Abstract
With the development of the current times, the demand for refrigeration is in every aspect. Nevertheless, the necessity for new refrigeration materials is immediate because of the greenhouse effect and the destruction of the atmosphere. Therefore, perovskite materials have been investigated. La0.8−xEuxCa0.1Sr0.1MnO3 (LECSMO) (x = 0.05, 0.10, 0.15) powder samples were prepared using the sol–gel method (S–G). The structure, magnetocaloric effect, and magnetic of LECSMO have been explored. X-ray diffraction (XRD) indicates that all samples belong to the rhombohedral perovskite structure of the Pbnm space group. The magnetic research of LECSMO revealed that the samples doped with Eu3+ exhibited a ferromagnetic (FM) to paramagnetic (PM) transition around the Curie temperature (Tc). As the amount of Eu3+ doping is added, Tc decreased from 264 to 194 K. Normalization and Banerjee’s criterion confirmed the second-order phase transition of the LECSMO near Tc. The maximum magnetic entropy change (\(-\varDelta {S}_{M}^{max}\)) for LECSMO (x = 0.05, 0.10, 0.15) is 5.39, 4.52, and 4.35 J/(kg·K) when the applied magnetic field (H) = 5 T, respectively. Further, the relative cooling power (RCP) of LECSMO is 282.20 J·kg− 1, 305.20 J·kg− 1, and 323.15 J·kg− 1, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
At present, refrigeration technology has a critical position and is closely related to the production and life of people. Refrigeration technology is used everywhere, for example, in medical equipment, refrigerators, the chemical and petroleum industry, the power industry, and precision instruments [1,2,3,4,5]. In daily life, the commonly used method is gas compression refrigeration. The extensive use of gas compression refrigeration (Freon) has caused severe damage to the environment. Therefore, it is vital to find newer cooling methods to achieve better cooling. Under these conditions, magnetic refrigeration technology was created. Since the physical properties of the magnetocaloric effect (MCE), magnetic refrigeration using MCE is becoming increasingly advantageous [6]. Magnetic refrigeration offers better cooling efficiency than conventional gas compression refrigeration and does not cause environmental pollution, making it greener and more energy efficient [7, 8]. The MCE is caused by the magnetized material’s demagnetization, cooling, or heating. Whereas, the MCE results from the coupling between the atomic lattice and the magnetic moment, the change in magnetic entropy of magnetic refrigeration materials in the presence of H. The change in entropy for isothermal processes and the change in temperature (T) for adiabatic processes are the basic indicators of performance [9,10,11,12]. As a result, researchers have developed new refrigeration materials with a wide operating temperature range and a considerable variation in magnetic entropy change (\(\varDelta {S}_{M}\)).
There are hundreds of MCE materials that have been discovered since the 21st century. Metal Gd and Gd-based alloys, La(FeSi)13, and Gd5Si2Ge2 all exhibit excellent MCE [13,14,15]. However, these materials are difficult to apply due to their high price, cumbersome synthesis methods, and bigger thermal hysteresis. ABO3 has attracted much attention and has been extensively investigated because of its low price, simple synthesis route, small magnetic hysteresis and thermal hysteresis, and significant \(\varDelta {S}_{M}\) [16,17,18,19]. Recently, researchers have become very interested in studying RE1−xAExMnO3 (RE = La, Pr, Nd, and Sm and AE = Ba, Sr, and Ca) in ABO3. Because the Tc of La1−xSrxMnO3 is suitable and thus could be a potential magnetic refrigeration material [20,21,22,23]. Szewczyk et al. found that with Sr2+ doping increased, Tc approached near room temperature. When H = 7 T, Tc = 305 K, \(-\varDelta {S}_{M}^{max}\)=7.90 J/(kg·K), and RCP=395 J·kg−1 [24]. A. Elghoul et al. study given Tc = 330 K for La0.8Sr0.2MnO3 when H = 2 T, \(-\varDelta {S}_{M}^{max}\)=1.99 J/(kg·K), RCP=69.07 J·kg−1 [17]. When doped with trace amounts of alkaline earth ions (Ca2+, Ba2+), the MCE of ABO3 can be significantly augmented without affecting the double-exchange interaction (DE) [10, 25, 26].
In order to investigate a refrigeration material with simple preparation, low cost, and axenic, the structural, magnetic, and magnetocaloric properties of LECSMO (x = 0.05, 0.10, 0.15), which the amount of Eu3+ doping increases, using La0.8Ca0.1Sr0.1MnO3 as a substrate have been deeply explored.
2 Experimental
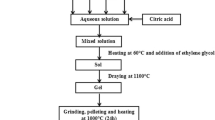
In this paper, samples of LECSMO polycrystalline powder were prepared using the S–G method. The amount required for each sample was first calculated from the stoichiometric ratios. (La(NO3)3·6H2O 10277-43-7, Ca(NO3)2·4H2O 13477-34-4, Sr (NO3)2 10042-76-9, Eu(NO3)3·6H2O 10031-53-5, and Mn(NO3)2 (50%) 10377-66-9. All chemicals used were of the Aladdin brand and were ≥ 99.5% pure). The gel was then stirred uniformly in a constant temperature water bath at 85 °C until a wet gel was formed. The wet gel was dried at 120 °C for 12 h to obtain a dry gel. The resulting dry gel was subjected to a heat treatment at 300 °C in a muffle furnace to remove the organic matter from the samples. The powder was allowed to cool naturally and then ground. The ground powder was then calcined in a muffle furnace at 950 °C for 10 h to obtain LECSMO polycrystalline powder. (Fig. 1)
XRD with Cu Kα radiation (λ = 1.54059Å) was used to determine the physical phase, structure, and cell parameters of LECSMO. The data were further processed by the Rietveld method. The characteristics of morphology and size of the samples were analyzed by scanning electron microscopy (SEM) (SEM, ZEISS, GeminiSEM 300). Finally, the LECSMO was magnetically tested using the integrated magnetic measurement system MPMS (Quantum Design (USA)).
3 Results and discussion
3.1 Structural exploration
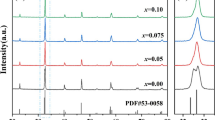
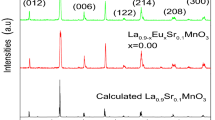
The XRD data of LECSMO at room temperature are shown in Fig. 2. Figure 2 confirms that the LECSMO are all single-phase perovskite and that no impurity phases are detected. Figure 2b is an enlarged figure of the characteristic diffraction peak of the LECSMO diffraction angle of 32–33.5°. The characteristic diffraction peak of LECSMO between 32° and 33.5° shifts slightly toward higher angles with increasing Eu3+ content, indicating that the cell volume (V) of samples is diminished. This is because the ionic radius of Eu3+ (1.15 Å) is smaller than La3+ (1.36 Å). When the smaller ionic radius of Eu3+ replaces the larger ionic radius of La3+, the average radius of the A-site ion decreases and therefore V decreases accordingly [27,28,29,30]. At the same time, the Mn–O bond length becomes larger, and the Mn–O–Mn bond angle decreases, resulting in a distortion of the LECSMO lattice. Figure 3 demonstrates how the cultural parameters vary with the amount of Eu3+ doping. Refinement of the XRD data from LECSMO by FULLPROF software confirmed that LECSMO is a rhombohedral perovskite structure with a Pbnm space group (Fig. 4).
Table 1 displays the calculated lattice constants, V, Mn–O–Mn bond angles, and Mn–O bond lengths and summarizes the fit χ2 for LECSMO. To determine the degree of stability of the LECSMO architecture, the tolerance factor (t) Goldschmidt introduced is defined [31]. The equation is as follows:
r O, rB, and rA are the average ionic radii at the O, B, and A positions in the LECSMO perovskite manganites, respectively. The average LECSMO ion radius size was calculated using the Shannon table and Eq. (1). The calculated t for LECSMO is less than 0.96, confirming that it is a stable rhombohedral perovskite structure. The bandwidth W is decided from the angle of Mn–O–Mn and the length of Mn–O, which is calculated as follows [32]:
Table 1 presents that as the amount of Eu3+ doping augments, the Mn–O–Mn bond angle declines, while the Mn–O bond length increases, leading to a decrease in W. The decrease in W reduces the overlap between the Mn 3d orbital and the 2p orbital of the O anion, reducing the Mn4+–O2−–Mn3+ exchange coupling and weakening the DE interaction, ultimately weakening, leading to a decrease in Tc.
Figure 5 manifests the SEM diagram of LECSMO. The particles of LECSMO show an irregular elliptical distribution with porosity. LECSMO is available in sub-micron crystalline size spanning from 84 to 171 nm. The average crystalline size (DXRD) of LECSMO can be obtained according to Scherrer’s formula [33]
K is the Scherrer constant, λ is the wavelength of the x-rays, θ is the Bragg diffraction angle, and β is the half-height width of the LECSMO diffraction peak. The \({D}_{XRD}\) range of LECSMO is between 50 and 100 nm. Results show that the size of the LECSMO particles viewed by SEM is bigger than the \({D}_{XRD}\) obtained by the Scherrer equation since each particle in LECSMO is made up of multiple grains [10, 17, 34]. Figure 6 indicates the scanning electron microscope mapping of La0.8−xEuxCa0.1Sr0.1MnO3 (x = 0.05, 0.10, 0.15). Verification of the presence of all elements in LECSMO by energy-dispersive spectrometer analysis (Fig. 7). Energy-dispersive spectrometer analysis confirmed the existence of La, Ca, Mn, Eu, Sr, and O elements in the LECSMO.
3.2 Magnetic and Magnetocaloric Exploration
LECSMO is magnetized in the temperature (T) scale of 140−300 K in a field of 100 Oe. As shown in Fig. 8, with T increased, the magnetization intensity (M) of LECSMO diminished sharply. They indicated an FM-PM transition of the polycrystalline powder LECSMO. Tc is determined by differentiating the M–T curve, and the smallest value of Tc corresponding to dM/dT is determined as a consequence of T. (Fig. 8). Figure 8 presents Tc for different Eu3+ doping amounts are 264, 226, and 194 K. The variation of Tc is related to the DE within the LECSMO material. As the radius of Eu3+ is smaller than that of La3+, as the amount of Eu3+ doping increased, the \({r}_{A}\) decreased, distorting the lattice of LECSMO. The degree of lattice distortion can be expressed in the magnitude of the mismatch coefficient (\({\sigma }^{2}\)), which is defined as follows [35]
Equation (4) calculates the σ2 for LECSMO (x = 0.05, 0.10, 0.15) as 0.6944, 0.7908, and 0.8715, respectively. The increase in \({\sigma }^{2}\) with Eu3+ doping indicates an enhanced disorder and increased distortion of the A-site ions in LECSMO. The distortion leads to a reduction in the Mn–O–Mn bond angle and an enlargement of the Mn–O bond length, affecting Mn3+–O2−–Mn4+, leading to a weakness in DE and a reduction in Tc. Since the Tc of LECSMO (x = 0.05, 0.10) is closer to that required for low to medium room temperature refrigeration, the magnetic behavior of LECSMO (x = 0.05, 0.10) was further investigated in depth using the Curie–Weiss law (C–W) (Fig. 9) [36, 37]:
C is the Curie constant and \({\theta }_{W}\) is the Weiss temperature. The value of \(c\) is greater than Tc (Table 2). The differences in \({\theta }_{W}\) and Tc may have some relevance to the presence of magnetic inhomogeneities. Table 2 shows that \({\theta }_{W}\) decreases significantly with increasing Eu3+ doping, showing a decreased DE, associated with a decreasing Tc. A positive value of \({\theta }_{W}\) indicates the presence of FM interactions.
C can be determined from C–W and the experimental effective magnetic moment \({\mu }_{eff}^{exp}\) is defined by equation [38]:
\({N}_{A}\) is the Avogadro constant, \({\mu }_{B}\) is the Bohr magneton, and \({K}_{B}\) is the Boltzmann constant. The following relation can determine the theoretical effective magnetic moment \({\mu }_{eff}^{the}\) [39]:
\({\mu }_{eff}^{the}\) and \({\mu }_{eff}^{exp}\) are shown in Table 2. \({\mu }_{eff}^{exp}\) gradually raised as the amount of Eu3+ doping was added. The change in \({\mu }_{eff}^{exp}\) is the opposite of the change in Tc. The variance can illustrate the phenomenon in the strength limit of the FM association between Mn3+ and Mn4+. \({\mu }_{eff}^{exp}\) for the LECSMO (x = 0.05, 0.10) samples are more remarkable than \({\mu }_{eff}^{the}\), also confirming the presence of FM interactions in the PM phase. The difference in the strength limit of the FM interaction between Mn3+ and Mn4+ can illustrate this phenomenon. LECSMO (x = 0.05, 0.10) samples with \({\mu }_{eff}^{exp}\) greater than \({\mu }_{eff}^{the}\) confirmed the presence of FM interactions in the PM phase. Figure 10 shows the isothermal magnetization profiles (M–H) of LECSMO at different T. The range of H is 0–5 T, with a curve measured at 10 K intervals. It was found that the maximum M of LECSMO increased from 76.5 Am2 kg− 1 (x = 0.05) to 81.6 Am2 kg− 1 (x = 0.15) as the Eu3+ doping content increased. When T of LECSMO is lower than Tc and H is lower than 0.3 T, M of LECSMO tends to rise rapidly with increasing H. When H is more incredible than 0.3 T, M increases slowly and eventually tends to saturate gradually. When T < Tc, LECSMO has a larger M and exhibits typical FM behavior. When T > Tc, the M of LECSMO is relatively small and exhibits typical PM behavior. At the same H, the higher the T, the smaller the M of the LECSMO, determining that the LECSMO underwent an FM-PM phase transition. Figure 10 demonstrates that M–H is not all linear when T > Tc, explaining the reason for the difference between \({\mu }_{eff}^{the}\) and \({\mu }_{eff}^{exp}\) and demonstrating the presence of FM clusters in PM.
To further investigate the use of magnetic phase transformation in LECSMO, M2-H/M curves (Arrott curves) were graphed in the critical temperature region of the phase transformation (Fig. 11). The Banerjee criterion allows the type of magnetic phase change of LECSMO to be discerned. In the Arrott curve, if the M2-H/M curve has a negative slope around Tc or if the curve is S shaped, then the type of magnetic phase transition of the sample is a first-order phase transformation. If the gradient of the M2-H/M curve around Tc is all positive, then the type of magnetic phase transformation of the material is a second-order phase transformation. The more negligible hysteresis of the second-order phase transition facilitates the practical application of LECSMO magnetically refrigeration materials.
The type of phase transition in LECSMO is determined using the Banerjee criterion and the field-following normalization curve proposed by Franco. The normalization curve assumes that if there is a normalization curve, the curves at various of H collapse into a single curve, and the corresponding phase transition for that material becomes the second-phase transformation. The curve at various H cannot be collapsed into a curve with a primary phase transformation. This is defined by Equations. [40, 41]:
T1 and T2 temperatures all need to satisfy \(\varDelta {S}_{M}\left({T}_{2}\right)=\varDelta {S}_{M}\left({T}_{1}\right)=\frac{1}{2}\varDelta {S}_{M}^{Max}\). The normalized curves of LECSMO at various H can all slump into one curve. Confirming that the phase transitions occurring in LECSMO are all second-order phase transitions, in agreement with the results obtained using the Banerjee criterion (Fig. 12).
The \(\varDelta {S}_{M}\) of LECSMO is obtained from the adiabatic change of T under a magnetic field. The equation for the \(\varDelta {S}_{M}\) is obtained using Maxwell’s thermodynamic relations as follows [42]:
The magnetization measurements are carried out at discrete magnetic fields and T separation, which can be circa by Eq. (9) as Eq. (10) [43].
where \({M}_{i}\) and \({M}_{i+1}\) are the experimental magnetization values at \({T}_{i}\) and \({T}_{i+1}\) temperatures, respectively. The relationship between the \(\varDelta {S}_{M}\) of LECSMO at different H and T are given in Fig. 13. At the same T, the \(\varDelta {S}_{M}\) of LECSMO increases gradually with increasing H. This is because increasing H increases the degree of ferromagnetic long-range ordering in LECSMO, which accelerates the change in M of the sample near Tc and ultimately leads to an increase in the \(\varDelta {S}_{M}\). The \(-\varDelta {S}_{M}^{max}\) of LECSMO is 5.39 J/(kg·K), 4.52 J/(kg·K), and 4.35 J/(kg·K) at H = 5 T. The \(\varDelta {S}_{M}\) of LECSMO decreases with increasing x, which is related to the weakening of DE inside the LECSMO sample. As the amount of Eu3+ doping increases, the MnO6 octahedral distortion becomes more and more severe, affecting the DE of Mn3+–O2−–Mn4+ and eventually leading to a decrease in the \(-\varDelta {S}_{M}^{max}\) variation of LECSMO.
For magnetic refrigeration materials, the relative cooling efficiency (RCP) is an essential parameter for judging their performance, and the RCP is calculated using formula [44]
where \(\delta {T}_{FWHM }\) is the magnitude of the half-peak width of the \(-\varDelta {S}_{M}^{max}\) curve. The RCP of LECSMO varies as a function of H, as shown in Fig. 14 . It can be seen from the graph that the RCP of LECSMO gradually rises as H added. According to the equation RCP = aHb, it is shown that RCP is reliant on H. The b-values obtained after the fit was similar to those previously reported. The RCP for LECSMO was greatest when H = 5 T. The RCP for LECSMO was 282.20 J·kg− 1 (x = 0.05), 305.20 J·kg− 1 (x = 0.10), and 323.15 J·kg− 1 (x = 0.15), respectively. The \(\varDelta {S}_{M}\) of LECSMO is gradually enhanced in the RCP as the amount of Eu3 + doping rises, although it is declining. The magnitudes of Tc, \(-\varDelta {S}_{M}^{Max}\), and RCP for LECSMO are listed in Table 3 for comparison with the values for other magnetic materials at H.
According to thermodynamic theory, the variations in magnetic entropy are obtained from the following equation for individual heat capacity.
The change in H leads to variations in specific heat (\({\Delta }{C}_{p}\)), which can be derived from the following equation [57]:
Figure 15 demonstrates that the \(\varDelta {C}_{p}\) of the LECSMO changes the sign from a positive sign to a negative sign around Tc. As H increases, the magnitude of \({\Delta }{C}_{p}^{max}\) as well as \({\Delta }{C}_{p}^{min}\) has monotonicity. Since both \(\varDelta {S}_{M}\) and dM/dT are considered negative, the situation’s total entropy drops during the magnetization process.
4 Conclusion
Polycrystalline powder samples of LECSMO were prepared using the S–G method. The crystal structure, magnetic properties, and magnetocaloric effect of LECSMO were investigated. XRD analysis showed that LECSMO is a rhombohedral perovskite structure in the Pbnm space group. Scanning electron microscope observed an irregular ellipsoidal distribution of LECSMO particles. Magnetic measurements demonstrated that the PM–FM transition occurs near Tc and that Tc reduces as the amount of Eu3+ doping rises. Both the method based on the Arrott curve and the normalized curve confirmed that LECSMO undergoes a second-order phase transition near Tc. With a progressive addition of Eu3+ doping, the \(-\varDelta {S}_{M}^{max}\) of LECSMO is reduced, but the RCP gradually increases.
Data availability
The datasets generated during and analyzed during the current study are available from the corresponding author on reasonable request.
References
Z.J. Xie, Z.G. Zou, B.R. He, L.L. Liu, Front. Mater. 8, 771941 (2021)
Z.J. Xie, Z.G. Zou, X.Y. Jiang, W.J. Zhang, B.R. He, X.N. Han, Physica B: Condensed Matter 639, 413985 (2022)
Z.J. Xie, Z.G. Zou, Z. Mao, X.Y. Jiang, W.J. Zhang, J. Mater. Res. Technol. 21, 2778 (2022)
Z.J. Xie, W.J. Zhang, Z.G. Zou, X.Y. Jiang, J. Magn. Magn. Mater. 536, 170014 (2022)
Z.J. Xie, X.Y. Jiang, Z.G. Zou, Eur. Phys. J. Plus. 137, 1350 (2022)
M.-H. Phan, S.-C. Yu, J. Magn. Magn. Mater. 308, 325 (2007)
K.A. Gschneidner Jr., V.K. Pecharsky, Int. J. Refrig. 31, 945 (2008)
I. Rasta, I. Susila, I. Subagia, J. Phys. Conf. Ser. 953, 012077 (2018)
A. Barman, S. Kar-Narayan, D. Mukherjee, Adv. Mater. Interfaces 6, 190021 (2019)
K. Laajimi, F. Ayadi, M. Kchaw, I. Fourati, M. Khlifi, M.H. Gazzah, J. Dhahri, J. Juraszek, Solid. State. Sci. 119, 106683 (2021)
P. Zhang, H.G. Piao, Y.D. Zhang, J.H. Huang, Acta Phys. Sin 70, (2021)
C. Henchiri, L.H. Omari, T. Mnasri, A. Benali, E. Dhahri, M.A. Valente, J. Alloys Compd. 905, 194196 (2022)
J. Meyers, S. Chumbley, W. Choe, G.J. Miller, Phys. Rev. B Condens. 66, (2002)
B. Podmiljsak, I. Skulj, B. Markoli, K.Z. Rozman, P.J. McGuiness, S. Kobe, J. Magn. Magn. Mater. 321, 300 (2009)
V.D. Buchel’nikov, A.N. Vasiliev, V.V. Koledov, S.V. Taskaev, V.V. Khovaylo, V.G. Shavrov, Phya-Usp. 49, 871 (2006)
G. Akça, S. Kılıç Çetin, A. Ekicibil, Ceram. Int. 43, 15811 (2017)
A. Elghoul, A. Krichene, N. Chniba Boudjada, W. Boujelben, Ceram. Int. 44, 12723 (2018)
Z. Liu, W.G. Lin, K.W. Zhou, J.L. Yan, Ceram. Int. 44, 2797 (2018)
A. Selmi, R. Mnassri, W. Cheikhrouhoukoubaa, N. Chniba Boudjada, A. Cheikhrouhou, Ceram. Int. 41, 7723 (2015)
J. Moradi, M.E. Ghazi, M.H. Ehsani, P. Kameli, J. Solid State Chem. 215, 1 (2014)
T. Raoufi, M.H. Ehsani, D.S. Khoshnoud, J. Alloys Compd. 689, 865 (2016)
X. Zhang, J. Fan, L. Xu, D. Hu, W. Zhang, Y. Zhu, Magnetic and magnetocaloric properties of nanocrystalline La0.5Sr0.5MnO3. Ceram 42, 1476 (2016)
G. Kadim, R. Masrour, A. Jabar, E.K. Hlil, Phys. A 573, 125936 (2021)
A. Szewczyk, M. Gutowska, B. Dabrowski, T. Plackowski, N.P. Danilova, Y.P. Gaidukov, Phys. Rev. B 71, 224423 (2005)
M. Jeddi, H. Gharsallah, M. Bekri, E. Dhahri, E.K. Hlil, J. Low Temp. Phys. 198, 135 (2020)
M.-H. Phan, Y. u Hur, NH Appl. Phys. Lett. 86, 072504 (2005)
F. Cao, H. Chen, Z. Xie, Y. Lu, J. Zhao, X. Jin, Chin. J. Phys. 65, 424–435 (2020)
A.B. Hassine, A. Dhahri, M.L. Bouazizi, M. Oumezzine, E.K. Hlil, Ceram. Int. 43(1), 1390–1393 (2017)
S. Vadnala, S. Asthana, J. Magn. Magn. Mater. 446, 68–79 (2018)
H. Chen, C. Li, J. Zhao, Y. Lu, F. Cao, W. Wang, X. Jin, J. Supercond. Nov. Magn. 34, 2651–2666 (2021)
V.M. Goldschmidt, Sci. Nat. 14, 477 (1926)
J.M.M. Medarde, P. Lacorre, S. Rosenkranz, P. Fischer, K. Gobrecht, Phys. Rev. B 52, 9248 (1995)
C. Kursun, M. Gogebakan, E. Uludag, M.S. Bozgeyik, F.S. Uludag, Sci. Rep. 8, 13083 (2018)
Z. Ma, J. Mohapatra, K. Wei, J.P. Liu, S. Sun, Chem. Rev. 123, 3094 (2023)
R.D. Shannon, Acta Crystallogr. Sect. A: Found. Crystallogr. 32, 751 (1976)
P.G. De Gennes, Phys. Rev. 118, 141 (1960)
M. Kochmański, T. Paszkiewicz, S. Wolski, Eur. J. Phys. 34, 1555 (2013)
S. Hcini, M. Boudard, S. Zemni, Appl. Phys. A 115, 985 (2014)
K.H.J. Buschow, F.R. Boer, Physics of magnetism and magnetic materials introduction, 1st end (Springer, Boston, 2003), pp.19–42
V. Franco, J.S. Blazquez, A. Conde, Appl. Phys. Lett. 89, 222512 (2006)
V. Franco, A. Conde, Int. J. Refrig. 33, 465 (2010)
K.A. Gschneidner, V. Pecharsky, A. Tsokol, Rep. Prog. Phys. 68, 1479 (2005)
M. Földeàki, R. Chahine, T. Bose, J. Appl. Phys. 77, 3528 (1995)
V. Franco, J. Blázquez, B. Ingale, A. Conde, Annu. Rev. Mater. 42, 305 (2012)
P.T. Phong, N.V. Dang, P.H. Nam, L.T.H. Phong, D.H. Manh, N.M. An, I.N. Lee, J. Alloys Compd. 683, 67–75 (2016)
S. Yu Dan’ kov, A.M. Tishin, V.K. Pecharsky, K.A. Jr. Gschneidner, Phys. Rev. B 57, 3478 (1998)
V.K. Pecharsky, K.A. Gschneidner, J. Phys. Rev. Lett. 78, 4494 (1997)
M.S. Kim, J.B. Yang, Q. Cai, X.D. Zhou, W.J. James, W.B. Yelon, P.E. Parris, D. Buddhikot, S.K. Malik, Phys. Rev. B 71, 014433 (2005)
X.Y. Luo, H.F. Yang, S.H. Hua, Q. Wu, N.J. Yu, P.Y. Zhang, H.L. Ge, Bull. Mater. 44, 1 (2021)
A. Bouderbala, J. Makni-Chakroun, W. Cheikhrouhou-Koubaa, M. Koubaa, A. Cheikhrouhou, S. Nowak, S. Ammar-Merah, Ceram. Int. 41, 7337 (2015)
A. Dhahri, M. Jemmali, E. Dhahri, M.A. Valente, J. Alloys Compd. 638, 221 (2015)
X. Jin, Y. Lu, Y. Sun, H. Wu, X. Sun, F. Cao, J. Zhao, J. Low Temp. Phys. 195, 403 (2019)
Y. Bitla, S.N. Kaul, L.F. Barquín, J. Gutiérrez, J.M. Barandiarán, A. Pena, New. J. Phys. 12, 093039 (2010)
A. Guedri, S. Mnefgui, S. Hcini, E.K. Hlil, A. Dhahri, J. Solid State Chem. 297, 122046 (2021)
H.E. Sekrafi, A. Ben, N. JaziaKharrat, W. Chniba-Boudjada, Boujelben, Solid. State. Sci. 105, 106274 (2020)
E. Bouzaiene, A.H. Dhahri, J. Dhahri, E.K. Hlil, Inorg. Chem. Commun. 132, 108824 (2021)
Y. Zhang, P.J. Lampen, T.-L. Phan, S.C. Yu, H. Srikanth, M.-H. Phan, J. Appl. Phys. 111, 063918 (2012)
Funding
The funding was provided by the National Natural Science Foundation of China, Grant No. 52162038.
Author information
Authors and Affiliations
Contributions
ZX contributed to conceptualization, data curation, writing of the original draft, and writing, reviewing, & editing of the manuscript. XJ contributed to data curation, supporting, and writing, reviewing, & editing of the manuscript. WZ contributed to writing, reviewing, & editing of the manuscript and supporting. ZZ contributed to conceptualization, funding acquisition, and writing, reviewing, & editing of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have not disclosed any competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xie, Z., Jiang, X., Zhang, W. et al. Structural, magnetic, and magnetocaloric properties of La0.8−xEuxCa0.1Sr0.1MnO3 (x = 0.05, 0.10, and 0.15): A-site doping. J Mater Sci: Mater Electron 34, 1514 (2023). https://doi.org/10.1007/s10854-023-10943-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10854-023-10943-4