response and behavior of HGFs, the cells within the fifth and tenth passages were used in this study. Such cells retain phenotypically similar features to their counterparts in vivo. This means that meaningful conclusions can be drawn from the observed cell behavior in vitro.
It is known that connective tissue cells normally interact through a protein environment and usually do not touch each other [21, 22]. In addition, when a prosthetic material such as an implant abutment is introduced, healing takes place and epithelial as well as connective tissue matures. Direct connective tissue attachment to the implant/abutment surface is intended to prevent apical migration of the junctional epithelium and prevent crestal bone resorption [23]. It was shown that implant abutment modification allows direct connective tissue attachment to the abutment surface [24]. Among different methods to modify the abutment surface (surface anodization, acid etching, UV and laser irradiation, use of biomolecules etc.), sandblasting is widely used for these purposes [25–28].
In our study, sandblasting was applied only to the titanium and zirconium oxide groups. Polished surfaces had a flat profile, whereas sandblasted surfaces showed distinct topographical differences such as strongly pronounced micro-scale roughness and wave-like configuration. Despite equal sandblasting protocol for Ti and ZrO groups, optic profiler images revealed rougher Ti surfaces. According to our data, cytotoxic effect was not influenced by surface modification. Titanium and zirconium oxide specimens showed similar biocompatibility which was in line with previous reports [29].
In this study, two shaking intensities were used to evaluate fibroblast adhesion strength to different materials and their surface modifications. Due to a high diversity of methods used to test cell adhesion strength it is impossible to make direct comparisons with other studies [30]. In our case, HGFs showed stronger adhesion to roughened titanium and zirconium oxide surfaces. These findings are in agreement with other authors [31, 32]. There is data showing that a higher number of focal contacts and a better organization of the cytoskeleton including stronger network of actin fibers of fibroblasts were observed on rough titanium surfaces compared to smooth surfaces [33]. Results of this study indicate that increased FAK expression in Ti–S group negatively correlate with the mean quantity of detached cells after shaking. This is in accordance with the data showing that expression of FAK may play an important role in the maintenance of a mucosal tissue barrier [34].
Various studies [35–38] report that micro-grooved surfaces may aid fibroblast attachment to substratum forming a more stable connective tissue zone that inhibits downgrowth of the epithelium. Visually, the growth of fibroblasts on the micro-grooved surfaces is orientated by fine irregularities, whereas cells on sandblasted surfaces seem to be randomly aligned. A study on HGF behavior, adhesion, orientation and proliferation on nickel-titanium alloys with different surface roughness showed a relation between the alignment and proliferation of fibroblasts and the surface roughness of nickel-titanium alloy [39]. Still, direct cell adhesion strength was not measured, thus, it may well be that the alignment itself may have significant impact on adhesion strength. In this study, however, surface roughness values were relatively small compared to the size of fibroblasts. Therefore, it was possible to directly compare groups with polished and sandblasted surfaces.
Reports show that fibroblasts grown on rougher titanium surfaces tend to proliferate slower, so initial attachment occurs later [40]. Despite the before mentioned fact, fibroblasts still proliferate, flatten and form cellular bridges with adjacent cells on surfaces with various roughness, indicating good attachment to surfaces. Optimal surface roughness (Ra) for fibroblasts is reported to range between 0.1 [41] and 0.15 μm [42]. In our study, surface roughnesses (Ra) of polished titanium and zirconium oxide were 0.04 and 0.1 μm, whereas for sandblasted specimens, it was 0.79 and 0.48 μm respectively. According to our data, sandblasted specimen surfaces favored stronger fibroblast adhesion. It was reported that fibroblasts could form actin fiber terminations both in the grooves and on the ridges [43]. It may be speculated that collagen produced by fibroblasts had better mechanical interlocking on rougher surfaces as well as better resistance for lateral shear force due to rough surface grooves that acted as anchoring sites. Still, this hypothesis should be confirmed by additional physiochemical studies.
Although Au and Cr–Co alloys had comparable roughnesses to polished ZrO and Ti specimens, fibroblast adhesion strength was weaker. These findings support the idea that material type has a strong influence on cellular adhesion strength [23, 44, 45]. The latter fact shows that the chemical composition of Au and Cr–Co alloys may be less compatible with gingival fibroblasts despite similar or even higher roughnesses compared to ZrO and Ti specimens. Welander et al. [23] studied mucosal barrier with various implant abutments in vivo and concluded that abutments made of ZrO and Ti favored better mucosal barrier than Au alloy because Au led to an apical shift of the barrier epithelium as well as marginal bone occurring between 2 and 5 months of healing.
Because alumina particles used for sandblasting could remain embedded into the surface of titanium or even zirconia specimens, it should be also considered as a factor, which could affect the measured outcomes. However, previous studies have failed to show the biocompatibility issues of implant abutment materials made from aluminum oxide [46, 47].A controversy exists among various researchers on the topic of surface roughness in relation to cell interaction quality. Some studies report that smoother surfaces increase fibroblast proliferation [48], while others oppose [49]. For instance, Meyle [50] reported that sandblasted titanium surface enhanced fibroblast as well as osteoblast proliferation and adhesion, whereas epithelial cells showed a contrary response to the same surfaces. Moreover, Cochran et al. [40] compared attachment and proliferation of human periodontal fibroblasts and epithelial cells cultured on titanium surfaces with different roughnesses (fine or coarse sandblasted/acid-etched vs. electro-polished) and found that initial adhesion of periodontal fibroblasts was greater on smooth titanium, however, after 5 days of incubation, there were no differences in cell numbers between all surfaces. In addition, it was found that epithelial cells had a lower growth rate on rough surfaces. It should also be noted that an increase in surface roughness facilitates biofilm formation on dental implant and abutment surfaces, as Ra values above 0.2 μm were associated with early plaque formation [51, 52]. Considering these facts, surface characteristics of implant abutment materials should enhance epithelial and connective tissue attachment and at the same time minimize bacterial adhesion. However, it is still not clear what is the recommended surface roughness for dental implant abutment materials in vivo. A study comparing roughened and turned surfaces of dental implants failed to demonstrate any clinically significant differences [53]. Moreover, it was shown that the location of rougher surface in relation to gingival margin is important, as rougher surfaces at subgingival areas have not influenced biofilm formation comparing with supragingival areas. This study has investigated modification methods of subgingival part of the implant abutment, therefore it could be hypothesized that negative influence of increased roughness would be limited [53, 54].
Fibroblasts from human gingival tissue were cultured in vitro to get a close imitation of in vivo circumstances. In this model, material type as well as surface processing techniques had an impact on gingival cell adhesion strength. In spite of that, it should be noted that cells from different species or other cell lines might behave in a different manner. Moreover, as this study was performed in an artificial environment, the findings should be verified further by in vivo investigations.
5 Conclusions
Within its limitations, the present study shows that material type and roughness have an effect on gingival fibroblast adhesion strength. There was a tendency of gingival fibroblast adhesion strength to decrease in the following order: sandblasted Ti, polished Ti, sandblasted ZrO, polished ZrO, Au alloy, Cr–Co alloy. Higher levels of total and phospho-FAK protein were registered in HGFs grown on roughened titanium.
References
Hallab NJ, Bundy KJ, O’Connor K, Moses RL, Jacobs JJ. Evaluation of metallic and polymeric biomaterial surface energy and surface roughness characteristics for directed cell adhesion. Tissue Eng. 2001;7:55–71.
Lange R, Luthen F, Beck U, Rychly J, Baumann A, Nebe B. Cell-extracellular matrix interaction and physico-chemical characteristics of titanium surfaces depend on the roughness of the material. Biomol Eng. 2002;19:255–61.
Elias CN. Implant dentistry—a rapidly evolving practice. In: Turkyilmaz I. Factors affecting the success of dental implants. InTech; 2011. p 319–365.
Goreish HH, Lewis AL, Rose S, Lloyd AW. The effect of phosphorylcholine-coated materials on the inflammatory response and fibrous capsule formation: in vitro and in vivo observations. J Biomed Mater Res A. 2004;68:1–9.
Wang YX, Robertson JL, Spillman WB, Claus RO. Effects of the chemical structure and the surface properties of polymeric biomaterials and their biocompatibility. Pharmaceut Res. 2004;21:1362–73.
Thevenot P, Hu W, Tang L. Surface chemistry influences implant biocompatibility. Curr Top Med Chem. 2008;8:270–80.
Wilson CJ, Clegg RE, Leavesley DI, Pearcy MJ. Mediation of biomaterial-cell interactions by adsorbed protein: a review. Tissue Eng. 2005;11:1–18.
Akiyama SK. Integrins in cell adhesion and signaling. Hum Cell. 1996;9:181–6.
Gumbiner BM. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345–57.
Burridge K, Chrzanowska-Wodnicka M. Focal adhesions, contractility, and signaling. Annu Rev Cell Dev Biol. 1996;12:463–519.
Thamilselvan V, Craig DH, Basson MD. FAK association with multiple signal proteins mediates pressure-induced colon cancer cell adhesion via a Src-dependent PI3K/Akt pathway. FASEB J. 2007;21:1730–41.
Schaller MD, Hildebrand JD, Parsons JT. Complex formation with focal adhesion kinase: a mechanism to regulate activity and subcellular localization of Src kinases. Mol Biol Cell. 1999;10:3489–505.
Hanks SK, Ryzhova L, Shin NY, Brábek J. Focal adhesion kinase signaling activities and their implications in the control of cell survival and motility. Front Biosci. 2003;8:982–96.
Schaller MD, Hildebrand JD, Shannon JD, Fox JW, Vines RR, Parsons JT. Autophosphorylation of the focal adhesion kinase pp125FAK, directs SH2-depentent binding of pp60src. Mol Biol Cell. 1994;14:1680–8.
Michael KE, Dumbauld DW, Burns KL, Hanks SK, García AJ. Focal adhesion kinase modulates cell adhesion strengthening via integrin activation. Mol Biol Cell. 2009;20:2508–19.
Ilic D, Damsky CH, Yamamoto T. Focal adhesion kinase: at the crossroads of signal transduction. J Cell Sci. 1997;110:401–7.
Maxson BB, Syed SA, Dominguez BL. Clinical and microbiologic comparisons of two dental implant systems. Oral Surg Oral Diagn. 1992;3:31–5.
Buser D, Mericske-Stern R, Bernard JP, Behneke A, Behneke N, Hirt HP, Belser UC, Lang NP. Long-term evaluation of non-submerged ITI implants. Part 1: 8-year life table analysis of a prospective multi-center study with 2359 implants. Clin Oral Implants Res. 1997;8:161–72.
Fartash B, Arvidson K. Long-term evaluation of single crystal sapphire implants as abutments in fixed prosthodontics. Clin Oral Implants Res. 1997;8:58–67.
Cabral MCT, Costa MA, Fernandes MH. In vitro models of periodontal cells: a comparative study of long-term gingival, periodontal ligament and alveolar bone cell cultures in the presence of β-glycerophosphate and dexamethasone. J Mater Sci Mater Med. 2007;18:1079–88.
Keller G, Sebastian J, Lacombe U, Toft K, Lask G, Revazova E. Safety of injectable autologous human fibroblasts. Bull Exp Biol Med. 2000;130:786–9.
Rompen E, Domken O, Degidi M, Pontes AE, Piattelli A. The effect of material characteristics, of surface topography and of implant components and connections on soft tissue integration: a literature review. Clin Oral Implants Res. 2006;17:55–67.
Welander M, Abrahamsson I, Berglundh T. The mucosal barrier at implant abutments of different materials. Clin Oral Implants Res. 2008;19:635–41.
Nevins M, Camelo M, Nevins ML, Schupbach P, Kim DM. Connective tissue attachment to laser-microgrooved abutments: a human histologic case report. Int J Prosthodont Restor Dent. 2012;32:385–92.
Jin C, Ren LF, Ding HZ, Shi GS, Lin HS, Zhang F. Enhanced attachment, proliferation, and differentiation of human gingival fibroblasts on titanium surface modified with biomolecules. J Biomed Mater Res B Appl Biomater. 2012;100:2167–77.
Watanabe H, Saito K, Kokubun K, Sasaki H, Yoshinari M. Change in surface properties of zirconia and initial attachment of osteoblast like cells with hydrophilic treatment. Dent Mater J. 2012;31:806–14.
Yazici H, Fong H, Wilson B, Oren EE, Amos FA, Zhang H, Evans JS, Snead ML, Sarikaya M, Tamerler C. Biological response on a titanium implant-grade surface functionalized with modular peptides. Acta Biomater. 2013;9:5341–52.
Baltriukiene D, Sabaliauskas V, Balciunas E, Melninkaitis A, Liutkevicius E, Bukelskiene V, Rutkunas V. The effect of laser-treated titanium surface on human gingival fibroblast behavior. J Biomed Mater Res A. 2014;102:713–20.
Moller B, Terheyden H, Acil Y, Purcz NM, Hertrampf K, Tabakov A, Behrens E, Wiltfang J. A comparison of biocompatibility and osseointegration of ceramic and titanium implants: an in vivo and in vitro study. Int J Oral Maxillofac Surg. 2012;41:638–45.
Meretoja VV, Rossi S, Peltola T, Pelliniemi LJ, Närhi TO. Adhesion and proliferation of human fibroblasts on sol–gel coated titania. J Biomater Res A. 2010;95:269–75.
Ungersbock A, Pohler O, Perren SM. Evaluation of the soft tissue interface at titanium implants with different surface treatments: experimental study on rabbits. Bio-Med Mater Eng. 1994;4:317–25.
Kim H, Murakami H, Chehroudi B, Textor M, Brunette DM. Effects of surface topography on the connective tissue attachment to subcutaneous implants. Int J Oral Maxillofac Surg. 2006;21:354–65.
Eisenbarth E, Linez P, Biehl V, Velten D, Breme J, Hildebrand HF. Cell orientation and cytoskeleton organization on ground titanium surfaces. Biomol Eng. 2002;19:233–7.
Miura S, Takebe J. Biological behavior of fibroblast-like cells cultured on anodized-hydrothermally treated titanium with a nanotopographic surface structure. J Prosthodont Res. 2012;56:178–86.
Chehroudi B, Gould TR, Brunette DM. A light and electron microscopic study of the effects of surface topography on the behavior of cells attached to titanium-coated percutaneous implants. J Biomed Mater Res A. 1991;25:387–405.
Chehroudi B, Gould TR, Brunette DM. The role of connective tissue in inhibiting epithelial downgrowth on titanium-coated percutaneous implants. J Biomed Mater Res. 1992;26:493–515.
Kawahara H, Kawahara D, Hashimoto K, Takashima Y, Ong JL. Morphologic studies on the biologic seal of titanium dental implants. Report I. In vitro study on the epithelialization mechanism around the dental implant. Int J Oral Maxillofac Surg. 1998;13:457–64.
Kawahara H, Kawahara D, Mimura Y, Takashima Y, Ong JL. Morphologic studies on the biologic seal of titanium dental implants. Report II. In vivo study on the defending mechanism of epithelial adhesions/attachment against invasive factors. Int J Oral Maxillofac Surg. 1998;13:465–73.
Ponsonnet L, Comte V, Othmane A, Lagneau C, Charbonnie M, Lissac M, Jaffrezic N. Effect of surface topography and chemistry on adhesion, orientation and growth of fibroblasts on nickel–titanium substrates. Mater Sci Eng Part C. 2002;21:157–65.
Cochran D, Simpson J, Weber HP, Buser D. Attachment and growth of periodontal cells on smooth and rough titanium. Int J Oral Maxillofac Surg. 1994;9:289–97.
Takamori ER, Cruz R, Goncalvez F, Zanetti RV, Zanetti A, Granjeiro JM. Effect of roughness of zirconia and titanium on fibroblast adhesion. Artif Organs. 2008;32:305–9.
Huang HH, Ho CT, Lee TH, Lee TL, Liao KK, Chen FL. Effect of surface roughness of ground titanium on initial cell adhesion. Biomol Eng. 2004;21:93–7.
Dunn GA, Brown AF. Alignment of fibroblasts on grooved surfaces described by a simple geometric transformation. J Cell Sci. 1986;83:313–40.
Kokoti M, Sivropoulou A, Koidis P, Garefis P. Comparison of cell proliferation on modified dental ceramics. J Oral Rehabil. 2001;28:880–7.
Scotchford CA, Ball M, Winkelmann M, Voros J, Csucs C, Brunette DM, Danuser G, Textor M. Chemically patterned, metal-oxide-based surfaces produced by photolithographic techniques for studying protein- and cell-interactions. II: protein adsorption and early cell interactions. Biomaterials. 2003;24:1147–58.
Abrahamsson I, Berglundh T, Glantz PO, Lindhe J. The mucosal attachment at different abutments. An experimental study in dogs. J Clin Periodontol. 1998;25:721–7.
Andersson B, Taylor A, Lang BR, Scheller H, Scharer P, Sorensen JA, Tarnow D. Alumina ceramic implant abutments used for single-tooth replacement: a prospective 1- to 3-year multicenter study. Int J Prosthodont. 2001;14:432–8.
Anselme K, Linez P, Bigerelle M, Le Maguer D, Le Mague A, Hardouin P, Hildebrand HF, Iost A, Leroy JM. The relative influence of the topography and chemistry of TiAl6V4 surfaces on osteoblastic cell behaviour. Biomaterials. 2000;21:1567–77.
Bachle M, Kohal RJ. A systematic review of the influence of different titanium surfaces on proliferation, differentiation and protein synthesis of osteoblast-like Mg63 cells. Clin Oral Implants Res. 2004;15:683–92.
Meyle J. Cell adhesion and spreading on different implant surfaces. Proceedings of the 3rd European Workshop on Periodontology, Berlin 1999;55–72.
Grossner-Schreiber B, Herzog M, Hedderich J, Duck A, Hannig M, Griepentrog M. Focal adhesion contact formation by fibroblasts cultured on surface-modified dental implants: an in vitro study. Clin Oral Implants Res. 2006;17:736–45.
Subramani K, Jung RE, Molenberg A, Hammerle CH. Biofilm on dental implants: a review of the literature. Int J Oral Maxillofac Surg. 2009;24:616–26.
Wennerberg A, Sennerby L, Kultje C, Lekholm U. Some soft tissue characteristics at implant abutments with different surface topography. A study in humans. J Clin Periodontol. 2003;30:88–94.
Elter C, Heuer W, Demling A, Hannig M, Heidenblut T, Bach FW, Stiesch-Scholz M. Supra- and subgingival biofilm formation on implant abutments with different surface characteristics. Int J Oral Maxillofac Implants. 2008;23:327–34.
Acknowledgments
This research was supported by Research Council of Lithuania, Grant No. MIP-11369.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rutkunas, V., Bukelskiene, V., Sabaliauskas, V. et al. Assessment of human gingival fibroblast interaction with dental implant abutment materials. J Mater Sci: Mater Med 26, 169 (2015). https://doi.org/10.1007/s10856-015-5481-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10856-015-5481-8