Abstract
The existing research explores the effect of annealing temperature on manganese oxide incorporated cobalt oxide thin films synthesized on stainless steel (SS) using potentiodynamic electrodeposition via aqueous route. Film thickness of synthesized material decreases with increase in annealing temperature. Contact angle measurements show decrease in the hydrophilicity with increase in annealing temperature. XRD of the synthesized samples reveals face centered cubic Co3O4 and orthorhombic MnO2 with polycrystalline nature. FESEM and TEM images display nano-spikes with nano-granular morphology. The presence of elemental composition was observed from EDX analysis. The AFM analysis also confirms the nano-granular morphology of fabricated thin film electrodes. The optimized electrodes were investigated for electrochemical characteristics. All CV curves show mixed capacitive behavior. Optimized sample shows maximum specific capacitance (SC) of 605.82 F/g at 2 mV/s using 1 M KOH electrolyte solution. The maximum designed values of energy density, power density and columbic efficiency were 25.20 Wh/kg and 31.10 kW/kg and 92.11% respectively. The observed internal resistance from Nyquist plot (Ri) was 0.64 Ω. The MnO2 incorporated Co3O4 thin films on SS substrates can be a potential candidate for cost-effective supercapacitor applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Now-a-days, whole the world is concerned about increasing environmental pollution, the depletion of fossil fuels and infectivity world which drastically affect socio-ecological systems on the earth. To surmount these problems researchers are developing a battery-supercapacitor hybrid device (BSH) for hybrid electric vehicles. Supercapacitor electrodes are fabricated with various morphologies using a variety of techniques [1,2,3,4,5,6]. The transition metal oxides (TMOs), have been proven as suitable electrode materials for pseudo-capacitor [7,8,9,10].
Amongst all these TMOs, Co3O4 and Mn3O4/MnO2 are easy and economical to prepare, it shows porous morphology, good chemical and physical stability with high specific capacitance [11]. Some researchers have reported porous/3D architecture, nano flowers, nanowires, flakes and spherical grains of Co3O4 and Mn3O4 using different techniques via aqueous routes [12,13,14,15,16,17,18,19,20]. Many authors reported the electrochemical measurements of cobalt oxide and manganese oxide synthesized via aqueous route using different methods [21,22,23]. Some investigators described the electrochemical measurements of manganese oxide prepared via aqueous route using different methods [15, 24, 25].
Pang et al. described the preparation and electrochemical investigation of cobalt oxide–manganese oxide composite which has a maximum specific capacitance (SC) 412 F/g [26]. Jun Chen et al. reported CoMn(CoMn)2O4 coated on reduced graphene oxide. Corresponding electrode exhibits 571 F/g SC at current density 1 A/g [27]. The details of related literature were studied and the comparison results are reported in Table 1 [28,29,30,31,32,33,34,35,36,37,38,39,40,41]. As per the available literature, there are least evidences found regarding the synthesis of MnO2:Co3O4 thin films by potentiodyanamic electrodeposition and their electrochemical applications which cognate to supercapacitor applications.
The present work focuses on synthesizing manganese incorporated cobalt oxide by potentiodynamic electrodeposition technique and exploring the outcome of annealing temperature on structural, physical and electrochemical parameters. The prepared samples were well characterized using sophisticated tools.
2 Experimental
2.1 Electrodes synthesis
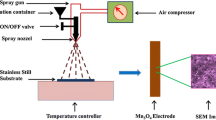
Figure 1 reports details of the synthesis of Mn incorporated cobalt oxide thin films carried out via potentiodynamic electrodeposition technique. Cobalt chloride hexa-hydrate and manganese chloride having AR grade were purchased from Thomas baker chemicals and used without any further purification. 0.1 M solutions of both manganese chloride and cobalt chloride were prepared in double distilled water. The working electrode is 1% Mn incorporated cobalt chloride solution, samples were deposited on SS substrate and counter electrode as a Platinum (Pt) wire and silver/silver chloride as a reference electrode. The potentiostat (CHI 600 D workstation) was used for the potentiodynamic electrodeposition. Deposition was carried out by exploiting cyclic voltammetry range from −1 to −0.1 V with respect to Ag/AgCl reference electrode at the scan rate 80 mV/s for 30 min. As the Annealing temperature is a key factor which may influence structure, morphology, component and mass change of the electrode, the deposited samples were subjected to different annealing temperatures to investigate its effect on crystallinity, surface morphology and electrochemical performance. The different annealing temperatures 473, 523, 573, 623, and 673 K were nomenclature as MAT1, MAT2, MAT3, MAT4 and MAT5. The mass loadings for MAT1- MAT5 electrodes are 0.0034, 0.00314, 0.00327, 0.00245 and 0.00236 gm respectively and the area of deposited material is 1.5 × 1.5 cm2. Prepared samples were characterized using XRD, FESEM, EDX, TEM and AFM and cyclic voltammetry to check their structural, morphological and electrochemical properties.
2.2 Characterizations
2.2.1 Physical characterizations
The diffractometer of Rigaku D/max 2550 Vb + 18 kW with Cu kα wavelength was used instrument in the 20°–80° range. Surface studies was done using FESEM (JEOL JSM 7600 FEG-SE). An elemental diffraction analysis (EDX) was carried out using AXFORD-SUK (Japan) at 20 kV to measure the percentage content of manganese incorporated in cobalt oxide. TEM and 2D-AFM images for topographical roughness of prepared samples. The contact angle measurement was carried out using HO-IAD-CAM-01B, Holmark Opto-Mechatronics, India. Tapson-100TS, USA analytical microbalance was used to find the weight of the deposited material.
2.2.2 Electrochemical characterizations
The CVs of the prepared electrodes were scanned at room temperature in the potential window −0.95 to 0.48 V Vs silver/silver chloride in 100 mM, 20 ml potassium hydroxide at the scan rate 2 mV/s. The SC of working electrodes was estimated with the assistance of CV [42].
where, SC is the specific capacitance, V is the scan rate (mV/s), I represents the current (mA), and ‘m’ denotes active mass on working electrode, td represents discharging time. The energy density, power density and coulombic efficiency (η) were intended.
Here, ‘V’ represents voltage, ‘Id’ denotes current and ‘td’ is discharge time. ‘W’ is the weight and ‘tc’ is the charging time.
3 Results and discussion
3.1 Chemical reaction kinetics
The manganese incorporated cobalt oxide thin films were potentio-dynamically electrodeposited at the scan rate 80 mV/s for 30 min. using −1.05 to −0.15 V Vs silver/silver chloride deposition window. During the electrodeposition, the cobalt and manganese ions or complexes are hydrolyzed by the distilled water solution to form the hydroxide on the working electrode. After annealing at various temperature (K) for 1.5 h, the Co(OH)2 and Mn(OH)2 was converted into Co3O4 and MnO2 respectively. The possible chemical reactions in the film formation are as given below [43]. After annealing at 473 K Mn(OH)2 gets converted into MnO2.
During electrochemical characterizations following reactions may occur
During electrochemical reaction the nano-composite oxide materials interact with K+ and OH− ion and form hydroxide and oxyhydroxide phases during the electrochemical reaction.
3.2 Thickness variation
Thicknesses of the samples were evaluated using gravimetric weight difference method. Figure 2 shows the variation in the film thickness for the samples from MAT1 to MAT5. From the figure, it was observed that film thickness decreases with increase in annealing temperature. It may be due to the loss of hydrated content in the film along with an increase in annealing temperature. The similar effect of various temperatures was described for Co3O4 thin films synthesized by spray pyrolysis [44].
3.3 XRD studies
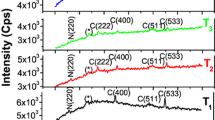
Figure 3 XRDs of MAT1, MAT2, MAT3, MAT4 and MAT5. The observed ‘d’ values were matched with standard ‘d’ values taken from JCPDS of Co3O4 and MnO2. All samples are polycrystalline in nature. Co3O4 samples exhibit characteristic peaks (220), (311), (400), (331), (440) and (533) at 31.18°, 36.46°, 44.23°, 47.40°, 65.82° and 75.15°. MnO2, samples exhibit characteristic peaks (101), (201), (210), (211), (202), (331) and (410) at 21.16°, 26.57°, 36.40°, 41.76°, 44.00°, 47.40° and 50.52°. Samples show strong orientations along (400) for Co3O4 or (202) for MnO2 for low temperature annealing and for high temperature annealing, orientations change strongly. Sample MAT4 shows strong orientation (211) for MnO2 and sample MAT5 shows strong orientation (311) for Co3O4. The calculated average value of crystallite size ‘D’ was around 18 nm for the plane (400) of Co3O4/(202) of MnO2. XRD reveals increase in crystallinity with increase in the annealing temperature. In the fluence of incorporating Mn elements in the Co system may results the more crystalline nature. The similar types of observations were reported for cobalt oxide thin films [45,46,47]. It may be at high annealing temperature, material gets enough energy to crystallize to orient in proper equilibrium sites, resulting in the upgrade of crystallinity and the degree of direction of the cobalt oxide films [48]. The calculated value of the crystallite size ‘D’ for plane (400) using Scherer’s formula and it is around 21 nm.
3.4 FE-SEM, EDX analysis
Figure 4a depicts FE-SEM images of manganese incorporated cobalt oxide samples annealed at various temperatures. Sample shows porous granular particles along with nano-spikes/nanorods at lower temperature. Inset of the image for sample MAT1 clearly shows the porous nature of the film at higher magnification. Such morphology possesses high surface area which can be structural base for electrochemical process [49]. But, with increase in annealing temperature particles are agglomerated to form clusters and nano-spikes/nanorods get disturbed. Porosity of the film drastically gets reduced at higher annealing temperature and it shows compact morphology. The elemental analysis of the MAT1 sample was carried out by using elemental diffraction analysis. Figure 4b shows the elemental mapping of MAT1 sample. From the figure it is confirmed that the reported elements are present in the elemental mapping. The contribution of elements for weight percent of Oxygen:18.46; Manganese:1.32; and Cobalt:80.22 and atomic percent contribution are Oxygen:45.44; Manganese:0.95; and Cobalt:53.61 etc.
3.5 TEM, SAED analysis
The micro-structural analysis of MAT1 electrode was made by using transmission electron microscopy. Figure 5a and b represent as and magnified TEM of MAT1 sample. It shows formation of granular type of grains corresponding to pure cobalt oxide, along with spikes originated from incorporation of Mn into the pure Co3O4 with average spike length − 3.00 × 102 nm. Similar nano-spikes/nanorods of MnO2 were reported by Kim et al. at higher magnifications in cobalt doped manganese oxide thin films [50]. Figure 5c shows the SAED pattern of Mn incorporated Co3O4 which confirms crystalline nature of the sample MAT1.
3.6 AFM analysis
Irregularity of the sample has single importance in the super-capacitive performance as it supports the ion exchange progression (faradaic reaction) in oxide material [51]. Figure 6 depicts 2D AFM micrograph of the sample MAT1. Micrograph reveals rough agglomerate granular surface morphology with roughness 111 nm. Figure 7.shows contact angle variation for Mn incorporated cobalt oxide thin films annealed at different annealing temperatures. This type of morphological roughness is highly suitable for easy ionic intercalation benefits for better supercapacitive application. The water contact angle depends on the morphology, surface roughness, and chemical properties of the material. In the present case it was observed that contact angle shows decrement in its value and hence decrement the hydrophilicity with increase in annealing temperature. Sample MAT1 shows super-hydrophilic nature which is feasible for supercapacitor application.
3.7 Electrochemical characterizations
3.7.1 Cyclic voltammetry (CV)
The CV curves for MAT1 to MAT5 electrodes were recorded as in Fig. 8a. From the CV curves, it was evidenced that as the annealing temperature increases, the area under CV curves decreases. Change in SC of the different annealed electrodes of MAT1, MAT2, MAT3, MAT4 and MAT5 were estimated to be 605.82, 431.52, 298.53, 218.36 and 214.58 F/g. The maximum calculated SC of 605.82 F/g was obtained for sample MAT1 which was decreased to 214.58 F/g for the sample MAT5. At lower annealing temperature, electrode shows maximum SC, it may be due to easy ionic intercalation. As evident from TEM, influence of MnO2 impurities i.e. porous spikes, hydrophilic nature observed from wettability study may be responsible for increase in surface area responsible to increases the SC as compared to un-doped cobalt oxide electrode.
a CVs of CM1 electrode annealed at different temperatures and scanned in 1 M KOH (CM1: 1% Mn incorporated cobalt oxide electrode, MAT1 to MAT5: 473 K to 673 K with the interval of 50 K), b CVs of MAT1 electrode observed at different scan rates in 1 M KOH (MAT1: 1% manganese doped Co3O4electrode annealed at 473 K), c CVs of MAT1 electrode in different electrolytes (MAT1: 1%), d CVs of MAT1 electrode observed in different concentrations of KOH. e Electrochemical stability curve of MAT1 electrode scanned in 1 M KOH at 100 mV/s scan rate
3.7.2 Effect of scan rate
To observe the effect of scan rate on electrochemical performance of the optimized electrode MAT1, CV was recorded at various scan rates from 2 to 100 mV/s. It was clearly observed that, area under the curve increases with increase in scan rate, at the same time peak was shifted towards negative potential as evidenced from Fig. 8b. The obtained SC values for different scan rates 2, 5, 10, 50 and 100 mV/s are 605.82, 312.09, 308.37, 196.56 and 144.67 F/g. The calculated maximum value of SC was 605.82 F/g at 2 mV/s. As the scan rate increases, SC value decreases. This may be attributed due to the improper ionic intercalation at higher scan rates which affects the charge storing performance of the electrode. At higher scan rates, accessibility of the ions inflowing into all the pores inside the electrode materials rises and thus the transportation of ions was partial due to their slow diffusion. Hence individual the external surface could be exploited for the charge storing [52, 53]. The CV curves of MAT1 electrode. In both cases it is possible to observe that the presence of redox peaks that can be assigned to CoOx-related redox reactions. For MAT1 electrode the redox peaks can be attributed to the transition Co(II)/Co(III) in the film, which is accompanied by insertion/de-insertion of hydroxyl ions, in order to preserve the electroneutrality of the material during the redox process [54]. It is observed that, with an increase in scan rate from 2 to 100 mV/s, the area under the curve and potential window increases up to − 1.6 V. A reduction reaction must be occurring causes H2 evolution. A similar result was reported by R. Della Noce et al. [55].
3.7.3 Effect of electrolytes and electrolytic concentration
To see the suitability of electrolyte for electrochemical performance, MAT1 electrode was analyzed in different aqueous inorganic 1 M electrolytes such as KCl, Na2SO4 and KOH etc. Figure 8c shows typical CVs of MAT1 electrode in 1 M solutions of KCl, Na2SO4 and KOH respectively. In all electrolytes, electrode shows redox peaks at the particular anodic and cathodic sweeps of CV curves endorses for the mixed-capacitive nature. The calculated values of SC at 2 mV/s in 1 M KOH, Na2SO4,and KCl electrolytes are 605.82, 57.56, and 3.66 F/g respectively. Electrode tested in 1 M KOH electrolyte shows maximum SC, which may be attributed due to easy ionic redox activity of aqueous KOH solution with high mobility of K+/OH− ions which supports maximum charge storage in the EDL at the electrode—electrolyte boundary and ion diffusion in the host material [22]. One of the most important properties of electrolytic solution is the effective ion size. The size of ions determines the accessibility of the pores to ions and influences on their mobility in the solution bulk. It has been reported [56]. Figure 8d shows the CV curves of MAT1 electrode carried in different concentrations of KOH. It was evidenced from the area under curve that at lower (less than 1 M) and higher (more than 1 M) concentrations of the electrolyte, capacitive performance was poor. Figure 8e Electrochemical stability curve of MAT1 electrode scanned in 1 M KOH at 100 mV/s scan rate. Form the figure it is observed that initially the value of SC is 144.67 F/g. With increase in number of cycles for same scan rate current density goes on decrease exponentially. After completion of 2500 number of cycles, the calculated SC is nearly about 140.10 F/g. Further increase in number of cycles the current density and SC associated with materials remains steady. The similar result has been reported [35]. The decrease in SC may be attributed to reduction in pore size and line spacing as evidenced from SEM due to intercalation of potassium ion and showing stable SC beyond 2500 cycles. These results can be due to the different mechanism for storing charge. Assuming that the main storage mechanism for MAT1 electrode involves bulk redox reactions, the shorter life cycle could be due to irreversible phase changes due to material pseudocapacitive nature that hinder the faradaic process since the continuous K + /OH − insertion/de-insertion will gradually affect the film structure [54].
3.7.4 Charge–discharge
Charge–discharge technique is used to understand the energy and power application of the prepared optimized electrode. Figure 9 of MAT1 electrode scanned at various current densities 10, 15, 20 mA/cm2 in 1 M KOH. From the figure it is observed that with increase in current density curve shifted towards the triangular side itself indicates the double layer behavior and the prepared electrode material itself indicates the power efficient at higher current density. And at lower current density material shows pseudocapacitive behavior itself represents energy efficient. The electric parameters associated with MAT1 electrode was calculated. The SC associated with electrode has been calculated it is − 590.0 F/g at current density 10 mA/cm2. The maximum calculated values of SE, SP and η were 25.20 Wh/kg and 31.10 kW/kg and 92.11% respectively.
3.7.5 Electrochemical impedance
The internal resistance of MAT1 electrode was studied using EIS at OCP—0.084103 V in 1 M KOH in the frequency 1 to 1 mHz. Figure 10 shows the Nyquist impedance plot of Z″vs Z′. Nyquist Plot composed of three regions, at maximum frequency region a depressed semicircle results from a parallel combination of the charge-transfer resistance (Rct) produced by the Faradaic reactions at the minimum frequency region. The observed internal resistance (Ri) is 0.64 Ω. The knee frequency (6.21 × 10−4 Hz with phase angle 24.4°) which reflect the extreme frequency at which capacitive behavior is govern. Portion of the curve having an inclination of − 45° with Z″ axis gives the value of Warburg resistance (Zw). The minimum frequency behavior shifting near ‘Y’ axis signified double layer capacitance (\({C}_{dl}\)).
4 Conclusions
Present work reports the successful synthesis of MnO2 incorporated cobalt oxide electrode using potentiodynamic electrodeposition technique. XRD reveals the FCC and orthorhombic crystal structures for cobalt oxide and manganese oxide respectively. Nano-spike type morphology observed from FESEM. The presence of elemental composition was observed from EDX analysis. TEM study and it signified the incorporation of MnO2 in cobalt oxide matrix and explored positive effect on the super capacitive effect of Co3O4 electrode. Porosity of the film drastically gets reduced at higher annealing temperature implies lower annealing temperature provides excellent capacitive behavior. Optimized electrode scanned in1M KOH electrolyte exhibited high SC 605.82 F/g at 2 mV/s, this specifies capacitive success in contradiction of pristine Co3O4 electrode (SC 441.17 F/g). The maximum intended values of SE SP and η were 25.20 Wh/kg and 31.10 kW/kg and 92.11% respectively. The observed internal resistance from Nyquist plot (Ri) is 0.64 Ω. The MnO2 incorporated Co3O4 electrodes have great potential to produce future energy storage devices.
Data availability
Research data is original and it made available on request.
References
P.R. Deshmukh, N.M. Shinde, S.V. Patil, R.N. Bulakhe, C.D. Lokhande, Supercapacitive behavior of polyaniline thin films deposited on fluorine doped tin oxide (FTO) substrates by microwave-assisted chemical route. Chem. Eng. J. 223, 572–577 (2013)
R. Kotz, M. Carlen, Principles and applications of electrochemical capacitors. Electrochim. Acta 45(15–16), 2483–2498 (2000)
C.N.R. Rao, K. Pramoda, Borocarbonitrides, BxCyNz, 2D nanocomposites with novel properties. Bull. Chem. Soc. Jpn. 92, 441–468 (2019)
U.T. Nakate, P. Patil, S.P. Choudhury, S.N. Kale, Microwave assisted synthesis of Co3O4 and NiO nanoplates and structural, optical, magnetic characterizations. Nano-Struct. Nano-Obj. 14, 66–72 (2018)
W. Wei, X. Cui, W. Chen, D.G. Ivey, Manganese oxide-based materials as electrochemical supercapacitor electrodes. Chem. Soc. Rev. 40, 1697–1721 (2011)
B.E. Conway, Electrochemical supercapacitors: scientific fundamentals and technological applications (Kluwer Academic/Plenum Publishers, New York, 1999)
P.R. Deshmukh, S.V. Patil, R.N. Bulakhe, S.N. Pusawale, J.-J. Shim, C.D. Lokhande, Chemical synthesis of PANI–TiO2 composite thin film for supercapacitor application. RSC Adv. 5, 68939–68946 (2015)
M.A. Mahdi, S.R. Yousefi, L.S. Jasim, M. Salavati-Niasari, Green synthesis of DyBa2Fe3O7.988/DyFeO3 nanocomposites using almond extract with dual eco-friendly applications: photocatalytic and antibacterial activities. Int. J. Hydrogen Energy 47, 14319–14330 (2022)
H. Xia, D. Zhu, Z. Luo, Y. Yu, X. Shi, G. Yuan, J. Xie, Hierarchically structured Co3O4@Pt@ MnO2 nanowire arrays for high-performance supercapacitors. Sci. Rep. 3, 2978 (2013)
H. Jiang, T. Sun, C. Li, J. Ma, Hierarchical porous nanostructures assembled from ultrathin MnO2 nanoflakes with enhanced supercapacitive performances. J. Mater. Chem. 22, 2751–2756 (2012)
R.C. Ambare, S.R. Bharadwaj, B.J. Lokhande, Spray pyrolysed Mn:Co3O4 thin film electrodes via non-aqueous route and their electrochemical parameter measurements. Measurement 88, 66–76 (2016)
J. Xu, L. Gao, J. Cao, W. Wang, Z. Chen, Preparation and electrochemical capacitance of cobalt oxide (Co3O4) nanotubes as supercapacitor material. Electrochim. Acta 56, 732–736 (2010)
S. Kumar, G. Saeed, L. Zhu, K. Nam Hui, N.H. Kim, J.H. Lee, 0D to 3D carbon-based networks combined with pseudocapacitive electrode material for high energy density supercapacitor: a review. Chem. Eng. J. 403, 126352 (2021)
T. Yousefi, A.N. Golikand, M.H. Mashhadizadeh, M. Aghazadeh, High temperature and low current density synthesis of Mn3O4 porous nano spheres: characterization and electrochemical properties. Curr. Appl. Phys. 12, 544–549 (2012)
S.V. Khavale, B.J. Lokhande, Electrochemical performance of potentio-dynamically deposited Co3O4 electrodes: influence of annealing temperature. J. Mater. Sci.: Mater. Electron. 28, 5106–5115 (2017)
X. Cui, F. Hu, W. Wei, W. Chen, Dense and long carbon nanotube arrays decorated with Mn3O4 nanoparticles for electrodes of electrochemical supercapacitors. Carbon 49, 1225–1234 (2011)
R.C. Ambare, B.J. Lokhande, Solution concentration and decomposition temperature dependent electrochemical behavior of aqueous route spray pyrolysed Mn3O4: supercapacitive approach. J. Mater. Sci.: Mater. Electron. 28, 12246–12252 (2019)
Y.F. Lee, K.H. Chang, C.C. Hu, Y.H. Chu, Designing tuneable microstructures of Mn3O4 nanoparticles by using surfactant-assisted dispersion. J. Power Sources 206, 469 (2012)
B. Wang, J. Park, C. Wang, H. Ahn, G. Wang, Mn3O4 nanoparticles embedded into graphene nanosheets: preparation, characterization, and electrochemical properties for supercapacitors. Electrochim. Acta 55(22), 6812–6817 (2010)
C.C. Hu, C.Y. Hung, K.H. Chang, Y.L. Yang, A hierarchical nanostructure consisting of amorphous MnO2, Mn3O4 nanocrystallites, and single-crystalline MnOOH nanowires for supercapacitors. J. Power Sources 196, 847–850 (2011)
W. Wei, X. Cui, X. Mao, W. Chen, D.G. Lvey, Morphology evolution in anodically electrodeposited manganese oxide nanostructures for electrochemical supercapacitor applications-effect of supersaturation ratio. Electrochim. Acta 56, 1619 (2011)
R.C. Ambare, S.R. Bhradwaj, B.J. Lokhande, Non-aqueous route spray pyrolyzed Ru:Co3O4 thin electrodes for supercapacitor application. Appl. Surf. Sci. 349, 887–896 (2015)
S.R. Yousefi, M. Ghanbari, O. Amiri, Z. Marzhoseyni, P. Mehdizadeh, M. Hajizadeh-Oghaz, M. Salavati-Niasari, Dy2BaCuO5/Ba4DyCu3O9.09 S-scheme heterojunction nanocomposite with enhanced photocatalytic and antibacterial activities. J. Am. Ceram. Soc. 104, 2952–2965 (2021)
S.R. Yousefi, H.A. Alshamsi, O. Amiri, M. Salavati-Niasari, Synthesis, characterization and application of Co/Co3O4 nanocomposites as an effective photocatalyst for discoloration of organic dye contaminants in wastewater and antibacterial properties. J. Mol. Liquids 337, 116405 (2021)
R.C. Ambare, S.R. Bharadwaj, B.J. Lokhande, Electrochemical characterization of Mn: Co3O4 thin films prepared by spray pyrolysis via aqueous route. Curr. Appl. Phys. 11, 1582–1590 (2014)
H. Pang, J. Deng, J. Du, S. Li, L. Juan, Y. Ma, J. Zhang, J. Chen, Porous nanocubic Mn3O4–Co3O4 composites and their application as electrochemical supercapacitors. Dal. Trans. 41, 10175 (2012)
J. Chen, Y. Cui, X. Wang, M. Zhi, M. Lavorgna, A.P. Baker, J. Wu, Fabrication of hierarchical porous cobalt manganese spinel graphene hybrid nanoplates for electrochemical supercapacitors. Electrochim. Acta 188, 704–709 (2016)
J.M. Luo, B. Gao, X.G. Zhang, High capacitive performance of nanostructured Mn–Ni–Co oxide composites for supercapacitor. Mater. Res. Bull. 43, 1125 (2008)
V. Gupta, S. Gupta, N. Miura, Potentiostatically deposited nanostructured CoxNi1−x layered double hydroxides as electrode materials for redox-supercapacitors. J. Power Source 175, 685 (2008)
G.X. Pan, X. Xia, F. Cao, P.S. Tang, H.F. Chen, Porous Co(OH)2/Ni composite nanoflake array for high performance supercapacitors. Electrochim Acta 63, 340 (2012)
V. Gupta, T. Kawaguchi, N. Miura, Synthesis and electrochemical behavior of nanostructured cauliflower-shape Co–Ni/Co–Ni oxides composites. Mater. Res. Bull. 44, 206 (2009)
G. Wang, L. Zhang, J. Kim, J. Zhang, Nickel and cobalt oxide composite as a possible electrode material for electrochemical supercapacitors. J. Power Source 217, 561 (2011)
G. He, J. Li, H. Chen, J. Shi, X. Sun, S. Chen, X. Wang, Hydrothermal preparation of Co3O4@graphene nanocomposite for supercapacitor with enhanced capacitive performance. Mater. Lett. 82, 61–63 (2012)
Q. Chu, W. Wang, X. Wang, B. Yang, X. Liu, J. Chen, Hierarchical NiCo2O4@nickel-sulfide nanoplate arrays for high-performance supercapacitors. J. Power Sources 276, 19–25 (2015)
D.P. Dubal, A.D. Jagadale, S.V. Patil, C.D. Lokhande, Simple route for the synthesis of supercapacitive Co–Ni mixed hydroxide thin films. Mater. Res. Bull. 47, 1245 (2012)
M. Zhang, D. Yanga, J. Li, Effective improvement of electrochemical performance of electrodeposited MnO2 and MnO2/reduced graphene oxide supercapacitor materials by alcohol pretreatment. J. Energy Storage 30, 101511 (2020)
D. Lim, T. Park, Y. Choi, E. Oh, S. Shim, S. Baeck, Cathodic electrochemical deposition of highly ordered mesoporous manganese oxide for supercapacitor electrodes via surfactant templating. J. Electrochem. Sci. Technol. 11(2), 148–154 (2020)
H. Fang, L. Zhang, Y. Xing, S. Zhang, S. Wu, Nanostructured manganese oxide films for high performance supercapacitors. Int. J. Electrochem. Sci. 13, 8736–8744 (2018)
H. Pang, J. Deng, J. Du, S. Li, J. Li, Y. Ma, J. Zhang, J. Chen, Porous nanocubic Mn3O4–Co3O4 composites and their application as electrochemical supercapacitors. Dalton Trans. 41, 10175–10181 (2012)
A. Singh, D. Kumar, A. Thakur, N. Gupta, V. Shinde, B. Saini, R. Kaur, Galvanostatic deposition of manganese oxide films for super capacitive application and their fractal analysis. Ionics 11, 148–154 (2021)
C. Sun, W. Pan, D. Zheng, R. Yao, Y. Zheng, J. Zhu, D. Jia, Low-cost MnO2 nanoflowers and La2O3 nanospheres as efficient electrodes for asymmetric supercapacitors. Energy Fuels 34, 14882–14892 (2020)
R.C. Ambare, P. Shinde, U.T. Nakate, B.J. Lokhande, R.S. Mane, Sprayed bismuth oxide interconnected nanoplates supercapacitor electrode materials. Appl. Surf. Sci. 453, 214–219 (2018)
S.V. Khavale, R.C. Ambare, B.J. Lokhande, Molar optimization of MnO2 to form composite with Co3O4 by potentiodynamic electrodeposition for better electrochemical characterizations. J. Mater. Sci.: Mater. Electro. 31, 7315–7323 (2019)
A. Elsakhi, S.M. Hamed, M.A. Siddig, A.A. Elbadawi, A.I. Mohamed, M.I. Elhadi, Preperation of cobalt oxide thin film using chemical spray pyrolysis method and the effect of annealing temperature in its optical properties. J. Sci. Res. Innov. Tech. 2, 112–116 (2015)
C. Guo, Y. Zhang, M. Yin, J. Shi, W. Zhang, X. Wang, Y. Wu, J. Ma, D. Yuan, C. Jia, Co3O4@Co3S4 core-shell neuroid network for high cycle-stability hybrid-supercapacitors. J. Pow. Sou. 485, 229315 (2021)
H. Shim, V.R. Shinde, H. Kim, Y. Sung, W. Kim, Porous cobalt oxide thin films from low temperature solution phase synthesis for electrochromic electrode. Thin Solid Films 516, 8573–8578 (2008)
S.R. Yousefi, H.A. Alshamsi, O. Amiri, M. Salavati-Niasari, Synthesis, characterization and application of Co/Co3O4 nanocomposites as an effective photocatalyst for discoloration of organic dye contaminants in wastewater and antibacterial properties. J Mol Liquids 337, 116405 (2021)
V.R. Shinde, S.B. Mahadik, T.P. Gujar, C.D. Lokhande, Supercapacitive cobalt oxide (Co3O4) thin films by spray pyrolysis. Appl. Surf. Sci. 252, 7487–7492 (2006)
U.M. Patil, K.V. Gurav, V.J. Fulari, C.D. Lokhande, O.S. Joo, Characterization of honeycomb-like “β-Ni(OH)2” thin films synthesized by chemical bath deposition method and their supercapacitor application. J. Power Sources 188, 338–342 (2009)
B. Kim, R.C. Justin, W. Cho, W. Lee, H. Jeong, Y.K. Hyun, Enhanced electrochemical properties of cobalt doped manganese dioxide nanowires. J. Alloys Comp. 617, 491–497 (2014)
Hu. Chi-Chang, T.-W. Tsou, The optimization of specific capacitance of amorphous manganese oxide for electrochemical supercapacitors using experimental strategies. J. Power Sources 115, 179–186 (2003)
V. Srinivasan, J. Weidner, Studies on the capacitance of nickel oxide films: effect of heating temperature and electrolyte concentration. J. Electrochem. Soc. 147, 880 (2000)
R.C. Ambare, S.V. Khavale, U.T. Nakate, M.B. Khanvilkar, B.J. Lokhande, Electrochemical investigations of spray pyrolysed ruthenium incorporated Co3O4 electrodes prepared via aqueous route. Colloids Surfaces A 615, 126215 (2021)
A. García-Gómez, S. Eugénioa, R.G. Duarte, T.M. Silva, M.J. Carmezim, M.F. Montemor, Electrodeposited reduced-graphene oxide/cobalt oxide electrodes for charge storage applications. Appl. Surf. Sci. 382, 34–40 (2016)
R. DellaNoce, S. Eugenio, T.M. Silva, M.J. Carmezim, M.F. Montemor, a-Co(OH)2/carbon nanofoam composite as electrochemical capacitor electrode operating at 2 V in aqueous medium. J. Power Sources 288, 234–242 (2015)
K. Torchała, K. Kierzek, J. Machnikowski, Capacitance behavior of KOH activated mesocarbon microbeads in different aqueous electrolytes. Electrochim. Acta 86, 260–267 (2012)
Acknowledgements
Authors are grateful to thanks Department of Science and Technology, New Delhi for providing financial supports through the project scheme DST-SERB sanction no. SB/EMEQ-331/2013.
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
SVK: Conceptualization, Investigation, Methodology, Writing—review & editing. RCA: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Writing—original draft, Writing—review & editing. UTN: Investigation, Formal analysis, Writing—review & editing. BJL: Investigation, Supervision, Writing—original draft, Writing—review & editing.
Corresponding authors
Ethics declarations
Competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
Indicate that our research manuscript has been conducted ethically, keeping in mind privacy, consent and appropriate reporting of those involved in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khavale, S.V., Ambare, R.C., Nakate, U.T. et al. Potentiodynamic electrodeposited MnO2:Co3O4 thin films electrodes for supercapacitor application. J Mater Sci: Mater Electron 34, 1345 (2023). https://doi.org/10.1007/s10854-023-10718-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10854-023-10718-x