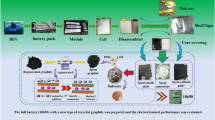
Abstract
The widespread utilization of lithium-ion batteries has led to an increase in the quantity of decommissioned lithium-ion batteries. By incorporating recycled anode graphite into new lithium-ion batteries, we can effectively mitigate environmental pollution and meet the industry’s high demand for graphite. Herein, a suitable amount of ferric chloride hexahydrate was employed as a catalyst precursor to facilitate the low-temperature graphitization process of spent graphite. This resulted in renewed graphite with abundant pores, a high degree of graphitization (89.5%), and exceptional electrochemical properties. This material exhibits minimal polarization and impedance while maintaining a specific capacity of 376 mAh/g after 260 cycles at a rate of 0.5 C. While highlighting the remarkable attributes of recycled graphite, we also address the limitations associated with the catalytic graphitization method to provide researchers with comprehensive and unbiased insights. Ultimately, we believe that this method will play a pivotal role in recycling spent graphite with severely damaged structure.








Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Data availability
Data will be made available on request.
References
R. Gopalakrishnan, S. Goutam, L.M. Oliveira, J.-M. Timmermans, N. Omar, M. Messagie, P. Van den Bossche, J. van Mierlo, A comprehensive study on rechargeable energy storage technologies. J. Electrochem. Energy Convers. Storage (2016). https://doi.org/10.1115/1.4036000
A. Masias, J. Marcicki, W.A. Paxton, Opportunities and challenges of lithium ion batteries in automotive applications. ACS Energy Lett. 6, 621–630 (2021). https://doi.org/10.1021/acsenergylett.0c02584
IEA, Global EV Outlook 2023-Trends in Batteries (2023), pp. License: CC BY 4.0. https://www.iea.org/reports/global-ev-outlook-2023/trends-in-batteries
Q. Wei, Y. Wu, S. Li, R. Chen, J. Ding, C. Zhang, Spent lithium ion battery (LIB) recycle from electric vehicles: a mini-review. Sci. Total Environ. 866, 161380 (2023). https://doi.org/10.1016/j.scitotenv.2022.161380
X. Zeng, J. Li, N. Singh, Recycling of spent lithium-ion battery: a critical review. Crit. Rev. Environ. Sci. Technol. 44, 1129–1165 (2014). https://doi.org/10.1080/10643389.2013.763578
Y. Gao, J. Zhang, H. Jin, G. Liang, L. Ma, Y. Chen, C. Wang, Regenerating spent graphite from scrapped lithium-ion battery by high-temperature treatment. Carbon 189, 493–502 (2022). https://doi.org/10.1016/j.carbon.2021.12.053
H. Hou, X. Qiu, W. Wei, Y. Zhang, X. Ji, Carbon anode materials for advanced sodium-ion batteries. Adv. Energy Mater. (2017). https://doi.org/10.1002/aenm.201602898
J. Liu, H. Shi, X. Hu, Y. Geng, L. Yang, P. Shao, X. Luo, Critical strategies for recycling process of graphite from spent lithium-ion batteries: a review. Sci. Total Environ. (2022). https://doi.org/10.1016/j.scitotenv.2021.151621
K.K. Jena, A. AlFantazi, A.T. Mayyas, Efficient and cost-effective hybrid composite materials based on thermoplastic polymer and recycled graphite. Chem. Eng. J. (2022). https://doi.org/10.1016/j.cej.2021.132667
J. Hao, X. Meng, S. Fang, H. Cao, W. Lv, X. Zheng, C. Liu, M. Chen, Z. Sun, MnO2-functionalized amorphous carbon sorbents from spent lithium-ion batteries for highly efficient removal of cadmium from aqueous solutions. Ind. Eng. Chem. Res. 59, 10210–10220 (2020). https://doi.org/10.1021/acs.iecr.9b06670
X. Chen, Y. Zhu, W. Peng, Y. Li, G. Zhang, F. Zhang, X. Fan, Direct exfoliation of the anode graphite of used Li-ion batteries into few-layer graphene sheets: a green and high yield route to high-quality graphene preparation. J. Mater. Chem. A 5, 5880–5885 (2017). https://doi.org/10.1039/c7ta00459a
Y. Wang, H. Cao, L. Chen, C. Chen, X. Duan, Y. Xie, W. Song, H. Sun, S. Wang, Tailored synthesis of active reduced graphene oxides from waste graphite: structural defects and pollutant-dependent reactive radicals in aqueous organics decontamination. Appl. Catal. B 229, 71–80 (2018). https://doi.org/10.1016/j.apcatb.2018.02.010
Y. Gao, S. Zhang, S. Lin, Z. Li, Y. Chen, C. Wang, Opportunity and challenges in recovering and functionalizing anode graphite from spent lithium-ion batteries: a review. Environ. Res. (2024). https://doi.org/10.1016/j.envres.2024.118216
H.A. Khayoon, M. Ismael, A. Al-nayili, H.A. Alshamsi, Fabrication of LaFeO3-nitrogen deficient g-C3N4 composite for enhanced the photocatalytic degradation of RhB under sunlight irradiation. Inorg. Chem. Commun. (2023). https://doi.org/10.1016/j.inoche.2023.111356
S.-J. Han, L. Xu, C. Chen, Z.-Y. Wang, M.-L. Fu, B. Yuan, Recovery of graphite from spent lithium-ion batteries and its wastewater treatment application: a review. Sep. Purif. Technol. (2024). https://doi.org/10.1016/j.seppur.2023.125289
A. Al-nayili, H.A. Khayoon, H.A. Alshamsi, N.M. Cata Saady, A novel bimetallic (Au-Pd)-decorated reduced graphene oxide nanocomposite enhanced rhodamine B photocatalytic degradation under solar irradiation. Mater. Today Sustain. (2023). https://doi.org/10.1016/j.mtsust.2023.100512
T. Zhao, Y. Yao, M. Wang, R. Chen, Y. Yu, F. Wu, C. Zhang, Preparation of MnO2-modified graphite sorbents from spent Li-ion batteries for the treatment of water contaminated by lead, cadmium, and silver. ACS Appl. Mater. Interfaces 9, 25369–25376 (2017). https://doi.org/10.1021/acsami.7b07882
A. Sarkar, I.C. Nlebedim, P. Shrotriya, Performance degradation due to anodic failure mechanisms in lithium-ion batteries. J. Power. Sources 502, 13 (2021). https://doi.org/10.1016/j.jpowsour.2020.229145
X.Q. Meng, Y.L. Xu, H.B. Cao, X. Lin, P.G. Ning, Y. Zhang, Y.G. Garcia, Z. Sun, Internal failure of anode materials for lithium batteries—a critical review. Green Energy Environ. 5, 22–36 (2020). https://doi.org/10.1016/j.gee.2019.10.003
S.J. An, J. Li, C. Daniel, D. Mohanty, S. Nagpure, D.L. Wood, The state of understanding of the lithium-ion-battery graphite solid electrolyte interphase (SEI) and its relationship to formation cycling. Carbon 105, 52–76 (2016). https://doi.org/10.1016/j.carbon.2016.04.008
H. Wang, Y. Huang, C. Huang, X. Wang, K. Wang, H. Chen, S. Liu, Y. Wu, K. Xu, W. Li, Reclaiming graphite from spent lithium ion batteries ecologically and economically. Electrochim. Acta 313, 423–431 (2019). https://doi.org/10.1016/j.electacta.2019.05.050
N. Cao, Y. Zhang, L. Chen, W. Chu, Y. Huang, Y. Jia, M. Wang, An innovative approach to recover anode from spent lithium-ion battery. J. Power. Sources (2021). https://doi.org/10.1016/j.jpowsour.2020.229163
R. Zhan, Z. Yang, I. Bloom, L. Pan, Significance of a solid electrolyte interphase on separation of anode and cathode materials from spent Li-ion batteries by froth flotation. ACS Sustain. Chem. Eng. 9, 531–540 (2020). https://doi.org/10.1021/acssuschemeng.0c07965
D.S. Ruan, F.M. Wang, L. Wu, K. Du, Z.H. Zhang, K. Zou, X.F. Wu, G.R. Hu, A high-performance regenerated graphite extracted from discarded lithium-ion batteries. New J. Chem. 45, 1535–1540 (2021). https://doi.org/10.1039/d0nj05434h
J.J. Roy, E.J.J. Tang, M.P. Do, B. Cao, M. Srinivasan, Closed-loop graphite recycling from spent lithium-ion batteries through bioleaching. ACS Sustain. Chem. Eng. 11, 6567–6577 (2023). https://doi.org/10.1021/acssuschemeng.2c07262
H.H. Tian, M. Graczyk-Zajac, D.M. De Carolis, C.M. Tian, E. Ricohermoso, Z.W. Yang, W. Li, M. Wilamowska-Zawlocka, J.P. Hofmann, A. Weidenkaff, R. Riedel, A facile strategy for reclaiming discarded graphite and harnessing the rate capabilities of graphite anodes. J. Hazard. Mater. (2023). https://doi.org/10.1016/j.jhazmat.2022.130607
X.D. Zhu, J. Xiao, Q.Y. Mao, Z.H. Zhang, Z.H. You, L. Tang, Q.F. Zhong, A promising regeneration of waste carbon residue from spent lithium-ion batteries via low-temperature fluorination roasting and water leaching. Chem. Eng. J. (2022). https://doi.org/10.1016/j.cej.2021.132703
C. Yi, P. Ge, X. Wu, W. Sun, Y. Yang, Tailoring carbon chains for repairing graphite from spent lithium-ion battery toward closed-circuit recycling. J. Energy Chem. 72, 97–107 (2022). https://doi.org/10.1016/j.jechem.2022.05.002
Y. Xiao, J. Li, W. Huang, L. Wang, J. Luo, Green & efficient regeneration of graphite anode from spent lithium ion batteries enabled by asphalt coating. J. Mater. Sci.: Mater. Electron. 33, 16740–16752 (2022). https://doi.org/10.1007/s10854-022-08533-x
H.J. Yu, H.L. Dai, Y. Zhu, H.W. Hu, R.R. Zhao, B.B. Wu, D.C. Chen, Mechanistic insights into the lattice reconfiguration of the anode graphite recycled from spent high-power lithium-ion batteries. J. Power. Sources (2021). https://doi.org/10.1016/j.jpowsour.2020.229159
R.D. Hunter, J. Ramirez-Rico, Z. Schnepp, Iron-catalyzed graphitization for the synthesis of nanostructured graphitic carbons. J. Mater. Chem. A 10, 4489–4516 (2022). https://doi.org/10.1039/d1ta09654k
M. Inagaki, M. Toyoda, T. Tsumura, Control of crystalline structure of porous carbons. RSC Adv. 4, 41411–41424 (2014). https://doi.org/10.1039/c4ra06730d
M. Sevilla, C. Sanchis, T. Valdes-Solis, E. Morallon, A.B. Fuertes, Direct synthesis of graphitic carbon nanostructures from saccharides and their use as electrocatalytic supports. Carbon 46, 931–939 (2008). https://doi.org/10.1016/j.carbon.2008.02.019
G. Hasegawa, K. Kanamori, K. Nakanishi, Facile preparation of macroporous graphitized carbon monoliths from iron-containing resorcinol-formaldehyde gels. Mater. Lett. 76, 1–4 (2012). https://doi.org/10.1016/j.matlet.2012.02.069
C.G. Zhang, J.J. Li, C.S. Shi, C.N. He, E.Z. Liu, N.Q. Zhao, Effect of Ni, Fe and Fe-Ni alloy catalysts on the synthesis of metal contained carbon nano-onions and studies of their electrochemical hydrogen storage properties. J. Energy Chem. 23, 324–330 (2014). https://doi.org/10.1016/S2095-4956(14)60154-6
Y. Lei, Z.H. Huang, W.C. Shen, Y.P. Zheng, F.Y. Kang, Synthesis of porous graphitic carbon from mesocarbon microbeads by one-step route. J. Porous Mater. 20, 1323–1328 (2013). https://doi.org/10.1007/s10934-013-9717-z
X. Guo, Y.X. Liu, X.D. Tian, Z.C. Tao, X. Yan, Z.J. Liu, In situ Ti assisted graphitization approach for the preparation of graphite foam with light weight and high thermal conductivity. RSC Adv. 13, 6075–6086 (2023). https://doi.org/10.1039/d2ra06164c
E. Thompson, A.E. Danks, L. Bourgeois, Z. Schnepp, Iron-catalyzed graphitization of biomass. Green Chem. 17, 551–556 (2015). https://doi.org/10.1039/c4gc01673d
L. Chen, Z.Y. Wang, C.N. He, N.Q. Zhao, C.S. Shi, E.Z. Liu, J.J. Li, Porous graphitic carbon nanosheets as a high-rate anode material for lithium-ion batteries. ACS Appl. Mater. Interfaces 5, 9537–9545 (2013). https://doi.org/10.1021/am402368p
K. Momma, F. Izumi, VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 44, 1272–1276 (2011). https://doi.org/10.1107/S0021889811038970
Q. Yan, J. Li, X. Zhang, E.B. Hassan, C. Wang, J. Zhang, Z. Cai, Catalytic graphitization of kraft lignin to graphene-based structures with four different transitional metals. J. Nanopart. Res. (2018). https://doi.org/10.1007/s11051-018-4317-0
C.W. Huang, L.C. Hsu, Y.Y. Li, Synthesis of carbon nanofibres from a liquid solution containing both catalyst and polyethylene glycol. Nanotechnology 17, 4629–4634 (2006). https://doi.org/10.1088/0957-4484/17/18/016
X.H. Zhong, W. Liu, J.W. Han, F. Jiao, W.Q. Qin, T. Liu, Pretreatment for the recovery of spent lithium ion batteries: theoretical and practical aspects. J. Clean. Prod. (2020). https://doi.org/10.1016/j.jclepro.2020.121439
D. Voiry, J. Yang, J. Kupferberg, R. Fullon, C. Lee, H.Y. Jeong, H.S. Shin, M. Chhowalla, High-quality graphene via microwave reduction of solution-exfoliated graphene oxide. Science 353, 1413–1416 (2016). https://doi.org/10.1126/science.aah3398
C.Y. Zhang, X.M. Zhong, P. Chen, S.J. Sun, Y. Jiang, X.M. Yan, Facile synthesis of porous graphite by calcium carbide and nitrogen gas for lithium-ion batteries. J. Energy Storage (2023). https://doi.org/10.1016/j.est.2023.107386
J. Yang, X.Y. Zhou, Y.L. Zou, J.J. Tang, A hierarchical porous carbon material for high power, lithium ion batteries. Electrochim. Acta 56, 8576–8581 (2011). https://doi.org/10.1016/j.electacta.2011.07.047
J. Yang, E. Fan, J. Lin, F. Arshad, X. Zhang, H. Wang, F. Wu, R. Chen, L. Li, Recovery and reuse of anode graphite from spent lithium-ion batteries via citric acid leaching. ACS Appl. Energy Mater. 4, 6261–6268 (2021). https://doi.org/10.1021/acsaem.1c01029
B. Markey, M. Zhang, I. Robb, P. Xu, H. Gao, D. Zhang, J. Holoubek, D. Xia, Y. Zhao, J. Guo, M. Cai, Y.S. Meng, Z. Chen, Effective upcycling of graphite anode: healing and doping enabled direct regeneration. J. Electrochem. Soc. (2020). https://doi.org/10.1149/1945-7111/abcc2f
A. Davoodabadi, J. Li, Y. Liang, D.L. Wood, T.J. Singler, C. Jin, Analysis of electrolyte imbibition through lithium-ion battery electrodes. J. Power. Sources 424, 193–203 (2019). https://doi.org/10.1016/j.jpowsour.2019.03.115
P. Liu, J. Wang, J. Hicks-Garner, E. Sherman, S. Soukiazian, M. Verbrugge, H. Tataria, J. Musser, P. Finamore, Aging mechanisms of LiFePO4 batteries deduced by electrochemical and structural analyses. J. Electrochem. Soc. 157, A499–A507 (2010). https://doi.org/10.1149/1.3294790
Acknowledgements
This work is supported by the Science and technology innovation plan of Hunan Province (No.2022RC1086), the Youth Foundation of Hunan Province (2021JJ40762), Natural Science Foundation of Hunan Province (2021JJ30794), Excellent youth funding of Hunan Provincial Education Department (Grant No. 22B0677). The authors would like to thank Ms. Luyu Zhang from Shiyanjia Lab (www.shiyanjia.com) for the XPS test.
Funding
This work is supported by the Science and technology innovation plan of Hunan Province (Grant No. 2022RC1086), the Youth Foundation of Hunan Province (Grant No. 2021JJ40762), Natural Science Foundation of Hunan Province (Grant No. 2021JJ30794), Excellent youth funding of Hunan Provincial Education Department (Grant No. 22B0677).
Author information
Authors and Affiliations
Contributions
Zhengyi Li: conceptualization, methodology, data curation, writing—original draft. Jian Li: supervision, writing—review & editing. Lihua Wang: investigation, writing—review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Z., Li, J. & Wang, L. Renewed graphite for high-performance lithium-ion batteries: catalytic graphitization approach. J Mater Sci: Mater Electron 35, 599 (2024). https://doi.org/10.1007/s10854-024-12370-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10854-024-12370-5