Abstract
Li2CaSiO4:Eu3+ phosphors with single phase were successfully synthesized by the solid state reaction method. Characterizations XRD, SEM, FTIR, Photoluminescence and CIE were studied of the prepared samples. SEM images showed irregular, non-homogeneous morphology and relatively big grain size of the samples. From the FTIR, it was observed that most of the bands are due to ligand ions (Ca–O, Si–O, Li–O) stretching or bending vibrations. The authors are interested in investigating the concentration effect of the activator ion in the host and also in the effect of the excitation wavelength on emission intensity of the synthesized phosphors. The experimental results of Li2CaSiO4:Eu3+ showed the different excitation wavelengths in the range 200–500 nm, especially in the ultraviolet region. Strong and sharp excitation peak was observed at 394 nm. The emission in the red spectrum and the CIE Chromaticity coordinate (0.68, 0.31) indicated that the phosphor has great potential in UV chip pumped white LEDs. A comparative investigation of the excitation and emission intensities of Li2CaSiO4:Eu3+ were studied. The CIE study of Li2CaSiO4:Eu3+ under the excitation of 394 nm was also studied. The CIE Chromaticity coordinates (0.68, 0.31) and (0.60, 0.39), (0.60, 0.38) indicated orange/red color. They are useful for the generation of white light for ultra-violet (394 nm) chip when pumped and multi-phosphors are converted into white LEDs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Phosphors converted into white light emitting diodes (LEDs) have attracted great interest in the present generation of solid state lighting technology. These white LEDs feature great potential due to their excellent properties such as low power consumption, high efficiency, long lifetime, non-mercury pollution and design flexibility. White LED products composed of a blue LED chip and YAG:Ce3+ phosphor have been commercially available. Therefore, the development of highly efficient blue, green, yellow and red phosphors for multi-phosphors converted white LEDs is of particular interest [1,2,3]. In order to develop such phosphors, rare earth doped silicates such as M2SiO4:Eu [4,5,6,7], M3SiO5:Eu [8,9,10,11], M3MgSi2O8:Eu, Mn [12,13,14], Li2MSiO4:Eu [15,16,17,18,19] (M = Ba, Sr, Ca or Mg) etc., have emerged themselves with excellent performances for possible use as down conversion luminescent materials for white LEDs. These phosphors can be efficiently excited by UV–Vis or blue light which covers exactly the emission spectra (350–460 nm) of typical UV or blue LED chips. Sr2SiO4:Eu showed stronger yellow emission better than YAG:Ce3+ [4]. Its emission band has been tuned by substituting Sr2+ by other ions like Ba2+ or Mg2+ and thus their performance in white LEDs improved [6].
The Sr3SiO5:Eu2+ phosphor emits deep yellow light under blue excitation and its combination with InGaN blue chip yields warm white light [8]. The Li2SrSiO4:Eu2+ was reported to be an excellent yellow phosphor and previous studies have also confirmed its application in white LEDs as yellow light source [16, 19]. The photoluminescence of Li2CaSiO4:Eu2+ has also been reported to be a bluish phosphor, however, the report about Li2CaSiO4:Eu3+ photoluminescence and its application in white LEDs are scant. Lithium-containing silicate compounds have attracted so much attention in recent years due to the demand for energy storage and illumination source applications (e.g., lithium ion batteries, LEDs, FEDs) [20,21,22,23]. These applications are based on lithium-containing silicates having a rigid structure and good electrical conductivity. The rigid structure of the lithium-containing silicate is formed by the LiO4 and SiO4 tetrahedra being interconnected by common edges or angles. The rigid structure can restrict the local relaxation of rare earth ions, which is a prerequisite for high efficiency and thermally stable emission. The good conductivity of lithium-containing silicates is due to the good mobility of lithium ions in the lattice network, which facilitates charge conduction and has a large saturation potential. Furthermore, the synthesis temperature of the lithium-containing silicate is low, usually at 800–1000 °C, which can greatly reduce the cost of the product. Therefore, based on these advantages, lithium-containing silicates have broad application prospects in WLEDs and FEDs [24, 25].
In this paper, we present a thorough study about the synthesis and specifically photoluminescence excitation and emission and application evaluation of the Eu3+ activator ion doped with different concentrations (0.01, 0.05, 0.10, 0.15 and 0.20 wt (in gram)) in the hosts Li2CaSiO4 phosphors. With the attempt of applying these phosphors in multi-phosphor converted white LEDs, the chromaticity coordinates which we found were reported and thus their application in white LEDs were investigated.
2 Materials and methods
To prepare Li2CaSiO4:Eu3+ phosphors, various concentrations of europium (0.01, 0.05, 0.10, 0.15 and 0.20 wt (in gram)), consists of heating stoichiometric amounts of reactant mixture was taken in alumina crucible and fired in air at 500 °C for 1 h in a muffle furnace. Every heating was followed by intermediate grinding using agate mortar and pestle. The Eu3+ activated Li2CaSiO4 phosphor was prepared via high temperature modified solid state diffusion. The starting materials were as follows: Li2CO3 (99.9%), CaCO3 (99.9%), SiO2 (99.9%) and Eu2O3 (99.99%) in molar ratio (0.01, 0.05, 0.10, 0.15 and 0.20 wt (in gram)) were used to prepare the phosphor. The mixture of reagents was ground together to obtain a homogeneous powder. After being ground thoroughly in stoichiometric ratios using an agate mortar by dry grinding for nearly 45 min, to ensure the best homogeneity and reactivity, powder was transferred to alumina crucible, and then heated in a muffle furnace at 900 °C for 2 h. The phosphor materials were cooled to room temperature naturally.
Conventional X-ray diffraction analysis (XRD, Bruker D8 Advance, CuKα radiation (λ = 1.5405 Å) was employed for phase identification. Scanning Electron Microscopy (SEM) was done for the morphology and size using JEOL JSM-6390LV and FT-IR was done using Perkin Elmer-Spectrum 100 FT-IR Spectrometer. The photoluminescence (PL) and photoluminescence excitation (PLE) and emission spectra were measured by using a spectrophotometer (SHIMADZU, RF5301). The Commission Internationaledel’Echairage (CIE) Chromaticity coordinates were calculated from the emission spectra of the phosphor samples using Radiant Imaging Software version 2.0 [26]. All the measurements were performed at room temperature.
3 Results and discussion
3.1 XRD studies
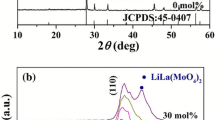
The crystal structure and phase purity of the Li2CaSiO4 base phosphor with Eu3+ (0.20 wt) concentration were studied by XRD are shown in Fig. 1a respectively. Li2CaSiO4 crystallizes in the tetragonal I-42 m space group. The structure is three dimensional. Li1+ is bonded to four equivalent O2− atoms to form distorted LiO4 trigonal pyramids that share corners with four equivalent SiO4 tetrahedra and corners with four equivalent LiO4 trigonal pyramids. All Li–O bond lengths are 1.98 Å. Ca2+ is bonded in eight coordinate geometry to eight equivalent O2− atoms. There are four shorter (2.40 Å) and four longer (2.76 Å) Ca–O bond lengths. Si4+ is bonded to four equivalent O2− atoms to form SiO4 tetrahedra that share corners with eight equivalent LiO4 trigonal pyramids. All Si–Obond lengths are 1.66 Å. O2− is bonded in a 3-coordinate geometry to two equivalent Li1+, two equivalent Ca2+, and one Si4+ atom [27]. All the diffraction peaks are indexed by JCPDS Card No. 027–290 (Li2CaSiO4, tetragonal, space group I-42 m). No second phase even at the trace level was found. These results indicate doping of Eu3+ in Li2CaSiO4 up to 0.20 wt concentrations do not influence the crystallinity of the host. When we compare the base and doped XRD pattern of the prepared phosphor, there is no variation found in the peak position. So, the impurity ions are not change the crystal structure. The crystal structure of the Li2CaSiO4 compound may be regarded as columns of SiO4 tetrahedra and CaO8 dodecahedra; the columns form a three-dimensional network, enclosing channels, parallel to (001), that contain lithium. Insertion of cerium takes place in the eight fold coordinated calcium site [28]. One can notice that there are two groups of Ca–O lengths, one at 2.68 Å, another at 2.40 Å. The dodecahedron site has D2d symmetry (Fig. 1b).
Here, in the Li2CaSiO4 host, Eu is expected to occupy the Ca site. The Ca atom in this site is coordinated to eight oxygen atoms which form the crystal surroundings and crystal field strength for Eu doping ions [29]. The excitation peaks in the UV–Vis range of the Li2CaSiO4:Eu3+ phosphors show sharp and strong peaks which are observed in the range 360–470 nm. The peak intensity increases with Eu3+ concentration which indicates that they originated from the 4f7→4f7 electronic transition [30]. With the perspective of UV chip based white LEDs application, the excitation energy of these phosphors qualify them as potential phosphor candidates since the excitation spectra confirm that they can be effectively excited by typical near ultraviolet LED chips.
3.2 SEM studies
Figure 2(a–c) shows the SEM images taken under different resolutions of the Eu3+ (0.20 wt) doped Li2CaSiO4 phosphor. From the images, the shape and size of the synthesized phosphors are studied. Figure shows non-homogeneous morphology and the relatively big grain size [31]. The surface morphology of the powders is irregular, which shows a grain growth tendency an agglomeration at higher temperatures. The grain size distribution is broad and the average size is about 1 μm to 100 nm. Aggregations are due to high temperatures involved in the synthesis of phosphor materials. Aggregation can cause strong light scattering and decrease luminescence efficiency. In order to break up the aggregation, long ball milling step is necessary to improve the quality of phosphors to make them suitable for use in solid state lighting.
3.3 FTIR studies
Figures 3 and 4 are the FTIR spectra of Li2CaSiO4 base phosphor and Li2CaSiO4:Eu3+ (0.2 wt) phosphors respectively. The FTIR technique is to measure the absorption of various infrared radiations by the target material, to produce an IR spectrum that can be used to identify functional groups and molecular structure in the phosphor. From the Fig. 3, it is observed that most of the bands are due to ligand ions (Ca–O, Si–O, Li–O) stretching or bending vibrations. The peak at 3441.4 cm−1 is due to single bond stretching vibrations between O–H and the peaks at 1418.8, 1097.80 and 870.96 cm−1 are lie in the finger print region. From the Fig. 4, it is observed that, when compared to both spectra, the spectrum of activator ion doped have more peaks in the finger print region belongs to ligand and metal ions (Ca–O, Eu–O) stretching or bending vibrations. The peak at 3441.4 cm−1 is due to single bond stretching vibrations between O–H and peaks at 1431.8, 1052.21, 941.24, 862.69 and 741.23cm−1 lie in the finger print region. As per the literature no researchers present the FTIR spectrum of this phosphor.
3.4 Photoluminescence studies
The photoluminescence excitation spectrum of Li2CaSiO4:Eu3+phosphor with fixed concentration (0.20 wt) is presented (Fig. 5). From the figure, the phosphor show excitation peaks at 271, 323, 361, 394 and 464 nm, which are monitored at 616 nm wavelength. Several sharp peaks are seen in the excitation spectrum along with a broad one at 271 nm. The broad one is due to charge transfer (CT band) between O2− and Eu3+. The sharp peaks are due to the intra 4f transitions of Eu3+ ions from the 7F0 level to 5H6, 5D4, 5G2, 5G3, 5L6, 5D4 and 5D2 levels respectively. Among these peaks, the peak at 394 nm (7F0→5L6) is a sharp one having high intensity. This is well matched with UV chip for the generation of white light available in the market [32]. The 464 nm excitation peak is also useful in the generation of white light and is well matched with the blue LED chip that is available in the market.
The full width at half maximum (FWHM) is much smaller than ordinary phosphors like YAG:Ce3+. Based on previous study, such a narrow emission with small FWHM is beneficial for high luminous output as they are applied in white LEDs [33]. The emission spectra show two main emission bands with good intensity at 594 (5D0 → 7F1) and 616 nm ((5D0 → 7F2) which are electrical and magnetic dipole moments of the Eu3+ ion due to the site symmetry. No concentration quenching of Eu3+is observed. The phosphors show the highest emission intensity at 0.20 wt concentration under 394 nm excitation wavelength (see in Fig. 6). The excitation spectra were monitored at emission wavelength of 616 nm. The excitation spectra clearly show that these phosphors have strong excitation in UV–Vis wavelength range (370–450 nm) with the maximum excitation intensity at 394 nm. These results agree well with the previous reported ones.
The influence of the Ca ion in the host material on the photoluminescence excitation and emission was studied by comparing their spectra. With different Eu3+ concentration, the excitation wavelength peaks are same, but the emission peak wavelengths are slightly changed, while the emission intensity varies a lot. This indicates that the crystal field strength in the host is relatively stable and is not influenced by doping of Eu3+. The emission intensity increases with increasing ‘Eu’ concentration. The highest emission intensity occurs when the excitation wavelength is 394 nm. This comparative study suggests that these phosphors would have the best performance with 0.20 wt of Eu3+ doping at 394 nm excitation wavelength (Fig. 7). The emission and excitation properties indicate that the phosphors can be effectively excited by ultra-violet LED chip (394 nm), if they are used as red phosphors in multi-phosphors converted into white LEDs. The PL intensity increases with increasing concentration of doping ions.
Figure 5 is the excitation spectrum of Li2CaSiO4:Eu3+ monitored at 616 nm, the following excitations are found 271, 323, 361, 394 and 464 nm having the intensities are 110, 20, 15, 60, and 42. The excitation spectrum of Li2CaSiO4:Eu3+ phosphor recorded by monitoring the 5D0→7F2 transition of Eu3+ at 616 nm. In excitation spectrum, a broad peak at 271 nm followed by some narrow peaks observed which are originating from the 7F0→5D4 (361 nm) and 7F0→5L6 (394 nm). The maximum intensity of the absorption spectrum was determined to be at 271, 323 and 394 nm. The excitation spectrum contains an intense broad band with a maximum at 271 nm (Fig. 5) arising from the charge transfer band (CTB) between Eu3+ and the neighbouring O2– and a group of lines in the longer wavelength region due to the f-f transitions within Eu3+ 4f6 configuration. The prominent excitation band observed at 394 nm (NUV) due to the transition of Eu3+ (7F0→5L6) and this clearly indicates that Li2CaSiO4:Eu3+ phosphors are effectively excited by near ultraviolet light emitting diodes (NUV–LEDs) also [34, 35].
The emission spectra of phosphors were recorded by excitations with 271 and 394 nm. The emission spectra are shown in Fig. 6 which is composed of 5D0→7FJ (J = 1, 2, and 4) emission lines of Eu3+. In general, when the Eu3+ ion is located at crystallographic site without inversion symmetry, its hypersensitive forced electric-dipole transition 5D0→7F2 red emission dominates in the emission spectrum. If the Eu3+ site possesses an inversion center, then 5D0–7F1 orange emission is dominant [36]. The distinct emission lines lying between 580 and 650 nm are observed due to transitions from excited 5D0 to the 7FJ (J = 0–3) levels of Eu3+ ions. The origin of these transitions (electric dipole or magnetic dipole) from emitting levels to terminating levels depend upon the location of Eu3+ ion in Li2CaSiO4 lattice and the type of transition is determined by selection rule. The most intense peak in the vicinity of 594 nm is ascribed to the magnetic dipole transition of 5D0 and 7F1 levels. The weak emission at 616 nm corresponds to the hypersensitive transition between the 5D0 and 7F2 levels due to forced electric dipole transition mechanism. Figure 8 shows the PL emission spectra of Eu3+ doped Li2CaSiO4 phosphors. It is found that at 394 nm excitation, spectra found the maximum intense peak. So, 394 nm excitation was chosen for further excitation.
Emission spectra consists of emission peaks in the range 550–650 nm, which result from 5D0→7FJ (J = 1, 2) transitions of Eu3+ ion, respectively. For an excitation wavelength of 394 nm, the emission spectra of Li2CaSiO4:Eu3+ consists of sharp emission lines at 594 and, 616 nm. The emission at 594 nm originates from the allowed magnetic dipole (MD) transition 5D0→7F1 which is having highest intensity. The peaks observed at 616 nm are due to the electric dipole 5D0→7F2 transition has double the intensity when compared to highest emission intensity peak which is at 594 nm. The ratio of 616 nm peak intensity to 594 nm peak is half. This result is interesting when compared the earlier work where the 594:612 nm peak intensities are nearly same. From the emission spectra, it is clearly observed that the emission intensity of magnetic dipole was lower than that of electric dipole transition due to Eu3+ ions occupy a lower symmetry site in Li2CaSiO4 host [37, 38]. Both magnetic dipole transition and electric dipole transitions are shown in the emission spectra. If the magnetic dipole transition 5D0→7F1 having the highest intensity, then Eu3+ ions in host lattice occupies an inversion centre. If the emission intensity of magnetic dipole transition was lower than that of electric dipole transition, which indicates that Eu3+ ions occupied without an inversion symmetric centres in the host. The increasing concentrations of Eu3+ ions have no change on the peak position of the emission spectra. The concentration quenching occurs at 0.20 wt of Eu3+ ion concentration. This concentration quenching was associated with the energy transfer which occurs from one Eu3+ ions to other ions and finally quenches the emission intensity. The results indicate that Li2CaSiO4:Eu3+ (0.20 wt) phosphor can be selected as a potential candidate for LED (Light Emitting Diode) application (Ex. 394 nm) [39, 40]. When we compare our results with Eu3+ activated SrY2O4 phosphor, the band near 254 nm is known to be a charge transfer (CT) process that is related to the excitation of an electron from the oxygen 2p state to Eu3+ 4f state [19, 41]. It shows the emission spectra in deep red region with excitation of 254 nm which touches the white light boundary and applicable for WLEDs [42]. At the same time YBO3 doped phosphor shows orange emission and useful for plasma display panels [43], but in this study phosphor for multi-phosphors converted white LEDs, e.g. red/green/blue phosphors covered and UV chip based white LEDs.
Figure 9 shows the various transitions of Eu3+ doped Li2CaSiO4 phosphor. The detailed effect of dopant on the structure evolution resulted in higher performance is expressed in detail in the form of band diagram to highlight the beneficial effect of the proposed method superior to others.
3.5 CIE studies
The Commission International del’Echairage (CIE) chromaticity coordination (x, y) coordinates were estimated from the emission spectra of the Li2CaSiO4:Eu (0.01 to 0.20 wt) phosphor CIE Chromaticity coordinates (0.68, 0.31) and (0.60, 0.39), (0.60, 0.38) indicated orange/red color are shown in Fig. 10. It is suggested that Li2CaSiO4:Eu2+ could be used as red phosphor for white LEDs. However, in our present study, the value of Li2CaSiO4:Eu3+ has an emission band peak at about 616 nm with a narrow bandwidth (FWHM is about 5 nm). It is clear from the CIE chromaticity diagram, the spectra lies in the red region with little orange ingredient. Therefore, the y value should be much smaller than 0.31. We have calculated the CIE chromaticity of Li2CaSiO4:Eu (0.01–0.20 wt) from its emission spectrum under the excitation of 394 nm and its final value was (0.68, 0.31). These are close to the reported by Mubiano Xie [24]. Therefore, we here propose that this should be used as red phosphor for multi-phosphors converted white LEDs, e.g. red/green/blue phosphors covered and UV chip based white LEDs [38, 39].
4 Conclusions
Eu3+ doped Li2CaSiO4 single phase phosphors were successfully synthesized by the solid state reaction method. Surface morphology of the powders showed that they are irregular and non-homogeneous. From the FTIR, it is observed that most of the bands are due to ligand ions (Ca–O, Si–O, Li–O) stretching or bending vibrations. The photoluminescence properties including the UV–Vis absorption, PLE and the CIE chromaticity coordinates of these phosphors were investigated thoroughly. The PLE spectra indicate that the Li2CaSiO4:Eu3+ can be effectively excited by near ultra-violet light which guarantees their application in UV-chip pumped white LEDs. Eu3+ doped into the Li2CaSiO4 host shows strong red emission band with the peak position at 616 nm. Based on the emission and CIE chromaticity coordination of (0.68, 0.31), we have proposed that this kind of phosphor can be used in red/blue/green phosphors and UV chip (394 nm) composed white LEDs. White light emission by Eu3+ doped Li2CaSiO4 phosphors was attempted and their photoluminescence properties were studied. These compound phosphors show red emission band and their CIE chromaticity coordinates varies slightly under 394 nm excitation wavelength. This shows the potential phosphor candidate in ultra-violet chip and multi-phosphors composed white LEDs.
Data availability
Data is available on request.
References
S. Nakamura, T. Mukai, M. Senoh, Appl. Phys. Lett. 64, 1687–1689 (1994)
M.G. Craford, Commerical Light Emitting Diode Technology (Kluwer Academic Publishers, Dordrecht, 1996)
L.S. Rohwer, A.M. Srivastava, Electrochem. Soc. Interf. 12, 36–39 (2003)
J.K. Park, J.T. Park, S.Y. Choi, Appl. Phys. Lett. 82, 683–685 (2003)
J.S. Kim, Y.H. Park, J.C. Choi, H.L. Park, J. Electrochem. Soc. 152, 135-H137 (2003)
H. He, R.L. Fu, X.F. Song, D.L. Wang, J.K. Chen, J. Lumin. 128, 489–493 (2008)
H. He, R.L. Fu, X.L. Zhang, X.F. Song, X.R. Zhao, Z.W. Pan, J. Mater. Sci. Mater. Electron. 20, 433–436 (2009)
J.K. Park, C.H. Kim, S.H. Park, H.D. Park, Appl. Phys. Lett. 84, 1647–1649 (2004)
J.K. Park, K.J. Choi, J.H. Yeon, S.J. Lee, C.H. Kim, Appl. Phys. Lett. 88, 043511 (2006)
S.D. Jee, J.K. Park, S.H. Lee, J. Mater. Sci. 41, 3139–3141 (2006)
H.S. Jang, Y.H. Won, S. Vaidyanathan, D.H. Kim, D.Y. Jeon, J. Electrochem. Soc. 156, 138–142 (2009)
J.S. Kim, P.E. Jeon, J.C. Choi, H.L. Park, Appl. Phys. Lett. 84, 2931–2933 (2004)
J.S. Kim, P.E. Jeon, Y.H. Park, J.C. Choi, H.L. Park, G.C. Kim, T.W. Kim, Appl. Phys. Lett. 85, 3696–3698 (2004)
J.S. Kim, A.K. Kwon, Y.H. Park, J.C. Choi, H.L. Park, G.C. Kim, J. Lumin. 122, 583–586 (2007)
K. Tada, Y. Kawakami, S. Kousaka, Y. Ito, A. Komeno, K. Uematsu, M. Sato, IEICE Trans. Electron. E89–C, 1406–1412 (2006)
M. Saradhi, U.V. Varadaraju, Chem. Mater. 18, 5267–5272 (2006)
X.L. Zhang, H. He, Z.S. Li, T. Yu, Z. Zou, J. Lumin. 128, 1876–1879 (2008)
H. He, R.L. Fu, D.L. Wang, X.F. Song, Z.W. Pan, X.R. Zhao, X.L. Zhang, Y.G. Cao, J. Mater. Res. 23, 3288–3291 (2008)
C. Kulshreshtha, A.K. Sharma, K.S. Sohn, J. Electrochem. Soc. 156, J52–J56 (2009)
J. Liu, J. Sun, C. Shi, Mater. Lett. 60(23), 2830–2833 (2006)
M. Avdeev, Z. Mohamed, C.D. Ling, J. Solid State Chem. 216, 42–48 (2014)
J. Zhang, W. Zhang, Z. Qiu et al., J. Alloys Compd. 646, 315–320 (2015)
Z. Wei, Y. Wang, X. Zhu et al., Chem. Phys. Lett. 648, 8–12 (2016)
Q. Wu, Q. Zhao, P. Zheng, W. Chen, D. Xiang, Z. He, J. Zhou, Ceram. Int. 46(3), 2845–2852 (2020)
E. Erdogmus, I. Pekgozlu, E. Korkmaz, Spectrosc. 29(3), 58–61 (2014)
R.W.G. Hunt, The Reproduction of Color in Photography, Printing and Television (Fountain Press, UK, 1987)
The Materials Project, Materials data on Li2CaSiO4 by materials project, United States: N. Web (2020). https://doi.org/10.17188/1291732
L. Pierron, A. Kahn-Harari, B. Viana, P. Dorenbos, C.W.E. Van Eijk, J. Phys. Chem. Solids. 64(9–10), 1743–1747 (2003)
J.A. Gard, A.R. West, J. Solid State Chem. 7, 422–427 (1973)
G. Blass, B.C. Grabmaier, Luminescent Materials (Springer, Berlin, 1994)
L. Zhou, P. Du, L. Li, Sci Rep. Nat. Res. 10, 20180 (2020)
M. Xie, T. Song, ECS J. Solid State Sci. Tech. 2(1), R29–R32 (2013)
T. Justel, H. Nikol, C. Ronda, Angew Chem. Int. Ed. Engl. 37, 3084–3103 (1998)
G.R. Dillip, S.J. Dhoble, L. Manoj, C.M. Reddy, B.D. Raju, J. Lumin. 132, 3072–3076 (2012)
K.N. Shinde, S.J. Dhoble, A. Kumar, J. Rare Earths. 29, 527–535 (2011)
K.G. Lee, B.Y. Yu, C.H. Pyun, S.I. Mho, Solid State Commun. 122, 485–488 (2002)
S. Shigeo, M. William, P. Handbook (CRC Press, Washington, 1998)
S.Z. Lu, J.S. Zhang, J. Lumin. 122, 500–502 (2007)
C. Kumari, J. Manam, S.K. Sharma, Mater. Sci. Semicond. Proc. 158, 107385 (2023)
M. Monisha, M.S. Murari, M.I. Sayyed, K. Naregundi, N.A. Harbi, S.D. Kamath, J. Non- Cryst. Solids. 599, 121971 (2023)
N.S. Kuriyan, M. Sabeena, J. Lumin. 249, 119038 (2022)
V. Dubey, J. Kaur, S. Agrawal, Mater. Sci. Semicond. Proc. 31, 27–37 (2015)
V. Dubey, J. Kaur, S. Agrawal, N.S. Suryanarayana, K.V.R. Murthy, Superlattices Microstruct 67, 156–171 (2014)
Funding
There are no funding sources for this article work.
Author information
Authors and Affiliations
Contributions
MPDP: She is main author and prepare sample for the study. MCR: He is guide of MPD Primala and analyzed structural analysis of materials and data interpretation. VD: Analysis and interpretation of PL studies and CIE technique. KVRM: Help for data recording and interpretation of morphological studies and IR spectrum.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Parimala, M.P.D., Rao, M.C., Murthy, K.V.R. et al. Structural and photoluminescence studies of lithium calcium silicate phosphor doped with Eu3+ ion for LED application. J Mater Sci: Mater Electron 34, 1486 (2023). https://doi.org/10.1007/s10854-023-10904-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10854-023-10904-x