Abstract
A study area in supercapacitor research is the design and manufacturing of composite electrode materials that combine the advantages of various materials. The rGO/CoFe2O4 composite material is produced by growing cobalt ferrite particles on the surface of rGO using a simple and highly efficient thermal reduction technique. The results show that the electrochemical properties of rGO are significantly improved by the incorporation of cobalt ferrite. rGO sheets are used as supporting frameworks for the CoFe2O4 nanoparticles, while the CoFe2O4 particles provide more active sites for redox reactions. The prepared rGO/CoFe2O4 show excellent electrochemical properties, with a high specific capacitance of 283 F/g at a current density of 0.1 A/g and a specific capacitance retention of 90.5% after 1000 cycles. The attractive performances exhibited by rGO/CoFe2O4 composites make them potential electrode materials for supercapacitors in future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Reduced graphene oxide (rGO) is a rapidly rising two-dimensional carbon material with the fascinating electronic, thermal, optical, magnetic, and chemical properties [1, 2]. Because of the outstanding properties, rGO finds prominent applications in the electronic devices, such as supercapacitor [3], battery [4], solar cell [5], and sensors [6]. In addition, other essential applications are printed electronics, conductive coatings [7], energy storage [8], etc.
Despite the fact that rGO has a lot of advantages, it still has limitations due to its intrinsic capacitance and comparatively slow charge–discharge rate. The charge–discharge rate as well as the specific capacitance for the rGO electrode can be increased by combining rGO with electrode materials that work on the pseudo-capacitance principle. The transition metal oxide is a typical pseudo-capacitive supercapacitor electrode material which can be used to modify rGO material.
The transition metal oxides commonly used to modify rGO include RuO2 [9], MnO2, and Co3O4 [10, 11]. However, single metal oxides have certain limitations, such as weak mechanical strength and short life cycle [12]. In recent years, multi-metal oxides such as ZnCo2O4, NiCo2O4, and CoFe2O4 have been widely used in order to modify carbon-based electrode materials, including rGO [13,14,15].
CoFe2O4 is one of the most well-known multi-metal oxides due to its high electrochemical activity and numerous active sites that are advantageous to redox processes. Numerous researchers are therefore interested in this oxide in an effort to modify carbon-based electrode materials and, in particular, to enhance capacitance performance [16].
Utilizing salts as precursors to bring metal ions to the surface of porous materials is an established technique for the fabrication of composites based on porous materials and metals. Chemical and thermal reduction can then be used to obtain composite materials with a variety of unique characteristics [13, 17, 18].
The RuO2/rGO nanocomposite material was fabricated by Kim et al. by the in situ and microwave method [19]. Zhao et al. also successfully fabricated RuO2/rGO composite materials by solution precipitation. The authors used NH3 solution to adjust the pH of RuCl3 and GO salts. GO was reduced to rGO by the microwave method [18]. Gao et al. dissolved Co(NO3)2 and Zn(CH3COO)2 salts into the GO dispersion. Urea is used to adjust the pH of the solution. The mixture was hydrothermally heated at 120 °C for 5 h to obtain ZnCo2O4/rGO material [13]. CoSe2 oxides were coated on nickel foam by hydrothermal method for 8 h. The CoSe-Se@Ni foam electrodes exhibited a specific capacitance of 1750.81 F.g−1 at 1 A.g−1 [20]. The CoFe2O4/rGO hydrogel material fabricated by Zheng et al. by the hydrothermal method has a specific capacitance of 356 F/g at a density of 0.5 A/g [21].
In this study, the rGO/CoFe2O4 aerogel was synthesized using a co-precipitation method in an aqueous solution, followed by ice template and heat reduction. This approach is simple, immediate, and enables the reduction of the material to graphene while simultaneously incorporating CoFe2O4 nanoparticles. The obtained materials are used to fabricate the supercapacitor electrodes, which have excellent electrochemical properties.
2 Experimental
2.1 Chemicals
Graphite powder, Fe(NO3)3·9H2O, Co(NO3)2·6H2O, and KMnO4 were supplied by Aladdin Chemical Co. H2SO4 98%, H3PO4 85%, HCl, H2O2 30%, and NH4OH 25% were commercially available from Fisher Scientific.
2.2 Preparation of rGO/CoFe2O4 composite
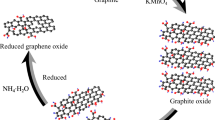
GO dispersion was produced by advanced Hummer method [22]. Firstly, 2.0-g graphite powder was added to 100-ml acid solution having 9:1 (volume/volume ratio) of concentrated H2SO4 and H3PO4 has been prepared and kept in an ice bath. Then, 6.0-g KMnO4 was gradually added while stirring. The addition rate of KMnO4 was carried out carefully to avoid sudden increase of temperature. After that, the reaction mixture is heated and stirred for 8 h at 50 °C. As the reaction extended, the mixture turned out to be paste. Finally, the distilled water is added into the mixture for stopping this reaction. To ensure the completion of reaction with KMnO4, the suspension was further treated with 30% H2O2 solution. The resulted mixture was washed with HCl and H2O, respectively, followed by filtration and drying, and thus GO sheets were obtained.
Accurately weigh the masses of Co(NO3)2 and Fe(NO3)3 compounds based on the molar ratio of Co(NO3)2:Fe(NO3)3 = 1:2 and dissolve in 50 mL of the GO dispersion. The mixture was stirred for 1 h. Adjust pH to 12 using an ammonia solution. The mixture is centrifuged and then washed with distilled water until neutral. A magnetic stirrer and ultrasound were used to redistribute the produced in 50 mL of distilled water. The mixture is poured into the containers, cooled to − 40 degrees Celsius for two hours, and then freeze-dried for twenty-four hours. rGO/CoFe2O4 composite was produced by calcining the mixture in a N2 gas atmosphere at 500 °C. Samples of materials with symbols rGO/CoFe2O4-x, where x is the concentration of Co(NO3)2 salt used (mmol/L), were obtained depending on the molar concentration of the salt. The synthetic route of rGO/CoFe2O4 composite is shown in Fig. 1.
When 50 mL of the GO mixture was replaced with 50 mL of distilled water, the aforementioned procedure was repeated in order to obtain CoFe2O4 powder.
2.3 Characterization
A Bruker D8-Advance (Germany) X-ray diffractometer with CuK radiation in the angle range 2θ from 10 to 70° was used to determine the crystal structure of rGO/CoFe2O4. Using a Tri-Star 3000 at 77 K and at a degassing temperature of 200 C for 5 h, the specific surface area and porosity distribution of materials were examined.
The morphologies and structural properties of the prepared materials were characterized by field emission scanning electron microscopy (FESEM; HITACHI S-4800) and high-resolution transmission electron microscopy (HRTEM; JEM 2100). The crystal structure of the obtained samples was determined on an X-ray diffractometer (XRD, Bruker Advance 8). The graphitization degree was determined by Laser Micro-Raman spectrometer (Raman Microscope - DXR3, Thermo Scientific).
2.4 Supercapacitor electrode preparation and electrochemical measurements
Two-electrode setups were employed to measure the electrochemical properties of the prepared sample. The nickel foil was pretreated with absolute ethanol and dried at 80 °C for 3 h. The rGO/CoFe2O4 composites were coated on the nickel foil with a size φ 8 mm. An aqueous solution of 6-M KOH was used as the electrolyte. Electrodes made by the method mentioned above were immersed into the electrolyte. Then, two electrodes were assembled in a cell lock.
The electrochemical performances of rGO/CoFe2O4 electrodes were investigated by Cyclic Voltammetry (CV), Galvanostatic Charging–Discharging (GCD), and Electrochemical Impedance Spectroscopy (EIS) measurements, which were carried out by Autolab PGSTAT309n (Metrohm, Switzerland). The specific capacitance (Cs) of the electrodes materials and energy density of supercapacitor (E) were calculated viz., Eq. (1) and Eq. (2), respectively [23].
where I, Δt, ΔV, and m represent current, discharging time, potential window, and the mass of the active material on electrode, respectively.
Where E is the energy density (Wh/kg).
3 Results and discussion
The hydroxide compounds of Co2+ and Fe3+ were precipitated on the surface of the rGO sheets in the basic environment. The concentration of the precursor salts employed directly affects the density and size of CoFe2O4 particles. rGO sheets are used as supporting frameworks for the CoFe2O4 nanoparticles, while the CoFe2O4 particles work as fillers to improve delamination efficiency and reduce rGO fragment aggregation. rGO serves as a conducting substrate to maintain the continuous diffusion of ions, while CoFe2O4 particles provide more active sites for redox reactions.
The results of X-ray diffraction (XRD) and Raman spectra are used to illustrate the properties of the phase composition of rGO/CoFe2O4 composites.
The XRD pattern of materials are shown in Fig. 2a. Only two distinctive peaks at 2θ = 25.6 and 42.9°, respectively, that are typical of high-graphite carbon materials (002) and (101) are visible in the XRD pattern of the rGO aerogel sample [24, 25]. Peaks typical for face-centered cubic CoFe2O4 crystals can be seen as (220, 311, 400, 422, 511, and 440) as well as the peaks intensity usual for rGO also dramatically reducing as the CoFe2O4 concentration rose in samples rGO/CoFe2O4-3 and rGO/CoFe2O4-4 [25].
The characteristic peaks at 1355 and 1590 cm−1 for the D and G bands of rGO may still be seen in the Raman spectra of rGO/CoFe2O4 samples (Fig. 2b) [22]. As the concentration of CoFe2O4 increased, the intensities of these two peaks are significantly reduced. The results of CoFe2O4 nanoparticles was produced with a cation distribution of [Fe0.69Co0.31](Co0.69Fe1.31)O4, as seen by the characteristic peaks for CoFe2O4 nanoparticles at positions 207, 307, 473, and 693 cm−1 [26].
Surface morphology of the rGO/CoFe2O4 samples was studied using TEM and SEM techniques. The results of SEM imaging demonstrate that the research successfully manufactured rGO and CoFe2O4 materials. The appearance of CoFe2O4 particles on the surface of rGO flakes is shown in Fig. 3. CoFe2O4 particle placement is fairly consistent. The CoFe2O4 particles with a size of about 10–20 nm are evenly distributed on the surface of the rGO sheet in the rGO/CoFe2O4-2 and rGO/CoFe2O4-3 samples. With particles that are about 50 nm in size, the rGO/CoFe2O4-4 sample seems to have larger ones. When the amount of Co(NO3)2 and Fe(NO3)3 salts employed in the fabrication process steadily rises, it can be seen that CoFe2O4 nanoparticles tend to grow in size and lose homogeneity. The aggregation of oxide particles during precipitation and heat treatment is what causes the above phenomena.
On the surface of the rGO thin sheets, visible CoFe2O4 particles can be seen in the TEM image of the rGO/CoFe2O4-3 material sample (Fig. 4).
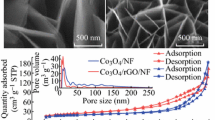
Specific surface area and pore distribution are fundamental and essential properties of rGO/CoFe2O4 materials that have a direct impact on their electrochemical properties when used as supercapacitor materials. Figure 5 illustrates the N2 gas adsorption–desorption isotherm and pore distribution of rGO/CoFe2O4 samples. The N2 gas adsorption–desorption curve of the rGO sample has the shape typical of materials with macropores, while the rGO/CoFe2O4 samples all have the shape typical of materials with an micropore structure management. The pore distribution diagram also reveals that rGO/CoFe2O4 samples contain almost no pores larger than 30 nanometers. The porosity of the material samples is primarily attributable to apertures ranging in size from 3 to 10 nm.
The results indicate that as the concentration of CoFe2O4 increases, the specific surface area of the material tends to decrease, from 162.4 m2/g for the rGO sample to only 43.4 m2/g for the rGO/CoFe2O4-4. In addition, the pore volume decreased substantially from 0.23 to 0.06 cm3/g. Increased concentration and aggregation of CoFe2O4 particles can cause the pores on the surface and between the sheets of rGO to become occluded. Nevertheless, the rGO/CoFe2O4-3 sample exhibits a greater surface area and pore volume than the other samples. The formation of CoFe2O4 particulates to some extent on the surface of GO sheets may be the delamination agent that causes the distance between GO layers to grow. From there, it is clear that selecting the appropriate content allows the application of a quantity of CoFe2O4 particles to completely cover the surface of the rGO sheet without having a significant impact on the surface area or porosity of the material.
Figure 6a shows the CV curves of prepared samples at scan rate of 20 mV/s. According to the results, the CV curve of highly symmetrical rGO/CoFe2O4 samples indicates that the reversibility of the electrochemical process is quite excellent. It can be identified that the mechanism of energy storage and release is mainly based on the adsorption and desorption processes on the surface of the material. The rGO/CoFe2O4-3 sample has the largest area of the CV curve, and the area representing the pseudo-capacitance characteristic is more evident compared to other samples. Figure 6b shows the CV curves of rGO/CoFe2O4-3 at different scan rates varying from 5 to 100 mV/s. The obvious increase of current with scan rate indicates a good rate capability for rGO/CoFe2O4 electrodes at high scan rate.
Figure 6c displays the findings of GCD curves of various rGO/CoFe2O4 at 0.2 A/g of scan rate. It is obvious that the charge curves are asymmetric to the corresponding discharge counterparts in the whole potential region. It can prove the pseudo-capacitor behavior of prepared materials. According to the charge/discharge curves, rGO and rGO/CoFe2O4-1, rGO/CoFe2O4-2, rGO/CoFe2O4-3, rGO/CoFe2O4-4, and CoFe2O4 samples have specific capacitances of 88, 120, 141, 265, 136, and 70 F/g, respectively.
Figure 6d shows GCD curves of prepared electrodes at different current densities varying from 0.1 to 1 A/g. It is clear that the material, even at high scanning speeds and current densities, maintains a decent scanning potential curve shape and a significant specific capacitance. At current densities of 0.1, 0.2, 0.3, 0.5, and 1.0 A/g, the rGO/CoFe2O4-3 electrode has specific capacitances of 283, 265, 253, 242, and 232 F/g, respectively. The specific capacitance remains at 82% compared to the 0.1-A/g current density when the current density reaches 1.0 A/g. This demonstrates the good performance of the produced material for a variety of discharge current densities.
The impedance measurement technique enables a deeper comprehension of the electrochemical properties of rGO/CoFe2O4 materials. The Nyquist diagram of supercapacitors with rGO, rGO/CoFe2O4, and CoFe2O4 electrodes makes it simple to compare the resistance properties of different materials. According to Fig. 6e, rGO, rGO/CoFe2O4, and CoFe2O4 exhibit internal resistances of 0.30, 0.48, and 0.81 ohms, respectively, and charge transfer resistances of 0.60, 0.46, and 0.58 ohms, respectively. The internal resistance and charge transfer resistance of rGO/CoFe2O4 materials are significantly reduced by the excellent conductivity of rGO and the rapid charge conversion rate of CoFe2O4 by redox reaction.
The excellent electrochemical properties of rGO/CoFe2O4 materials are reflected in the long charging life of the supercapacitor electrode. After 1000 cycles of continuous charging, the rGO/CoFe2O4 electrode’s specific capacitance is still 90.5% of the original (Fig. 6f).
Compared to previous analogous reports on rGO/CoFe2O4 composite electrodes, this result was quite favorable (Fig. 7). Therefore, the prepared rGO/CoFe2O4 composites exhibit excellent electrochemical performance and can be used as electrode material for supercapacitors.
Ragone plot of the energy density and power density of rGO/CoFe2O4 nanocomposite. Comparisons of supercapacitors composed of ZnCo2O4-rGO composite [13], CoFe2O4/rGO hydrogel [21], rGO/CoFe2O4 nanocomposites [27], PEDOT:PSS/rGO [28], rGO/CoFe2O4 paper [29], and ZnCo2O4 quantum dots/reduced graphene oxide [30]
The Ragone plot (Fig. 7). demonstrates the correlation between energy density and power density at different discharge current densities. A supercapacitor based on the prepared rGO/CoFe2O4 composite exhibits a high specific energy density of 29 Wh/kg at a specific power density of 855 W/kg. According to the Ragone plot, it can be seen that the rGO/CoFe2O4 material exhibit excellent electrochemical characteristics compared to similar studies.
4 Conclusion
The rGO/CoFe2O4 composite materials were fabricated by a simple thermal reduction process. The rGO/CoFe2O4 sample shows porous structure and high specific surface area. CoFe2O4 particles of approximately 30 nm in size are distributed on the surface of GO sheets uniformly. The prepared rGO/CoFe2O4 showed excellent electrochemical properties, with a high specific capacitance of 283 F/g at a current density of 0.1 A/g and a specific capacitance retention of 90.5% after 1000 cycles. The rGO/CoFe2O4 composite material has the potential to be used in the production of high-performance supercapacitor electrodes.
Data availability
All the data analyzed are included in this manuscript.
References
M.A. Worsley et al., Synthesis of graphene aerogel with high electrical conductivity. J. Am. Chem. Soc. 132(40), 14067–14069 (2010)
M.J. Allen, V.C. Tung, R.B. Kaner, Honeycomb carbon: a review of graphene. Chem. Rev. 110(1), 132–145 (2010)
C. Liu et al., Graphene-based supercapacitor with an ultrahigh energy density. Nano Lett. 10(12), 4863–4868 (2010)
M.F. El-Kady, Y. Shao, R.B. Kaner, Graphene for batteries, supercapacitors and beyond. Nat. Reviews Mater. 1(7), 1–14 (2016)
S. Alex Pandian, M. Sivakumar, Hoisting photovoltaic performance of perovskite BaSnO3 nanoparticles wrapped reduced graphene oxide: Efficient photoelectrode for dye-sensitized solar cell Materials Today: Proceedings, (2023)
Q. Li et al., Highly sensitive graphene-based ammonia sensor enhanced by electrophoretic deposition of MXene. Carbon. 202, 561–570 (2023)
H.J. Salavagione et al., Scalable graphene-based nanocomposite coatings for flexible and washable conductive textiles. Carbon. 167, 495–503 (2020)
R. Padma Priya, A. Baradeswaran, A. Bagubali, Energy storage improvement of graphene based super capacitors. Materials Today: Proceedings. 78, 919–923 (2023)
J. Zhao et al., Ultra-fine ruthenium oxide quantum dots/reduced graphene oxide composite as electrodes for high-performance supercapacitors. Nanomaterials 12(7), 1210 (2022)
L. Xie et al., Self-assembled 3D graphene‐based aerogel with Co3O4nanoparticles as high‐performance asymmetric Supercapacitor Electrode. ChemSusChem. 8(17), 2917–2926 (2015)
Y. Wang et al., Mesoporous transition metal oxides for supercapacitors. Nanomaterials. 5(4), 1667–1689 (2015)
J. Ran et al., MnO2@MoS2/RGO hollow structure as high-performance supercapacitor electrode materials. J. Energy Storage 64, 107216 (2023)
Z. Gao et al., ZnCo2O4 -reduced graphene oxide composite with balanced capacitive performance in asymmetric supercapacitors. Appl. Surf. Sci. 442, 138–147 (2018)
J. Pan et al., The NiFe2O4/NiCo2O4/GO composites electrode material derived from dual-MOF for high performance solid-state hybrid supercapacitors. Colloids Surf., A 609, 125650 (2021)
X. Mo et al., A facile microwave hydrothermal synthesis of ZnFe2O4/rGO nanocomposites for supercapacitor electrodes. Nanomaterials (Basel) 13(6), 1034 (2023)
I. Ayman et al., CoFe2O4 nanoparticle-decorated 2D MXene: A novel hybrid material for supercapacitor applications. Energy & Fuels. 34(6), 7622–7630 (2020)
S. Subhadarshini et al., Silver nanodot decorated dendritic copper foam as a hydrophobic and mechano-chemo bactericidal surface. Langmuir 37(31), 9356–9370 (2021)
J. Zhao et al., Ultra-fine ruthenium oxide quantum dots/reduced graphene oxide composite as electrodes for high-performance supercapacitors. Nanomaterials (2022). https://doi.org/10.3390/nano12071210
J.-Y. Kim et al., In situ chemical synthesis of ruthenium oxide/reduced graphene oxide nanocomposites for electrochemical capacitor applications. Nanoscale 5(15), 6804–6811 (2013)
S. Subhadarshini et al., One-pot facile synthesis and electrochemical evaluation of selenium enriched cobalt selenide nanotube for supercapacitor application. Ceram. Int. 47(11), 15293–15306 (2021)
L. Zheng et al., One-pot synthesis of CoFe2O4/rGO hybrid hydrogels with 3D networks for high capacity electrochemical energy storage devices. RSC Adv. 8, 8607–8614 (2018)
N.I. Zaaba et al., Synthesis of graphene oxide using modified hummers method: Solvent influence. Procedia Eng. 184, 469–477 (2017)
P. Simon, Y. Gogotsi, Materials for electrochemical capacitors. Nat. Mater. 7(11), 845–854 (2008)
X. Wu et al., High-rate capacitive performance of graphene aerogel with a superhigh C/O molar ratio. J. Mater. Chem. 22(43), 23186–23193 (2012)
X. Feng et al., Hierarchical CoFe2O4/NiFe2O4 nanocomposites with enhanced electrochemical capacitive properties. J. Mater. Sci. 53, 2648–2657 (2018)
P. Chandramohan et al., Cation distribution and particle size effect on Raman spectrum of CoFe2O4. J. Solid State Chem. 184(1), 89–96 (2011)
I. Kotutha et al., Electrochemical properties of rGO/CoFe2O4 nanocomposites for energy storage application. Ionics. 25(11), 5401–5409 (2019)
G. Liu et al., Fabrication of PEDOT:PSS/rGO fibers with high flexibility and electrochemical performance for supercapacitors. Electrochim. Acta. 365, 137363 (2021)
J. Song et al., The continuous porous PEDOT:PSS film improves wettability and flexibility of the rGO/CoFe2O4 paper electrodes for symmetric supercapacitors. Appl. Surf. Sci. 568, 150915 (2021)
X. Ma et al., Role of N doping on the electrochemical performances of ZnCo2O4 quantum dots/reduced graphene oxide composite nanosheets. Chem. Eng. J. 327, 1000–1010 (2017)
Acknowledgements
The financial support of Science and Technology Development Project (number:788/2021) is gratefully acknowledged.
Funding
This work was supported by Science and Technology Development Project (Grant no.: 788/2021)
Author information
Authors and Affiliations
Contributions
HVN contributed to material preparation, data interpretation, analysis, and writing and original draft preparation; HTL contributed to data analyzing and writing, reviewing, and editing of the manuscript; THL, TTV, and KTP contributed to discussion and data curation; HTN and TXP contributed to reviewing and editing of the manuscript and supervision. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Van Ngo, H., Nguyen, H.T., Le, H.T. et al. Enhancement of electrochemical properties of porous rGO by controlled growth CoFe2O4 nanoparticles. J Mater Sci: Mater Electron 34, 1707 (2023). https://doi.org/10.1007/s10854-023-11071-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10854-023-11071-9