Abstract
In this study, calcium modified strontium barium niobate (CaxSr0.75−xBa0.25Nb2O6, CSBN-x, 0 ≤ x ≤ 0.20) lead-free ferroelectric relaxor ceramics were prepared by conventional solid-state reaction technique and their crystal structure, morphology, dielectric, ferroelectric and energy storage properties were studied comprehensively. The modified CSBN ceramics present pure tetragonal tungsten bronze structure (TTBS) in the whole studied composition range. The introduction of calcium elevates the ferroelectric to paraelectric transition temperature remarkably while has little influence on the relaxor behavior of strontium barium niobate system. Although the maximum polarization (Pmax) and remnant polarization (Pr) of CSBN-x increase with x, the difference of the two values (Pmax−Pr) is also heightened, thus improving its energy storage performance. The optimal energy storage properties, i.e. high recoverable energy storage density Wrec (1.21 J/cm3) and energy storage efficiency η (87.7%), ultrahigh temperature stability (the fluctuations on Wrec and η are within 2% and 3% over the range of 25 °C–120 °C, respectively) are achieved in Ca0.05Sr0.70Ba0.25Nb2O6 composition. These excellent performance properties qualify this environment-friendly SBN-based ferroelectric relaxor ceramics as a promising alternative in energy storage application.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Due to their high-power density, fast charge–discharge speed and good temperature stability, dielectric capacitors are considered to be promising for energy storage application, including electric vehicles, mobile electronics, pulsed power devices and [1, 2]. Generally, linear dielectrics (LDs), ferroelectrics (FEs), anti-ferroelectrics (AFEs) and relaxor ferroelectrics (RFEs) are considered as four kinds of promising dielectrics in these fields [3, 4]. The recoverable energy density (Wrec) and energy storage efficiency (η) are key parameters of a dielectric capacitor to evaluate its energy storage performance, entirely depending on the saturation polarization (Pmax), remnant polarization (Pr), and breakdown strength (BDS) of the materials [5, 6]. Relaxor ferroelectrics with polar nanoregions (PNRs) usually demonstrate diffuse phase transition and slim hysteresis loops, which result in low Pr and high Pmax, thus expecting superior energy storage densities and energy conversion efficiencies [6,7,8]. Moreover, due to the diffusion phase transition nature, the permittivity of RFEs is usually insensitive to temperature change within a certain range, which favors the improvements of temperature stability in energy storage application [9]. Over the past decades, huge research progress for dielectric energy storage capacitors has been made in perovskite structure RFEs including BaTiO3-based, NaNbO3-based, Bi0.5Na0.5TiO3-based and K0.5Na0.5NbO3-based ceramics [8, 9, 10, 11, 12, 13].
Tetragonal tungsten bronze structure (TTBS) ferroelectrics are the second largest group of ferroelectrics after perovskite structure, which features connection of BO6 octahedrons by sharing common vertex and forming three types of interstices (A1, A2 and C sites) accessible for different cations. According to cations occupancy situation, this kind of materials can be categorized into three groups: fully filled (A and C sites all occupied), filled (all A sites occupied and C sites empty) and unfilled (partial A sites occupied and C sites empty) [14]. Many investigations have been performed among filled TTBS ceramics for energy storage owing to their superior dielectric properties [15]. For unfilled TTBS compounds, the special structure of A-site vacancies and multi-interstices becomes the origin of relaxation and provides great freedom for composition design and performance improvement, [16] suggesting the potential as candidates for energy storage capacitors. Thus, the innovative design of unfilled TTBS energy storage ceramics may help to understand the underlying mechanism among structure, composition and performance, and provide an effective material for high performance energy storage application.
As a typical unfilled TTBS compound, SrxBa1−xNb2O6 (SBN, x = 0.25 − 0.75) solid solution has been widely studied for its excellent electro-optic, pyroelectric and photorefractive properties [17,18,19,20]. The crystallographic non-equivalent sites of SBN structure and its compositional flexibility provide various possibilities of physical properties modification [21, 22]. For example, changing the Sr/Ba ratio could have a strong impact on the phase transition and properties due to the preferential occupation of A-site ions. When increasing Sr/Ba ratio, SBN materials experience a change from normal ferroelectrics to relaxor ferroelectrics. The higher Sr/Ba ratio is, the greater the phase transformation diffusion is. Meanwhile in the high Sr compositions, the ferroelectric-paraelectric phase transformation temperature decreases while the dielectric maximum at this point increases [18, 20]. All facts above suggest that SBN with high Sr composition, as typical relaxor ferroelectrics, should be favorable for energy storage application. Bai et al. [23] synthesized (Sr0.5Ba0.5)1+xNb2−xFexO6 ceramics that acquired a Wrec of 0.680 J/cm3 and an η of 83.6% at x = 0.04. Tang et al. [24] obtained a Wrec of 0.19 J/cm3 and an η of 86.37% in Sr0.60Ba0.40Nb2O6 ceramics under 60 kV/cm electric field. Rao et al. [25] reported that Sr0.70Ba0.30Nb2O6−x wt%MgO ceramics exhibit a Wrec = 0.93 J/cm3 and an η = 89.4% at 157 kV/cm when x = 0.5. In general, due to the slim characteristics of the hysteresis loops of the SBN ceramics with high Sr compositions, the energy storage efficiency η is high. However, in consequence of suffering from low polarization in these compositions, the recoverable energy density Wrec is quite poor.
Several methods, including doping and utilizing various synthesis techniques, [23, 25, 26] have been introduced to modify electric properties of SBN. Among them, Ca-substitution is considered as an effective way to enhance the dielectric, pyroelectric and ferroelectric properties of SBN, since (Ca,Ba) Nb2O6 (CBN) crystal exhibits higher spontaneous polarization (~ 35 μC/cm2) than SBN crystal (~ 27 μC/cm2) [27]. Our previous results showed that Ca-substitution at the A sites of unfilled SBN can improve the ferroelectric and pyroelectric performance in Ca0.15(Sr0.5Ba0.5)0.85Nb2O6 ceramics [28]. Further studies in CaxSr0.3−xBa0.7Nb2O6 system also revealed that the saturated polarization increased monotonically with increasing Ca content, indicating that the ferroelectricity in unfilled SBN can be greatly enhanced by Ca addition [29]. It is also reported that Ca-substitution can be capable of improving the ferroelectric properties of filled SBN systems, such as Sr2−xCaxNaNb5O15 (SCNN) and (Sr0.6Ba0.4)4Na2Nb10O30 (SBNN) [30, 31].
In this article, we introduced Ca into the typical TTBS Sr0.75Ba0.25Nb2O6 lead-free ferroelectric relaxor ceramics in order to improve the energy storage performance. Effect of Ca content on the structure and electric properties of CaxSr0.75−xBa0.25Nb2O6 (x = 0, 0.05, 0.10, 0.15, 0.20 abbreviated as CSBN-0, CSBN-5, CSBN-10, CSBN-15, CSBN-20) ceramics was systematically investigated. Enhanced energy storage performance is achieved in Ca-substituted SBN matrix with high Sr composition, indicating this environmentally friendly ferroelectric relaxor ceramic as a promising alternative in energy storage application.
2 Experimental procedures
The CSBN-x ceramics are synthesized by conventional solid-state reaction method using Nb2O5 (99.3%), BaCO3(99%), SrCO3(99%), and CaCO3(99%) as raw materials and weighted stoichiometrically. The starting materials are mixed in polyurethane jars and ball milled via ethanol for 24 h using agate balls with a rotation speed of 115 rpm. The mass ratio of the starting materials to milling ball is 1: 1.5. The starting materials are mixed and ball milled for 24 h via ethanol. The mixed powder is calcined at 1260 °C for 2 h and then re-milled and pressed into circular pellets with a diameter of 13 mm. The pellets are air sintered at 1360 °C for 4 h. The sintered pellets are polished to 0.15 mm thickness and coated with silver paste in order to take the electric properties measurement.
The crystal structure of the samples is confirmed by X-ray diffraction (XRD, D/MAX-2550 V; Rigaku, Tokyo, Japan) technique using Cu Kα radiation. The microstructure of the ceramics is observed by the TM3000 Tabletop Microscope (Hitachi, Tokyo, Japan) on the fresh surface of samples. The porosity percentage of the samples were tested and calculated via Archimedes method. Measurements of the dependence of dielectric constant and loss on temperature are performed in the range 20 °C–200 °C using a Hewlett Packard LCR meter (Model HP4284A; Agilent Technologies Japan Ltd., Hyogo, Japan) at different frequencies from 100 Hz to 1 MHz. Polarization hysteresis loops are characterized by applying an electric field of sinusoidal waveform at a frequency of 10 Hz using commercial ferroelectric analyzer (TF Analyzer 2000, aixACCT, Aachen, Germany) assisted with high-voltage power supply (Trek Inc, Medina, NY).
3 Results and discussion
3.1 Phase structure and microstructure
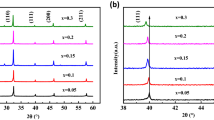
Figure 1a displays the XRD pattern of CSBN-x ceramics. The diffraction peaks, being indexed according to JCPDF39-0265, present a pure tetragonal tungsten bronze structure. No obvious splits of diffraction peaks can be detected in all samples. The enlarged patterns for the (410) diffraction peak (around 29°–30°) of all samples shown in the Fig. 1b are found to shift towards higher 2θ angles, indicating the shrinkage of lattice with the increase of the Ca content. The detailed structure properties are further analyzed via Rietveld refinement method using FullProf software. The R-pattern Rp, R-weighted pattern Rwp and Goodness-of-fit χ2 are used as the numerical criteria of fitting. The important structural parameters such as lattice parameters and cell volume values are summarized in Table 1. The fitting results are proved to be reliable with P4bm space group since the Rp, Rwp and χ2 is relatively low. It is evident that the lattice parameters a, b, c and cell volume decrease with Ca content, also demonstrating the fact that Ca2+ ions are incorporated into the lattice (Fig. 2).
Figure 3 illustrates the SEM image of fresh surface of CSBN-x ceramics. The surface morphology of all samples presents a mixed characteristics of ordinary grains and rod-like grains. The sizes of two kinds grains show little difference among all samples. The average size of ordinary grains is about 2–3 μm while the mean size of rod-like grains significantly increases to 5–8 μm. As Ca content increased from 0.00 to 0.20, the porosity percentage of the samples varies slightly (4.37%, 3.82%, 4.02%, 3.63% and 3.77% for x = 0.00, 0.05, 0.10, 0.15 and 0.20, respectively).
3.2 Dielectric properties
The temperature dependences of dielectric constant and loss for CSBN-x ceramics at the range of 100 Hz to 1 MHz are depicted in Fig. 4. The main dielectric phase transition parameters are listed in Table 1. The Tm representing the ferroelectric to paraelectric phase transition temperature is determined as the corresponding temperature of maximum dielectric constant (εm) at 1 kHz. As can be seen from Fig. 3, the Tm increases monotonically with Ca-substitution amount, which is 40.7 °C for x = 0.00 and 90.0 °C for x = 0.20. In CSBN crystal lattice, smaller Ca2+ (0.99 Å) and part of Sr2+ (1.13 Å) prefer to occupy the smaller A1-site (tetragonal interstices, with 12-fold coordination), while Ba2+ (1.35 Å) and residual Sr2+ jointly fill the larger A2-site (pentagonal interstices, with 15-fold coordination). When more and more Ca2+ cations substitute Sr2+ cations in A1-site, the radius difference between A1-and A2-site cations will become larger, which is suggested to be the reason for elevating ferroelectric transition temperature in TTBS ceramics [29].
For ferroelectric relaxors, it is well known that the degree of the diffuseness can be described by a modified Curie–Weiss relationship: [32]
where εm is the maximum value of dielectric constant at the transition temperature, C represents the Curie constant and γ stands for the diffusivity parameter. Normally, γ = 1 is for normal ferroelectrics and γ = 2 is for ideal ferroelectric relaxors. The γ values, derived from fitting the plots of \(\mathit{ln}(1/{\varepsilon }_{r}-1/{\varepsilon }_{m})\) as a function of \(\mathit{ln}(T-{T}_{m})\), are shown in Table 2. All the values are around 1.71–1.85 for both pure SBN and Ca-substituted SBN ceramics, indicating that all samples exhibit a typical diffuse transition and Ca-substitution does not affect the relaxor behavior of SBN matrix.
In addition, the ferroelectric relaxor feature can also be depicted by the parameters of \(\Delta {T}_{relax}\). The specific symbols of the relaxation degree are defined as [33]:
The values of \(\Delta {T}_{relax}\) are calculated from experimental results and listed in Table 1 as well. With increasing Ca content, the value of \(\Delta {T}_{relax}\) varies slightly, which is consistent with the diffusivity parameters. By using first-principle density functional calculations, G. H. Olsen et al. [34] found that the relaxor behavior in Sr-rich SBN originated from Sr displacement inside pentagonal channels (A2-sites) in the TTBS. Since Ca2+ is inclined to incorporate into A1-site, the amount of Sr2+ occupying A2-site in Sr-rich compositions remains constant after Ca-substitution, thus scarcely influencing the relaxation property of the samples.
3.3 Ferroelectric and energy storage properties
Figure 4a shows the polarization versus applied electrical field (P-E) hysteresis loops of CSBN-x measured under 200 kV/cm at 10 Hz and room temperature and Fig. 4b demonstrates the variations of Pr, Pmax, Pmax-Pr estimated from the loops. It can be seen that all samples present slim hysteresis loops. Compared with pure SBN sample, the introduction of Ca has greatly elevated the maximum polarization Pmax from 13.2μC/cm2 for x = 0 to 17.9μC/cm2 for x = 0.15. Meanwhile, the remnant polarization Pr and coercive electric field Ec also increase monotonically in different degrees with increasing Ca content, indicating that the ferroelectricity is significantly enhanced by Ca addition. The value of Pmax-Pr shows a tendency of increasing first and then decreasing and achieves the maximum at x = 0.05. It has been reported that the ferroelectricity in SBN system is determined by the atomic displacement (Δz) of the Nb5+ which is linearly proportional to the ferroelectric polarization [35]. Smaller Ca2+ substituting Sr2+ in A1-site could cause the twisting of the octahedral framework and changes in the O–Nb–O bonds, which probably gives rise to larger Δz and thus an enhancement in spontaneous polarization. The important ferroelectric polarization features extracted from P-E loops are summarized in Table 3 (Fig. 5).
The energy storage performance can be evaluated by following equations:
where Wtotal, Wrec and η are the total energy density, the recoverable energy storage density and the energy storage efficiency, respectively. In ferroelectric ceramic capacitors, due to noncoincidence of charging and discharging paths in hysteresis P-E loops, Wtotal representing the total charged energy cannot be released completely, while Wrec is the energy density that could be totally discharged and utilized. The Wloss equals to the difference between Wtotal and Wrec, which is wasted during the process and will generate heat within capacitors degrading the properties of capacitors. Energy storage efficiency η is the ratio of the discharged energy density (Wrec) to the charged energy density (Wtotal). Therefore, Wrec and η are important metrics to evaluate dielectrics’ performance in energy storage application. The energy storage behaviors of CSBN-x ceramics are studied via P-E loops as shown in Fig. 6. The important energy storage properties are also listed in Table 3. Both Wtotal and Wrec increase after Ca-substitution. Particularly, Wtotal achieves the maximum values (~ 1.52 J/cm3) in CSBN-15 composition, where Wrec is 1.15 J/cm3 at ambient temperature. Wrec achieves the maximum values (~ 1.21 J/cm3) at x = 0.05 and decreases slightly when further increasing the value of x, of which the trend is fully in line with that of Pmax-Pr. Due to the elevation of Pr by introduction of Ca, the η shows a downward trend with increasing Ca content. The maximum η = 88.99% is obtained in CSBN-0 samples, while the η drops to 72.37% at x = 0.20. In CSBN-5 samples, the energy storage efficiency η reaches as high as 87.68%, nearly equivalent to the value of pure SBN. In the whole, CSBN-5 composition presents the optimal overall energy storage properties with high Wrec (1.21 J/cm3) and η (87.68%) at room temperature. Figure 6b benchmarks the energy storage performance of the filled and unfilled TTBS ceramics systems in the recent literatures [15, 23,24,25, 36,37,38,39,40,41,42,43,44,45,46]. In comparison with the filled and unfilled TTBS ceramics systems, it is evident that the CSBN-5 ceramics simultaneously achieve high Wrec and high η, making it very attractive for energy storage application.
The good thermal stability is also an important parameter for practical energy storage application, for which guarantee the reliable operation of energy storage devices over a wide temperature range. To evaluate the thermal stability performance of CSBN-5 sample, its hysteresis loops are measured in the temperature range of 25 °C –20 °C at 200 kV/cm, as shown in Fig. 7a. The slim hysteresis loops are maintained throughout the testing temperature range, with a slight decrease in Pmax and Pr. Specifically, the Pmax of CSBN-5 is 17.20 μC/cm2 at 25 °C while it still maintains 89.5% of the initial value at 120 °C (15.4 μC/cm2). In the meantime, the Pr has reduced from 1.03 μC/cm2 around ambient temperature to 0.70 μC/cm2 at 120 °C. The difference between Pmax and Pr (Pmax−Pr) at each temperature declines to a small extent over this range, implying tiny reduction in recoverable energy storage density. The Wrec, Wloss and η of CSBN-5 composition at each temperature calculated from the P-E loops are also shown in Fig. 7b. The flat curve reflects the stable Wrec value over the test temperature with a maximum of 1.21 J/cm3 and a minimum of 1.19 J/cm3. Of particular interest is that the η slightly increases towards elevated temperature due to the declined Pr. The room temperature η is 87.68% while it reaches as high as 90.32% at 120 °C. Overall, the variations of Wrec and η are respectively on the order of < 2% and 3% within the temperature range, demonstrating quite good thermal stability performance of this composition.
4 Conclusions
Tetragonal tungsten bronze structure CaxSr0.75−xBa0.25Nb2O6 (x = 0.00, 0.05, 0.10, 0.15, 0.20) lead-free ferroelectric relaxor ceramics have been synthesized via conventional solid-state reaction route. Enhanced dielectric and ferroelectric properties with increased ferroelectric transition temperature Tm and maximum polarization Pmax are achieved in CSBN-x ceramics due to the enhancement of structure distortion by smaller Ca2+ substituting Sr2+. The optimal energy storage properties, i.e. high recoverable energy storage density Wrec (1.21 J/cm3) and energy storage efficiency η (87.68%), ultrahigh temperature stability (the fluctuations on Wrec and η are within 2% and 3% over the range of 25 °C–120 °C, respectively) are obtained in Ca0.05Sr0.70Ba0.25Nb2O6 composition, unveiling the potential of CSBN-5 lead-free ferroelectric relaxor ceramics for energy storage application.
Data availability
The data used to support the findings of this study are included within the article.
References
B.J. Chu, X. Zhou, K.L. Ren, B. Neese, M.R. Lin, Q. Wang, F. Bauer, Q.M. Zhang, Science 313, 334 (2006)
L.T. Yang, X. Kong, F. Li, H. Hao, Z.X. Cheng, H.X. Liu, J.F. Li, S.J. Zhang, Prog. Mater. Sci. 102, 72 (2019)
Z.H. Yao, Z. Song, H. Hao, Z.Y. Yu, M.H. Cao, S.J. Zhang, M.T. Lanagan, H.X. Liu, Adv. Mater. 29, 1601727 (2017)
Z. Liu, T. Lu, J.M. Ye, G.S. Wang, X.L. Dong, R. Withers, Y. Liu, Adv. Mater. Technol. 3, 1800111 (2018)
M.X. Zhou, R.H. Liang, Z.Y. Zhou, X.L. Dong, J. Mater. Chem. C 6, 8528 (2018)
T. Wang, L. Jin, C. Li, Q.Y. Hu, X.Y. Wei, J. Am. Ceram. Soc. 98, 559 (2015)
P.Y. Zhao, H.X. Wang, L.W. Wu, L.L. Chen, Z.M. Cai, L.T. Li, X.H. Wang, Adv. Energy Mater. 9, 1803048 (2019)
Q.Y. Hu, Y. Tian, Q.S. Zhu, J.H. Bian, L. Jin, H.L. Du, D.O. Alikin, V.Y. Shur, Y.J. Feng, Z. Xu, X.Y. Wei, Nano Energy 67, 104264 (2020)
W. Wang, L.Y. Zhang, R.Y. Jing, Q.Y. Hu, D.O. Alikin, V.Y. Shur, X.Y. Wei, G. Liu, Y. Yan, L. Jin, Chem. Eng. J. 434, 134678 (2022)
A.W. Xie, R.Z. Zuo, Z.L. Qiao, Z.Q. Fu, T.F. Hu, L.F. Fei, Adv. Energy Mater. 11, 2101378 (2021)
D. Hu, Z.B. Pan, X. Zhang, H.R. Ye, Z.Y. He, M.K. Wang, S. Xing, J.W. Zhai, Q. Fu, J.J. Liu, J. Mater. Chem. C 8, 591 (2020)
A. Zhang, H.B. Yang, Y. Lin, Q.B. Yuan, H.L. Du, Energy Stor. Mater. 45, 861 (2022)
Y. Slimani, A. Selmi, E. Hannachi, M.A. Almessiere, G. Alfalah, L.F. Alousi, G. Yasin, M. Iqbal, J. Phys. and Chem. Solids 156, 110183 (2021)
M.P. Trubelja, E. Ryba, D.K. Smith, J. Mater. Sci. 31, 1435 (1996)
L. Cao, Y. Yuan, Z.Y. Yang, E.Z. Li, S.R. Zhang, Ceram. Int. 46, 6108 (2020)
J. Gardner, F.D. Morrison, Dalton Trans. 43(30), 11687 (2014)
A.M. Glass, Appl. Phys. Lett. 13(4), 147 (1968)
A.M. Glass, J. Appl. Phys. 40(12), 4699 (1969)
P. Molina, M.D. Ramirez, L.E. Bausa, Adv. Funct. Mater. 18(5), 709 (2008)
J. Zhang, G.S. Wang, F. Gao, C.L. Mao, X.L. Dong, Ceram. Int. 39(2), 1971 (2013)
S. Podlozhenov, H.A. Graetsch, J. Schneider, M. Ulex, M. Wohlecke, K. Betzler, Acta Cryst. B 62, 960 (2006)
T.S. Chernaya, B.A. Maksimov, T.R. Volk, L.I. Ivleva, V.I. Simonov, Phys. Solid State 42(9), 1716 (2000)
H. Bai, J. Li, Y. Wu, Y. Hong, K.Z. Shi, Z.X. Zhou, Ceram. Int. 45, 11109 (2019)
H. Tang, X.G. Tang, M.D. Li, Q.X. Liu, Y.P. Jiang, J. Alloys Compd. 791, 1038 (2019)
Y. Rao, H.X. Liu, H. Hao, Z.H. Yao, X.X. Zhou, M.H. Cao, Z.Y. Yu, Ceram. Int. 44, 11022 (2018)
S. Dursun, E. Mensur-Alkoy, S. Alkoy, J. Eur. Ceram. Soc. 36, 2479 (2016)
Y.J. Qi, C.J. Lu, J. Zhu, J. Zhu, X.B. Chen, X.G. Xu, Appl. Phys. Lett. 87, 167 (2005)
J. Zhang, X.L. Dong, F. Cao, S.B. Guo, G.S. Wang, Appl. Phys. Lett. 102, 3495 (2013)
H. Chen, S.B. Guo, X.L. Dong, F. Cao, C.L. Mao, G.S. Wang, J. Alloys Compd. 695, 2723 (2017)
R.J. Xie, Y. Akimune, K. Matsuo, T. Sugiyama, N. Hirosaki, T. Sekiya, Appl. Phys. Lett. 80, 835 (2002)
Y.B. Yao, C.L. Mak, J. Alloys Compd. 544, 87 (2012)
K. Uchino, S. Nomura, Ferroelectr. Lett. 44, 55 (1982)
W. Chen, X. Yao, X.Y. Wei, Appl. Phys. Lett. 90, 2289 (2007)
G.H. Olsen, U. Aschauer, N.A. Spaldin, S.M. Selbach, T. Grande, Phys. Rev. B 93, 180101 (2016)
S.C. Abrahams, S.K. Kurtz, P.B. Jamieson, Phys. Rev. 172, 551 (1968)
R. Li, Y.P. Pu, Q.W. Zhang, W. Wang, J.W. Li, X.Y. Du, M. Chen, X. Zhang, Z.X. Sun, J. Eur. Ceram. Soc. 40, 4509 (2020)
S.D. Xu, S.J. Shen, C.W. Huang, Y. He, X.L. Chao, D. Wu, P.F. Liang, Z.P. Yang, J.B. Lu, L.L. Wei, ACS Appl. Interfaces Mater. (2023). https://doi.org/10.1021/acsami.2c18827
B. Yang, S.L. Hao, P. Yang, L.L. Wei, Z.P. Yang, Ceram. Int. 44, 8832 (2018)
S.D. Xu, S.J. Shen, R. Hao, Z.H. Peng, F.D. Zhang, D. Wu, P.F. Liang, X.L. Chao, L.L. Wei, Z.P. Yang, Chem. Eng. J. 433, 133812 (2022)
L. Cao, Y. Yuan, B. Tang, E.Z. Li, S.R. Zhang, J. Eur. Ceram. Soc. 40, 2366 (2020)
W.B. Feng, Y.F. Xu, X.G. Li, Y.Y. Wu, J.J. Yang, J. Mater. Sci.: Mater. Electron 34, 117 (2023)
X. Zheng, Z.H. Jin, Y.J. Dan, C.Z. Hu, L.J. Liu, L. Fang, J. Mater. Sci.: Mater. Electron 34, 148 (2023)
S.D. Xu, R. Hao, Z. Yan, S.T. Hou, Z.H. Peng, D. Wu, P.F. Liang, X.L. Chao, L.L. Wei, Z.P. Yang, J. Eur. Ceram. Soc. 42, 2781 (2022)
S.T. Hou, S.D. Xu, L.J. Yang, X. Liu, L.L. Wei, X.L. Chao, D. Wu, P.F. Liang, Z.P. Yang, Ceram. Int. (2022). https://doi.org/10.1016/j.ceramint.2022.06.148
Y.Y. Zhao, J.P. Wang, L.X. Zhang, X.J. Shi, S.J. Liu, D.W. Zhang, Ceram. Int. 42, 16697 (2016)
G.H. Chen, J. Zheng, Z.C. Li, J.W. Xu, Q.N. Li, C.R. Zhou, C.L. Yuan, Q. Feng, J. Mater. Sci.: Mater. Electron 27, 2645 (2016)
Funding
This work was supported by the Key Deployment Projects of Chinese Academy of Sciences (Grant No. ZDRW-CN-2021-3-1-18) and the National Natural Science Foundation of China (NSFC) (Grant No. 61475176).
Author information
Authors and Affiliations
Contributions
SG: implemented the research scheme and wrote the manuscript. CY: helped to make material preparation. FC: provided support for the electrical properties measurements. ZL: reviewed and edited the article. GW: reviewed and edited the article.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
This article does not contain any studies involving humans and animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guo, S., Liu, Z., Yao, C. et al. Enhanced energy storage properties on calcium modified strontium barium niobate lead-free ferroelectric relaxor ceramics. J Mater Sci: Mater Electron 34, 1271 (2023). https://doi.org/10.1007/s10854-023-10681-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10854-023-10681-7