Abstract
Bimetallic Au@Pt nanostructures (Au@Pts) are potential candidates for optical, electrical, catalytic and biological applications. However, methods for the fabrication of Au@Pts using total tea polyphenols (TPPs), studies of the mechanism of action of Au@Pts on biological systems and studies on the application of Au@Pts in cancer diagnosis and therapy are sparse. In this study, we developed a simple, eco-friendly and low-cost method for the synthesis of Au@Pts to examine the cytotoxic effect of these Au@Pts on human cervical cancers in vitro. The gold and platinum ions were successfully reduced simultaneously using TPPs at room temperature. The prepared Au@Pts were characterized using UV–Vis spectrophotometery, X-ray diffractometery (XRD), energy-dispersive X-ray spectroscopy (EDS), and transmission electron microscopy (TEM). EDS and XRD confirmed the formation of the Au@Pt. Formation of Au@Pts with a size of 5–20 nm was confirmed using TEM. The cytotoxic properties of the Au@Pts were evaluated in human cervical cancer cells (SiHa). The cell viability results revealed that Au@Pts induce cell death in a dose- and time-dependent manner. The morphological features of the Au@Pt-exposed SiHa cells were observed and indicated cell death via cell shrinkage, intranucleosomal DNA fragmentation and chromatin condensation. During progression of the different phases of the cell cycle, the proportion of cells in the G2/M phase of the treated SiHa cells was significantly increased, which strongly confirmed that the Au@Pts induced apoptosis through the G2/M phase check points. Our findings demonstrate the activity of Au@Pts against cervical cancer cells and reveal strategies for the development of highly active bimetallic nanostructures for cancer therapeutics.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Uterine cervical cancer, the second most common malignant tumor in women worldwide, has a particularly high mortality rate in developing countries. There are recommended therapies, including chemo- and radio-therapy, but the effects of these treatments on prognosis are not yet fully known [1–3]. Moreover, current cancer treatment strategies, primarily mono-drug chemotherapy, result in only minimal improvement in patient survival, and these treatments lack specific targets, causing an imbalance in the redox system and various other side effects. For instance, platinum-based materials are clinically efficient anti-cancer drugs, and they act via redox-mediated cell death mechanisms, such as apoptosis and necrosis [4]. However, platinum-based chemotherapeutic drugs can induce adverse toxic effects, such as nephrotoxicity, neurotoxicity and ototoxicity [5]. Therefore, researchers are engaged in identifying multiple targeted therapies to treat people suffering from cancer. Cervical cancer is a complex multifactorial disease caused by various biochemical, microbial (human papilloma virus) and genetic factors [6]. Therapeutic strategies for cervical cancer would require the simultaneous use of two or more materials with functionalized agents to induce a synergistic effect while also reducing the toxicity levels.
Nanomedicine, an emerging field of research in nanotechnology, involves diagnosing, treating and preventing diseases using nanoscale tools [7]. Currently, multi-functionalized nanomaterials have caused a revolution in the treatment and diagnosis of various chronic illnesses and have overcome conventional medical limitations, such as rapid nonspecific clearance, lack of selectivity, poor pharmacokinetics and undesirable side effects [8]. Notably, noble metal-based nanoparticles are versatile tools for biomedical applications, including targeted drug delivery, gene delivery, molecular imaging and diagnosis [9]. Bimetallic nanostructures are a combination of two diverse monometallic nanostructures that exhibit unusual optical, electrical and catalytic properties entirely distinct from their corresponding monometallic nanostructures. Bimetallic nanostructures serve as potential candidates for magnetic sensors, catalysts, optical detectors and biomedical applications [10–14]. Among the bimetallic nanostructures, noble metal-based bimetallic nanostructures are the most promising materials because of their exceptional optical, electrical, biological and catalytic properties [15–17]. In particular, Au@Pt is of great interest because of its wide variety of physicochemical properties. Moreover, Au@Pt may exhibit synergistic effects on optical, electrical and catalytic properties, which are in complete contrast to their corresponding metal nanoparticles [18]. Gold nanoparticles exhibit shape- and size-dependent surface plasmon resonance (SPR) and tunable optical, electrical, catalytic, and biological properties, which make them potential biocompatible material candidates for biomedical applications, such as immunoassay, protein assay, cancer diagnosis, drug delivery and cancer therapy [19–23]. Particularly, surface modified gold nanoparticles with specific biomolecules, such as antibodies, enzymes, or organic probes have been exploited for the diagnosis and thermo-photodynamic therapy of cancer cells [24]. Manikandan et al. reported that 5–6 nm platinum nanoparticles are effective for the photothermal treatment of Neuro 2A cells and have been recommended for cancer therapy [25]. Hikosaka et al. demonstrated that platinum nanoparticle activity is similar to that of oxidizing NADH and reducing CoQ [26].
A number of methods have been reported for the synthesis of bimetallic nanostructures, including reverse micelles [27], borohydride reduction [28], alcohol reduction [29], polyol processes [30], sonochemical methods [31], hydrothermal methods [32], radiolytic reduction [33], and laser ablation [34]. Recently, the green synthesis of nanostructures has become a rapidly growing area of research in nanobiotechnology because of advantages such as nontoxicity, eco-friendliness, biocompatibility, simplicity and cost effectiveness. Several types of green agents have been utilized for the fabrication of nanostructures, such as phytochemicals, bacteria, fungi, and biopolymers [35–37]. Generally, these green agents have been utilized to fabricate metal and metal oxide nanostructures. However, few studies have reported that green agents have been used to prepare Au–Pd, Ti/Ni and Eu/Au bimetallic nanostructures [38–40]. Tea polyphenol (TPP) contains several catechins, including (+)-catechin, (−)-epicatechin, (−)-gallocatechin, (−)-epicatechingallate, (−)-epigallocatechin and (−)-epigallocatechingallate [41]. TPPs are effective scavengers of free radicals and singlet oxygen. In addition, TPPs may prevent a variety of diseases, including cardiovascular, hepatic, renal, neural, pulmonary and intestinal diseases, cancer, diabetes, and arthritis [42, 43]. Previous studies, several studies suggested that tea leaf extract could be exploited for monometallic nanostructure formation. Nadagouda et al. demonstrated that tea extracts can reduce silver, iron and palladium ions into nanostructures [44, 45]. Moreover, tea extract has also been utilized for the preparation of noble metals, such as platinum, gold and silver nanoparticles at ambient temperature [46]. Currently, Au–Pt fabrication using TPPs has not yet been explored. This has directed the interests of our research group towards the screening of functionalized anticancer drugs, which may induce apoptosis or produce a multifunctional synergistic anticancer effect in human cervical cancer cells. In this study, we synthesized Au@Pt nanostructures using TPP as both a reducing and stabilizing agent at ambient temperature. The physicochemical properties of the Au@Pt were then analyzed by UV–Vis spectrophotometry, X-ray diffractometery (XRD) and transmission electron microscopy (TEM). The cytotoxic effect of the Au@Pt nanostructures on human cervical cancer cells (SiHa) was assessed by cell viability (MTT assay), cellular morphology (fluorescent microscopy, PI staining) and cell cycle (flow cytometry, PI staining) for the screening of novel anticancer drugs with special reference to apoptosis.
2 Materials and methods
Chloroauric acid (HAuCl4·4H2O) and chloroplatinic acid (H2PtCl6·6H2O) were purchased from Sigma Aldrich and were used as the precursors for the preparation of Au@Pt nanostructures. Total TPPs were used as the reducing agent. All chemicals were of analytical grade. Double-distilled water was used throughout the study.
2.1 Preparation of Au@Pt nanostructures
Au@Pt nanostructures were fabricated using a one pot synthetic approach at ambient temperature. Approximately 25 ml of 0.001 M HAuCl4 aqueous solution was mixed with 25 ml of 0.001 M H2PtCl6 solution with magnetic stirring. Subsequently, 10 ml of aqueous total TPPs was added to the gold and platinum ion mixture. The color of the solution then changed, which indicated that the Au@Pt nanostructures were obtained. The prepared colloids were centrifuged at 12,000 rpm for 5 min and the obtained pellet was washed twice with double-distilled water. The Au@Pt was then subjected to physiochemical characterization and in vitro anti-cancer drug screening studies.
2.2 Instrumentation
The UV–Visible absorption spectrum of the Au@Pts was recorded using an Agilent UV–Vis-NIR spectrophotometer. A pair of 1 cm optical path length quartz cuvettes was used under warm conditions (40 °C) for the UV–Visible absorption spectral studies between 300 and 800 nm. A Shimadzu X 600 Powder X-Ray diffractometer with Cu-Kα radiation 1.5414 Å was used for the X-ray studies for phase identification of the Au@Pt nanostructures. For TEM analysis, a drop of the Au@Pt nanostructures was dispensed directly onto a carbon-coated copper grid and allowed to dry completely in a vacuum desiccator. The prepared sample was analyzed using a JEOL transmission electron microscope (Model JEM 2100, Japan).
2.3 Maintenance of SiHa cells
The cervical cancer cell line (SiHa) was kindly provided by Prof. M.A. Akbarsha, Director of the Mahatma Gandhi–Doerenkamp Center for Alternatives to Use of Animals in Life Science Education, Bharathidasan University, India. The cell line was maintained and propagated in 90 % DMEM (Thermoscientific, USA) containing 10 % fetal bovine serum (FBS) (Thermoscientific, USA) and 1 % penicillin/streptomycin (Biochrom AG, Germany and ATCC, USA). The cells were cultured as an adherent monolayer at approximately 70–80 % confluence at 37 °C in a humidified atmosphere of 5 % CO2 (Thermoscientific, USA). The cells were harvested after brief trypsinization. All experiments were performed using cells from passage 15 or less.
2.4 Cell viability assay
The MTT assay was performed as previously described [47]. Briefly, the SiHa cells were seeded at a density of 1 × 104 cells per well in 200 μL fresh culture medium. After growth overnight, the cells were treated with different concentrations (25–200 μg/mL) of Au@Pt for 24 and 48 h. After incubation, 20 μL MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) solution [5 mg/mL in phosphate-buffered saline (PBS)] was added to each well. The plates were wrapped with aluminum foil and incubated at 37 °C for 4 h. The plates were then centrifuged, and the purple formazan product was dissolved by the addition of 100 μL DMSO to each well. The absorbance was monitored at 570 nm (measurement) and 630 nm (reference) using a 96-well plate reader (Bio-Rad, CA, USA). The data collected for the three replicates for each concentration of Au@Pts were used to calculate the mean percentage viability. The percent inhibition (IC50 and IC75 values) was calculated using the Calcusyn software (Biosoft, UK) and MS Excel (Microsoft Corp, USA).
2.5 Detection of cell death by epi-fluorescence microscopy
The nuclear morphology of the treated SiHa cells was analyzed after exposure of the cells to Au@Pt for 24 h. The control cells were grown under the same condition but without Au@Pt. The cells were trypsinized and fixed with 70 % cold ethanol overnight. After rehydration with PBS, the cell nuclei were then stained with 1 mg/mL propidium iodide (Sigma, USA) at 37 °C for 15 min in the dark. The stained cells were photographed and examined under a fluorescence inverted microscope (Carl Zeiss, Jena, Germany).
2.6 Cell cycle analysis
The SiHa cells were plated at 2 × 105 cells/mL in a 12-well plate. After culturing for 24-h at 37 °C in a 5 % CO2 atmosphere, the cells were treated with D1 and D2 of the Au@Pt for 24 h. Following trypsinization, the cells were centrifuged at 1000×g for 10 min, and the pellet was resuspended in 0.5 mL of PBS. Fixation was completed by adding 4.5 mL of cold 70 % ethanol for at least 16 h at 4 °C. The fixed cells were centrifuged at 1000×g for 10 min, and the pellet was resuspended in 5 mL of PBS. After being washed with PBS, the pellet was resuspended in 1 mL of propidium iodide (PI) staining solution. After incubation at 37 °C for 15 min, the cells were analyzed to determine the cell cycle stage using a flow cytometer (BD FACSCanto II; BD Biosciences, USA) with an excitation wavelength of 488 nm and emission at 670 nm. The acquired data were analyzed using the BD FACSDiva flow cytometry analysis software (BD Biosciences, USA). The presented data are representative of at least three independent experiments conducted in triplicate.
3 Results and discussion
3.1 Synthesis and characterization of TPP functionalized Au@Pt nanostructures
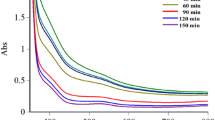
Phytochemicals have been explored for fabrication of monometallic nanoparticles. Several green synthetic methods have been evaluated for metal nanoparticle fabrication [40]. Previously, several studies suggested that tea leaf extract could be exploited for monometallic nanostructure formation. Nadagouda et al. demonstrated that tea extracts can reduce silver, iron and palladium ions into nanostructures [44, 45]. Moreover, tea extract has also been used for the preparation of noble metals, such as platinum, gold and silver nanoparticles at ambient temperature [46]. In this study, we use total TPPs as both a reducing and stabilizing agent for Au@Pt fabrication. Generally, TPP contains several polyphenols, such as epigallocatechin-3-gallate, (−)-epigallocatechin, epicatechin-3-gallate, and other catechins, and exhibits antioxidant properties. Figure 1 shows the mechanism of Au@Pts formation. Supporting evidence from earlier studies demonstrates that various plant polyphenols, including TPPs, have been used as reducing agents for monometallic nanoparticle synthesis [48–50]. In particular, Au@Pt is of great interest because of its wide variety of physicochemical properties. Moreover, Au@Pt may exhibit synergistic effects on optical, electrical and catalytic properties, which are in complete contrast to their corresponding metal nanoparticles [18]. The optical properties of Au@Pt were confirmed by UV–Vis spectrometry as shown in Fig. 2. A SPR peak was observed at 580 nm, which corresponds to the gold nanoparticles. The peak intensity showed a time-dependent red shift, which confirms the formation of Au@Pt nanostructures with increased particle size. These results are consistent with earlier studies showing that noble metal nanoparticles have tunable optical properties based on their size and shape [19]. Gao et al. also reported that bimetallic nanoparticles exhibit tunable optical properties due to the size and shape-dependent SPR [51]. Our results revealed that TPPs can potentially reduce and stabilize the gold and platinum ions simultaneously at ambient temperature. The crystalline nature of the prepared Au@Pt was analyzed using XRD. The XRD pattern of the sample showed 4 peaks at 39.5° (1 1 1), 44.39° (2 0 0), 64.58° (2 2 0) and 77.55° (3 1 1). The corresponding gold and platinum nanoparticle peaks were slightly shifted, which may be due to the formation Au@Pt nanostructures (Fig. 3). This XRD result is consistent with previous reports [52]. The morphology of the Au@Pt bimetallic nanostructures was analyzed using TEM. Figure 4 shows the TEM images of the Au@Pt nanostructures with sizes ranging between 5 and 20 nm (Fig. 3a, b, c). High resolution-TEM (HR-TEM) was also used to characterize the lattice arrangement and crystallinity of the Au@Pt nanostructures. Figure 4d shows the HR-TEM image of the bimetallic nanostructures. The HR-TEM images show the lattice planes for the gold and platinum particles with lattice spacings of 0.229 and 0.177 nm, respectively, which correspond to the (111) lattice plane in both materials. The elemental profile of the nanostructures was analyzed using TEM with an EDX setup. The EDX results (Fig. 5) show Au, Pt, C and Cu peaks, which suggests the presence of Au@Pt nanostructures. The Cu and C peaks correspond to the copper grid and TPP, respectively. Therefore, these observations all confirm the formation of spherical shaped Au@Pts 20–50 nm in size at ambient temperature.
3.2 Cytotoxicity studies
The cytotoxic effect of the Au@Pts on the human cervical carcinoma cell line SiHa was evaluated using an MTT assay. Different concentrations (12.5, 25, 50, 100 and 200 µg/ml) of purified Au@Pts were used to treat the SiHa cells for 24 and 48 h (Fig. 6). The Au@Pts had an inhibitory effect on proliferation and reduced the viability of the SiHa cells in a dose- and time- dependent manner as shown in Fig. 6. The IC50 and IC75 values of the Au@Pts were higher for the 24 h treatment groups and ranged from 25 to 30 μg/mL, whereas for the 48 h treatment groups, the IC50 and IC75 values ranged from 20 to 25 μg/mL. The dose- and time-dependent cytotoxicity clearly indicated that functionalized Au@Pts could interact with the intracellular environment by varying the mode of action and extent of interactions with the DNA and specific proteins. Moreover, a number of metal nanoparticles have been reported to induce cell death depending on their size, shape and surface area [53, 54]. Our results only apply to spherical shaped Au@Pt 20–50 nm in size that induce cytotoxicity of human cervical cancer cells.
3.3 Cellular morphological analysis using PI staining
To determine the mechanism of cell death, the cells were treated with IC50 and IC75 concentrations of Au@Pt for 24 h and were examined for induced cytological changes, such as chromatin fragmentation, mitotic phase chromosome arrangements, binucleation, nuclear swelling, and late apoptosis as indicated by dot like chromatin condensation with propidium iodide staining. The fluorescence microscope images of the Au@Pts treated and untreated (control) SiHa cells are shown in Fig. 7. The results reveal that the Au@Pts causes about nuclear morphological changes (Fig. 7) and that the cells are committed to a specific mode of cell death. All complexes also display both necrotic and apoptotic cell death. When compared to the control, the treated cells show a higher percentage of abnormal nucleated cells with increasing concentrations of Au@Pts. Chromosomal condensation, internucleosomal fragmentation and metaphase (mitotic) cells were observed in Au@Pts treated cells, which indicates that Au@Pts induces apoptotic cell death (programmed cell death). The data collected from the manual counting of cells with normal and abnormal nuclear features are shown in Fig. 7 and show that apoptotic cells are increased in a dose-dependent manner.
3.4 Effect of Au@Pt nanostructures on cell cycle progression
To identify the molecular mechanisms underlying the cell cycle specific inhibitory effects of Au@Pts, we analyzed the different cell cycle phases by flow cytometry, which is based on quantitative measurements of the nuclear DNA content of cells. The human cervical cancer cells were treated with different concentrations of Au@Pts (dose 1 and dose 2) for 24 h. The cell cycle distribution is shown in Fig. 8a, b, c. The percentage of cells significantly decreases during the G0/G1 and S phases in the Au@Pt exposed cells, which is accompanied by an increase of the number of cells in the G2/M phase. The Au@Pt exhibits dose dependent cell cycle distributions in the G0/G1 and G2/M phase, although no significant changes were observed in the S phase. Our results suggest that Au@Pts induces G2/M phase cell cycle arrest and subsequently accumulates in the sub-G1 phase. This is supported by the fragmentation of the nucleus (apoptotic cells) observed in the microscopic analyses of the cells treated with Au@Pts. Our present study suggests that the Au@Pts exhibits an antiproliferative effect demonstrated by reduced cell viability, internucleosomal DNA fragmentation, G2-M cell cycle arrest and hypo-diploid accumulation. Au@Pts may be a potential therapeutic agent for human cervical cancer. Several emerging pathophysiological pathways have identified within the aberrant apoptotic and cell cycle regulatory networks, which are frequently encountered in malignant tumor cells and may be targets for novel anticancer drug discovery. The inhibition of cell cycle check points (crucial proteins) and the induction of apoptosis have been successfully achieved with multifunctional nanoparticles that have advanced into clinical trials for cancer therapy [55, 56]. Previous studies demonstrated that silver and selenium nanoparticles induced G2/M phase cell cycle arrest [57, 58]. Clearly, G2-M arrest during cell cycle progression is important, and apoptosis by Au@Pts may act through mitosis. In future studies, the multi-functionalized Au@Pts effects in in vitro and in vivo cancer models will be evaluated at both the cell and molecular levels.
Cell cycle distribution analysis of SiHa cells. Representative histograms show the cell population according to the DNA content as determined by propidium iodide staining. a Control; b dose 1 (25 µg/ml); c dose 2 (100 µg/ml). d Bar diagram showing the cell distribution in the sub-G0, G0/G1, S, and G2/M phases for SiHa cells treated with and without Au@Pt. The data represent the mean ± SD of triplicate
4 Conclusions
The biosynthesis of metal nanoparticles using tea phytochemicals is an inexpensive, simple and eco-friendly process. In this study, Au@Pt nanostructures were fabricated using TPP as both the reducing and stabilizing agent. The nanostructures were characterized by various techniques, including UV–Vis spectroscopy, TEM, XRD, and EDX. The optical properties of the Au@Pt nanostructures showed a red shift of the SPR. In the TEM images, 5–20 nm Au@Pt nanostructures with different shapes were observed. The Au@Pt nanostructures possess cytotoxic potential through inhibition of cell proliferation and they induced apoptosis in human cervical cancer cells. The antiproliferative and morphological effects of Au@Pts appear to be target-specific killing through cell cycle and apoptotic pathways in uterine human cervical cancer cells. These findings suggest that bimetallic Au@Pt nanostructures have potential anticancer properties and have implications for novel cancer therapeutic applications. From these perspectives, further mechanistic studies are necessary to fully understand the detailed molecular mechanism behind the redox and TPP interactions. Appropriate molecular techniques, including the gene expression of pro- and anti-apoptotic genes, caspases, and cell cycle related gene pathways, with special reference to the influence of bimetals and TPP antioxidants on synergism, will need to be used to study the Au@Pts. In the future, various polyphenol specific functionalization of Au@Pt may be useful for multiple target-based cancer therapy because the mechanism of cell death appears to be apoptosis and because cell cycle arrest is a desired endpoint in novel anti-cancer drug screening.
References
WHO/ICO. Summary report on HPV and cervical cancer statistics in Saudi Arabia. 2007; 4:3–12.
Thomas GM. Improved treatment for cervical cancer concurrent chemotherapy and radiotherapy. N Engl J Med. 1999;340:1198–200.
Ren G, Zhao YP, Yang L, Fu CX. Anti-proliferative effect of clitocine from the mushroom Leucopaxillus giganteus on human cervical cancer HeLa cells by inducing apoptosis. Cancer Lett. 2008;262:190–200.
Wang X, Guo Z. Targeting and delivery of platinum-based anticancer drugs. Chem Soc Rev. 2013;42:202–24.
Kelland L. Broadening the clinical use of platinum drug-based chemotherapy with new analogues Satraplatin and picoplatin. Expert Opin Investig Drugs. 2007;16:1009–21.
Matos A, Moutinho J, Pinto D, Medeiros R. The influence of smoking and other cofactors on the time to onset to cervical cancer in a southern European population. Eur J Cancer Prev. 2005;14:485–91.
Jee J, Na J, Lee S, Kim S, Choi K, Yeoa Y, Kwon IC. Cancer targeting strategies in nanomedicine: design and application of chitosan nanoparticles. Curr Opin Solid State Mater Sci. 2012;16:333–42.
Gupta AS. Nanomedicine approaches in vascular disease: a review. Nanomed-Nanotechnol. 2011;7:763–79.
Arvizo RR, Bhattacharyya S, Kudgus RA, Giri K, Bhattacharya R, Mukherjee P. Intrinsic therapeutic applications of noble metal nanoparticles: past, present and future. Chem Soc Rev. 2012;41:2943–70.
Sun SH, Murray CB, Weller D, Folks L, Moser A. Monodisperse FePt nanoparticles and ferromagnetic FePt nanocrystal superlattices. Science. 2000;287:1989–92.
Wang DS, Li YD. Bimetallic nanocrystals: liquid-phase synthesis and catalytic applications. Adv Mater. 2011;23:1044–60.
Chen L, Chabu JM, Liu Y. Bimetallic AgM (M=Pt, Pd, Au) nanostructures: synthesis and applications for surface-enhanced Raman scattering. RSC Adv. 2013;3:4391–9.
Duan S, Wang R. Bimetallic nanostructures with magnetic and noble metals and their physicochemical applications. Prog Nat Sci Mater Int. 2013;23:113–26.
Li W, Kuai L, Qin Q, Geng B. Ag–Au bimetallic nanostructures: co-reduction synthesis and their component-dependent performance for enzyme-free H2O2 sensing. J Mater Chem A. 2013;1:7111–7.
Du B, Zaluzhna O, Tong YJ. Electrocatalytic properties of Au@Pt nanoparticles: effects of Pt shell packing density and Au core size. Phys Chem Chem Phys. 2011;13:11568–74.
Ji X, Xu S, Wang L, Liu M, Pan K, Yuan H, Ma L, Xu W, Li J, Bai Y, Li T. Immunoassay using the probe-labeled Au/Ag core-shell nanoparticles based on surface-enhanced Raman scattering. Colloid Surf A. 2005;257–258:171–5.
Chen L, Zhao W, Jiao Y, He X, Wang J, Zhang Y. Characterization of Ag/Pt core-shell nanoparticles by UV–vis absorption, resonance light-scattering techniques. Spectrochim Acta A. 2007;68:484–90.
Wanjala BN, Luo J, Fang B, Mott D, Zhong CJ. Gold platinum nanoparticles: alloying and phase segregation. J Mater Chem. 2011;21:4012–20.
El-Brolossy TA, Abdallah T, Mohamed MB, Abdallah S, Easawi K, Negm S, Talaat H. Shape and size dependence of the surface plasmon resonance of gold nanoparticles studied by photoacoustic technique. Eur Phys J. 2008;153:361–4.
Hirsch LR, Jackson JB, Lee A, Halas NJ, West JL. A whole blood immunoassay using gold nanoshells. Anal Chem. 2003;75:2377–81.
Tang D, Yuan R, Chai Y. Biochemical and immunochemical characterization of the antigen-antibody reaction on a non-toxic biomimetic interface immobilized red blood cells of crucian carp and gold nanoparticles. Biosens Bioelectron. 2007;22:1116–20.
Patra CR, Bhattacharya R, Mukhopadhyay D, Mukherjee P. Review Fabrication of gold nanoparticles for targeted therapy in pancreatic cancer. Adv Drug Deliv Rev. 2010;62(62):346–61.
Eghtedari M, Liopo AV, Copland JA, Oraevsky AA, Motamedi M. Engineering of hetero-functional gold nanorods for the in vivo molecular targeting of breast cancer cells. Nano Lett. 2009;9:287–91.
Yang J, Eom K, Lim EK, Park J, Kang YD, Yoon S, et al. Langmuir. 2008;24:12112.
Manikandan M, Hasan N, Wu H. Platinum nanoparticles for the photothermal treatment of Neuro 2A cancer cells. Biomaterials. 2013;34:5833–42.
Hikosaka K, Kim J, Kajita M, Kanayama A, Miyamotoa Y. Platinum nanoparticles have an activity similar to mitochondrial NADH:ubiquinone oxidoreductase. Colloid Surf B. 2008;66:195–200.
Wu ML, Chen DH, Huang TC. Synthesis of Au/Pd bimetallic nanoparticles in reverse micelles. Langumir. 2001;17:3877–83.
Liz-Marzan LM, Philipse AP. Stable hydrosols of metallic and bimetallic nanoparticles immobilized on imogolite fibers. J Phys Chem. 1995;99:15120–28.
Chen CW, Takezako T, Yamamoto K, Serizawa T, Akashi M. Colloids Surf A. 2000;169:107–16.
Silvert P-Y, Vijayakrishnan V, Vibert P, Herrera-Urbina R, Elhsissen KT. Nanostruct Mater. 1996;7:611.
Mizukoshi Y, Okitsu K, Maeda Y, Yamamoto TA, Oshima R, Nagata Y. J Phys Chem B. 1997;101:7033.
Rath C, Sahu KK, Anand S, Date SK, Mishra NC, Das RP. J Magn Magn Mater. 1999;202:77–84.
Mirdamadi-Esfahani M, Mostafavi M, Keita B, Nadjo L, Kooyman P, Remita H. Bimetallic Au-Pt nanoparticles synthesized by radiolysis: application in electro-catalysis. Gold Bullet. 2010;43:49–56.
Peng Z, Spliethoff B, Tesche B, Walther T, Kleinermanns K. Laser-assisted synthesis of Au-Ag alloy nanoparticles in solution. J Phy Chem B. 2006;110:2549–54.
Mohanpuria P, Rana NK, Yadav SK. Biosynthesis of nanoparticles: technological concepts and future applications. J Nanopart Res. 2008;10:507–17.
Kharissova OV, Rasika Dias HV, Kharisov BI, Olvera Pérez B, Jiménez Pérez VM. The greener synthesis of nanoparticles. Trends Biotechnol. 2013;31:240–8.
Padalkar S, Capadona JR, Rowan SJ, Weder C, Won YH, Stanciu LA, Moon RJ. Natural biopolymers: novel templates for the synthesis of nanostructures. Langmuir. 2010;26:8497–502.
Ascencio JA, Mejia Y, Liu HB, Angeles C, Canizal G. Bio-reduction synthesis of Eu-Au nanoparticles. Langmuir. 2003;19:5882–6.
Schabes-Retchkiman PS, Canizal G, Herrera-Becerra R, Zorrilla C, Liu HB, Ascencio JA. Biosynthesis and characterization of Ti/Ni bimetallic nanoparticles. Opt Mater. 2006;29:95–9.
Zhan G, Huang J, Du M, Abdul-Rauf I, Ma Y, Li Q. Green synthesis of Au–Pd bimetallic nanoparticles: single-step bioreduction method with plant extract. Mater Lett. 2011;65:2989–91.
Ninomiya M, Unten L, Kim M. Chemical and physicochemical properties of green tea polyphenols. In: Yamamoto T, Juneja LR, Chu DC, Kim M, editors. Chemistry and Applications of Green Tea. New York: CRC; 1997. p. 23–35.
Periasamy VS, Alshatwi AA. Tea polyphenols modulate antioxidant redox system on cisplatin-induced reactive oxygen species generation in a human breast cancer cell. Basic Clin Pharmacol Toxicol. 2013;112:374–84.
Higdon JV, Frei B. Tea catechins and polyphenols: health effects, metabolism, and antioxidant function. Crit Rev Food Sci Nutr. 2003;43:89–143.
Nadagouda MN, Varma RS. Green synthesis of silver and palladium nanoparticles at room temperature using coffee and tea extract. Green Chem. 2008;10:859–62.
Nadagouda MN, Castle AB, Murdock RC, Hussain SM, Varma RS. In vitro biocompatibility of nanoscale zerovalent iron particles (NZVI) synthesized using tea polyphenols. Green Chem. 2010;12:114–22.
Sanchez-Mendieta V, Vilchis-Nestor AR. In: Su YH, editor. Green synthesis of noble metal (Au, Ag, Pt) nanoparticles, assisted by plant-extracts, noble metals. Croatia: INTECH Open Access Publisher; 2012.
Blagosklonny MV, El-Deiry WS. In vitro evaluation of a p53-expressing adenovirus as an anti-cancer drug. Int J Cancer. 1996;67:386–92.
Park Y, Hong YN, Weyers A, Kim YS, Linhardt RJ. Polysaccharides and phytochemicals: a natural reservoir for the green synthesis of gold and silver nanoparticles. IET Nanobiotechnol. 2011;5:69–78.
Begum NA, Mondal S, Basu S, Laskar RA, Mandal D. Biogenic synthesis of Au and Ag nanoparticles using aqueous solutions of black tea leaf extracts. Colloid Surf B. 2009;71:113–8.
Nune SK, Chanda N, Shukla R, et al. Green nanotechnology from tea: phytochemicals in tea as building blocks for production of biocompatible gold nanoparticles. J Mater Chem. 2009;19:2912–20.
Gao J, Ren X, Chen D, Tang F, Ren J. Bimetallic Ag–Pt hollow nanoparticles: synthesis and tunable surface plasmon resonance. Scripta Mater. 2007;57:687–90.
Wang L, Qi B, Sun L, Sun Y, Guo C, Li Z. Synthesis and assembly of Au–Pt bimetallic nanoparticles. Mater Lett. 2008;62:1279–82.
Jensen TR, Malinsky MD, Haynes CL, Van Duyne RP. Nanosphere lithography: tunable localized surface plasmon resonance spectra of silver nanoparticles. J Phys Chem B. 2000;104:10549.
Smitha SL, Nissamudeen KM, Philip D, Gopchandra KG. Studies on surface plasmon resonance and photoluminescence of silver nanoparticles. SpectrochimActa A. 2008;71:186–90.
Fernandez AF, Manchanda R, McGoron AJ. Theranostic applications of nanomaterials in cancer: drug delivery, image-guided therapy and multifunctional platforms. Appl Biochem Biotechnol. 2011;165:1628–51.
Qiao W, Wang B, Wang Y, Yang L, Zhang Y, Shao P. Cancer therapy based on nanomaterials and nanocarrier systems. J Nanomat. 2010;9:796303.
Lee YS, Kim DW, Lee YH, Oh JH, Yoon S, Choi MS, et al. Silver nanoparticles induce apoptosis and G2/M arrest via PKCζ-dependent signaling in A549 lung cells. Arch Toxicol. 2011;85:1529–40.
Wu H, Zhu H, Li X, Liu Z, Zheng W, Chen T, Yu B, Wong K. Induction of Apoptosis and Cell Cycle Arrest in A549 Human Lung Adenocarcinoma Cells by Surface-Capping Selenium Nanoparticles: an Effect Enhanced by Polysaccharide-Protein Complexes from Polyporus rhinoceros. J Agric Food Chem. 2013;61:9859–66.
Acknowledgments
We gratefully acknowledge the financial support of the Deanship of Scientific Research, King Saud University, Saudi Arabia (Project No: RG-1435-044).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alshatwi, A.A., Athinarayanan, J. & Periasamy, V.S. Green synthesis of bimetallic Au@Pt nanostructures and their application for proliferation inhibition and apoptosis induction in human cervical cancer cell. J Mater Sci: Mater Med 26, 148 (2015). https://doi.org/10.1007/s10856-015-5468-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10856-015-5468-5