Abstract
High electrical conductivity, high transparency and mechanical flexibility are important properties in generations of transparent conductive electrodes. Currently the most efficient and widely used transparent conductive material is Indium Tin Oxide. Searching for alternative materials has been being followed due to the lack of Indium resources, ITO inflexibility and its high manufacturing costs. In the present study, silver nanowire as a promising alternative to ITO was synthesized through polyol method and then silver nanowire layer was applied on glassy substrates using drop-casting method. Adhesion between the layer and the substrate was established using polyaniline. Characterization of the thin film was conducted using X-ray diffraction methods, field emission scanning electron microscopy, UV–Vis spectroscopy and a four-point probe. The results show that corrective deoxidation operations and separation of nanowires from nanoparticles, leads to an increase in transparency from 79 to 81.4% and decrease sheet resistance of 89 to 76 Ω/□.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Transparent conductive electrodes(TCEs) play a critical role in many modern devices such as solar cells, monitors, smart touch screens and transparent heaters. Requirement of transparent conductive materials (TCMs) have made industrialists and scientists seek for the best alternatives to ITO, the most efficient and common material so far. This matter is originated from economics and physical factors: i.e., ITO's cost increases due to the scarcity of Indium resources. Moreover, its fragility makes it incompatible with the flexible sublayers that are becoming more and more practical [1,2,3].
In order to pursue alternative next-generation TCEs, various attempts have been made using new types of functional and nanoscale materials, such as carbon nanotubes, graphene conductive polymers and metal oxides. Among the candidates, silver nanowires (AgNWs) have been attracting more and more attention because of their intriguing electrical, thermal, and optical properties [4].
Interconnected metal nanowires' network (MNW) brings high flexibility, significant optical transparency and suitable electrical conductivity altogether. Metal nanowires represent good transparency and conductivity similar to ITO-based electrodes because of their long length and their low thickness, whereas the amount of consumable materials compared to ITO is less. furthermore, metal nanowire’s network shows impressive adaptability with deposition processes and could be used on a large scale [4,5,6].
So far, various metal nanowires such as copper, gold, nickel-copper nanowires have been synthesized and deposited on transparent surfaces with promising properties. Most of the studies are focused on silver nanowire's network due to the privileged physical properties of silver, which has the most electrical conductivity in the room temperature and second, its scalable synthesis [7,8,9,10].
After the deposition process, metal nanowire network's electrical resistance is usually high. This problem is related to insufficient contact between nanowires or presence of organic impurities like PVP crusts at junctions. A decrease in network's electrical resistance could be achieved through some methods like heating during the chemical process, sintering by laser, induced plasma welding with light in the scale of Nano or mechanical pressure [11,12,13,14].
In 2016 Bari et al. [15] synthesized Ag nanowire with 200 to 500 µm of length with an average diameter of 45–65 nm. They used spin coating method to deposit the film on a glass substrate and the film lacked of adhesion. To obtain an alternative transparent conductive electrode to ITO, presenting at least a transparency of 80% and a sheet resistance of less than 100 Ω/□ is required.
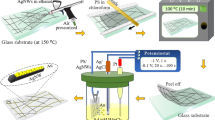
In the present study, after silver nano wire was synthesized, drop-casting is used instead of spin coating method, which is a cheaper method and leads to less consumption of electrical energy, and polyaniline was deposited onto the film to improve the adhesion between the film and the substrate so that the film can be used widely in industrial application. Also, deoxidation operations and separation of nanowires from nanoparticles were used to increase in transparency and decrease sheet resistance of synthesized silver nanowire transparent conductive electrodes. Moreover, annealing process was performed as a post treatment process to decrease the sheet resistance at the junctions.
2 Experimental
2.1 Synthesis of silver nanowires
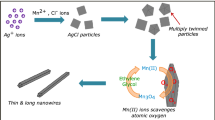
The required amounts of different materials to synthesis nanowires are 4 g of Poly vinyl pyrrolidone, 0.2038 g Silver Nitrate, 0.1402 g Sodium Chloride and 0.48 g Glucose. Poly vinyl pyrrolidone, Sodium Chloride, Glucose and Silver Nitrate are, respectively, dissolved in 20 ml, 60 ml, 20 ml and 60 ml of distilled water separately at room temperature except for polyvinyl pyrrolidone that is dissolved at 65 °C.
First, Glucose solution was added to Silver Nitrate solution and mixed with a stirrer for 8 min. Next, the Poly vinyl pyrrolidone solution was brought to room temperature and then was added to the stirring solution. 20 min later, sodium chloride solution was added dropwise to the solution, and stirred for 15–20 min until the reactions are gradually complete.
The final solution was put in an autoclave with a capacity of 200 ml and placed in the oven for 22 h at 160 °C. The solution was poured equally into 6 centrifuge special tubes. Tubes were centrifuged for an hour in 2500 rpm until the separation operation is complete.
2.2 Silver nanowire deposition
After separating the nanowires from the solution in each tube, they were washed three times with distilled water and three times with isopropanol to ensure that the Poly vinyl Pyrrolidone has been removed completely. Each step of washing involves dispersing the nanowires in distilled water and then in ethanol. Then they were centrifuged at 2500 rpm for 10 min. The washing operation continues until the primary transparency of ethanol is seen which indicates the complete removal of poly vinyl pyrrolidone from the solution. In case of any problems at this step deposition won't be successful and will increase sheet resistance due to the presence of Poly vinyl pyrrolidone.
After washing stage, the resulted white substance was dispersed in 30 ml of isopropanol, and deposited onto the samples with various concentrations. Sample preparation includes washing the glassy samples with degreasing liquid and then with ethanol twice. Drop-casting was used as the deposition method. In this method, after silver nanowire synthesis, inks with different amounts of 35, 28, 20, 10 and 5 ml of isopropanol were prepared and with each of these amounts, a sample was coated. The coated sample of the most diluted ink (35 ml) was named A. Similarly, the sample coated from a solution of 28 ml B, 20 ml C, 10 ml D and the sample coated from the 5 ml ink was named E. In order to measure the sheet resistance of the above samples, a Four-point probe was used. After that, the transmission was measured at wavelengths from 300 to 900 nm by a Jasco invisible-ultraviolet spectrometer, made in Japan model V-670. To identify the phases in the sample, XRD test was done by a Bruker device made in Germany model D8 Advance.
In order to improve the results, the effect of annealing at different temperatures was considered. In order to achieve the optimal annealing temperature, three samples named P, N and M with random amounts of silver nanowire ink, were coated and then, before and after annealing at 120,160 and 180 °C, Four-point probe test was done and it was determined that the temperature of 160 °C is the optimal temperature for annealing after the optimal annealing temperature was achieved, inks containing 28, 20 and 10 ml isopropanol were produced and samples D2, C2, B2 were coated using the same drop-casting method. Annealing process was applied as well. Then a Four-point probe test was done in order to obtain optimal results. In the final step, in order to improve adhesion between the silver nanowire and the glassy sublayer, 0.3 g of polyaniline dissolved in 10 ml of distilled water, was drop casted onto the samples and then, a Four-point probe test was done. Also, FE-SEM analysis was used to show silver network in great detail.
3 Results and discussion
3.1 Materials characterization
After nanowire was obtained through centrifuge and was washed with distilled water and isopropanol, it was dispersed in different amounts of isopropanol and after the glassy samples were prepared, deposition was done using drop-casting method. Figure 1 shows the coated samples 5 inks were prepared with different amounts of 35 ml, 28 ml, 20 ml, 10 ml and finally 5 ml of isopropanol. The light transmission amount and the sheet resistance of the samples are influenced by the concentration of the inks. Different densities in different samples that are due to the different ink concentration are shown in Fig. 1. The more nanowire disperses in a smaller amount of isopropanol solution, the higher the density of film will be. Therefore, in the FE-SEM images of Fig. 2, it can be clearly seen that with decreasing the concentration of solution, the density of nanowires in the film increases and this increase in density will affect the transparency as well as sheet resistance of the samples. A denser film will contain more electrical paths and as a result, higher conductivity. On the other hand, with the increase in the concentration of nanowires, the surface roughness will increase and therefore the scattering and reflection from the surface will increase. As a result, transparency is reduced.
After FE-SEM tests, in order to identify the present phases in the samples, XRD test was done. Figure 3 a shows the peaks extracted from XRD test. (By matching the peaks with the reference samples, the phases in sample E have been identified by xpert software and are presented in Table 1.
As shown in Table 1, in addition to silver metal, the sample also contains chloride and oxide phases. The resulting peaks at 38, 44, 64 and 78 degrees are, respectively, assigned to the plates (111), (200), (220) and (311) of body centered cubic (BCC) silver. According to Xpert software, peaks of 25, 32 and 34, respectively, indicate the presence of silver chloride and silver oxide.
3.2 Influence of removing particles
In the FE-SEM images, it is obvious that the film contains both nanowires and nanoparticles. In Fig. 4, it can be clearly seen that sample B with the lowest film density compared to samples C, D and E has the highest transparency, which is due to the less amount of nanowires that cause less scattered and reflected light beam. In fact, an inverse relationship between the density of nanowires and the amount of light transmission is considered. Furthermore, as mentioned, an inverse relationship between the nanowire density and the sheet resistance of the sample is conceivable. By combining these, it can be concluded that there is a direct relationship between sheet resistance and transparency. Similarly, sample E, which is the most dense sample, has the lowest sheet resistance of 22 Ω/□ and it also has the lowest transparency of 47%.
As seen in Fig. 4 the transparency in silver nanowire samples remains constant at different wavelengths between 360 and 700 nm and even higher (1700 nm) wavelengths; however, ITO samples do not show such stability [16, 17]. This is what makes silver nanowire much more practical in various industries compared to ITO.
The problem in synthesis of nanowires is the existence of the ineluctable nanoparticles, which not only reduces sheet resistance, but also reduces the transparency of the samples. it’s been one of the major issues to avoid and remove the aggregates. As shown in the EF-SEM figures, aggregate removal process is an essential requirement for the present samples. Therefore, efforts to solve this problem were made just before centrifuge stage, as follows:
First, deionized water was added and after dilution, 80 ml acetone was added. The resulting water–acetone density is more than the density of trapped water inside the agglomerate, so agglomerated particles float on the top of liquid. Finally, the upper part was removed via a pipette quite easily the precipitated part, was dispersed in distilled water. Then centrifuge was done in order to remove Poly vinyl pyrrolidone.
Figure 5a,b are FE-SEM images before and after the processes above. Figure 5a refers to the unprocessed ink so that much space is occupied by the aggregates and Fig. 5b refers to the optimal sample achieved by the process. As a matter of fact, since aggregates were separated, it is going to enhance the transparency of the sample.
As shown in Fig. 6, The maximum transmission in sample B is 91.03%, which occurs at the wavelength of 526 nm, while the maximum transparency of the sample with the same concentration of nanowires after removal process (named B2) is 92.8%.
Similarly, transparency increases in samples C and C2 from 78.7 to 81.4% and in samples D and D2 from 63.4 to 67.1%. This increase in transparency is due to the reduction of nanoparticles in the film.
3.3 Influence of annealing
3.3.1 Deoxidation
Researches show that the chloride unwanted phase does not adversely affect the desired properties of the transparent electrode like conductivity and transparency. However, increasing the reaction time, and the resulting pressure in the autoclave or even sun light, leads to the decomposition of the silver chloride phase [15].
Unlike the chloride phase, silver oxide, which is a p-type semiconductor with a blackish-brown color can increase the sheet resistance of the sample and makes the result unfavorable for use as a transparent conductive electrode[18]. Although silver is normally considered a noble metal (other than at its melting point) and does not have a significant reactivity, it is considered the most reactive metal among noble metals. According to the reversibility of the composition of silver oxide reaction, the oxide phase decomposes into metallic silver and oxygen gas at temperatures between 160 and 300 °C [19] this is the exact reverse of the composition reaction, which is achieved through annealing at 160 °C for 15 min. In order to eliminate the chloride phase, the reaction time in the oven was increased from 18 to 24 h, so that the chloride phase is decomposed and converted to pure metallic silver. After performing the actions above, XRD test was done again. The result of this test is presented in Fig. 3 b. It is determined by X-pert software that the only phase in the film structure is the silver phase, while the chloride and oxide phase have been removed from the sample. Table 2 is extracted from X-pert software and indicates the existence of the only phase which is silver.
3.3.2 Junctions
As the density of silver film increases, more conductive paths are created, which lead to more conductivity [15]. Therefore, sheet resistance of a sample with more density would be less than a sample with less density. Of course, this increase in density will lead to an increase in the collision of nanowires and the created junctions. In fact, the junctions are the places of electricity loss and will cause an increase in sheet resistance. Using a Four-point probe, the sheet resistance of the samples was measured; the results of this test are reported in Table 1.
Since junctions are the susceptible areas where most energy loss occurs, stronger connection at junctions should be created.
Not only deoxidation but also stronger connection at junctions could be achieved by annealing process, which can lead to less sheet resistance. It is clearly shown in Fig. 7a, b that stronger connection between nanowires as the result of annealing process is achieved.
As shown, Fig. 7a refers to the first sample which was not annealed, whereas Fig. 7b refers to the annealed sample (Table 3).
As shown in Fig. 7a, the connection between nanowires is extremely weak because of not doing annealing process and the weak connection that was mentioned, increases energy loss and therefore increases sheet resistance of the sample. Table 4 shows the sheet resistance of different annealed and non-annealed samples. Although annealing at 120 °C reduced the sheet resistance of the sample, it has not been significant, and this is due to the insufficient temperature and time for diffusion. Diffusion, which means displacement of mass due to the transfer of atoms, is directly dependent on temperature.
It is expected that by increasing the temperature over 160 °C, sheet resistance decreases due to the easier diffusion process. However, Table 4 shows that, there has not been a reduction, but instead there has been an increase in the sheet resistance of sample P, that could be because of a change in the hexagonal structure and its formation. As the temperature continues to increase, the nanowires become unstable and the phenomenon of Rayleigh instability occurs [20], leading to the spheroidisation of the nanowires. Indeed, the AgNW network minimises its surface energy and the nanowires break down into small semi-spherical particles forming a line of spheroids this leads to the destruction of network conduction, since the nanowires are no longer interconnected.
3.4 Electrical and optical properties
4 Samples that share the same concentration with samples D, C, and B with the mentioned optimizing processes were made and were named D2, C2, B2.
These samples were tested before and after annealing at 160 °C for 15 min in order to investigate the effect of the processes. In Table 5 the result of 4-point probe test is reported.
As shown in Table 5, the sheet resistance of sample B reduced from 130 to 117 Ω/□ by annealing at 160 °C for 15 min.
Similarly, in sample C, with a 13-unit decrease in the sheet resistance by annealing and thus reducing energy loss at junctions, sheet resistance reached 76 Ω/□.
In sample D, due to the high density of nanowires and more junctions in this sample compared to the other samples, the energy loss is higher, so by annealing at the optimum temperature of 160 °C for 15 min and creating stronger connections at junctions, the greatest reduction in the sheet resistance in this sample has been achieved (17%) which is 43 Ω/□.
To evaluate transparent conductive electrodes, the Figure of merit (FoM) is a typical parameter commonly used, which applies to optimize electrode designs. The FoM is calculated with the sheet resistance (Rs) and the optical transmittance (T) as defined by Haacke as follows [21].
As shown in Table 5 the figures of merit for samples C2 with a sheet resistance of 76 Ω/□ and transparency of 81.4% and B2 with a sheet resistance of 117 and transparency of 94.5% are 1.8 × 10−3 and 4.8 × 10−3, respectively, and could have various usages in different industries.
Lack of adhesion between silver nanowires and glassy substrates has always been a problem of silver nanowires. A glassy sample was coated after the surface was prepared and its sheet resistance was measured.
After the sheet resistance in different parts of the sample was measured, 0.5 ml of polyaniline solution, with the intention of bringing adhesion and preventing from oxidation, was coated onto 2 cm2 of the sample using Mayer-rod coating method.
After the sample dried up, a 4-point probe test was conducted, and the results are given in Table 6. The results indicate about threefold increase in the sheet resistance of the sample after polyaniline was coated, which is due to the diffusion of polyaniline into the junctions of the nanowire network.
5 Conclusion
In the present study, silver nanowires were synthesized using polyol method and silver nanowires were deposited onto glassy samples using drop-casting method, with the aim of maintaining transparency and bringing electrical conductivity in glassy samples.
-
The optimal sample was made with a sheet resistance of 76 Ω/□ and a transparency of 81.4%.
-
Deoxidation caused an approximate reduction of 8 to 9% in the sheet resistance of the sample.
-
Annealing operation at the optimal temperature caused 4% reduction in sheet resistance.
-
There was a 5% increase in the transparency of the sample when nanoparticle aggregates were removed.
-
A threefold increase in the sheet resistance of samples were reported after polyaniline was coated onto the silver nanowire film.
Data availability
All data generated or analysed during this study are included in this published article.
References
X. Li, J. Zhou, D. Yan, Y. Peng, Y. Wang, Q. Zhou, K. Wang, Effects of concentration and spin speed on the optical and electrical properties of silver nanowire transparent electrodes. Materials 14, 2219 (2021)
Y. Zhang, J.N. Guo, D. Xu, Y. Sun, F. Yan, one-pot synthesis and purification of ultralong silver nanowires for flexible transparent conductive electrodes. ACS Appl. Mater. Interfaces 9, 25465–25473 (2017)
F. Qiang, M. Jinlei, L. Xuhua, Z. Xingwei, Z. Wenxiao, T. Mingwei, Z. Shiefeng, Q. Lijun, Z. Xueji, Biomimetic hierarchically silver nanowire interwoven MXene mesh for flexible transparent electrodes and invisible camouflage electronics. ACS Nano Lett. 22, 740–750 (2022)
M. Oh, W. Jin, H. Jeong, M. Jeong, J. Kang, H. Kim, Silver nanowire transparent conductive electrodes for high-efficiency III-nitride light-emitting diodes. Sci. Rep. 5, 13483 (2015)
D.H. Kim, N.H. Park, T.W. Kim, Highly efficient flexible organic light-emitting devices based on PEDOT:PSS electrodes doped with highly conductive Pyronin B. Nano Energy 65, 104027 (2019)
S. Fahad, H. Yu, L. Wang, Recent progress in the synthesis of silver nanowires and their role as conducting materials. Mater. Sci. 54, 997–1035 (2019)
F. Fiévet, F. Ammar-Merah, R. Brayner, The polyol process a unique method for easy access to metal nanoparticles with tailored sizes, shapes and compositions. Chem. Soc. Rev. 47, 5187–5233 (2018)
L. Zhang, T. Song, L. Shi, N. Wen, Z. Wu, C. Sun, D. Jiang, Z. Guo, Recent progress for silver nanowires conducting film for flexible electronics. Nanostruct. Chem. 11, 323–341 (2021)
J. Han, J. Yang, W. Gao, H. Bai, Ice-Templated, Large-area silver nanowire pattern for flexible transparent electrode. Adv. Func. Mater. 31, 2010155 (2021)
S. Lim, Cu-based multilayer transparent electrodes: a low-cost alternative to ITO electrodes in organic solar cells. Sol. Energy Mater. Sol. Cells 101, 170–175 (2013)
R. Yin, Sh. Yang, Q. Li, Sh. Zhang, H. Liu, J. Han, Ch. Liu, Flexible conductive Ag nanowire nanofibril hybrid nanopaper for strain and temperature sensing applications. Sci. Bull. 65, 899–908 (2020)
R. Chen, S.R. Das, Ch. Jeong, M.R. Khan, D.B. Janes, M.A. Alam, Co-Percolating graphene-wrapped silver nanowire network for high performance, highly stable transparent conducting electrodes. Adv Funct. Mater. 23, 5150 (2016)
J. Kim, S. Kim, H. Kil, Y. Kim, J. Park, Highly conformable, transparent electrodes for epidermal electronics. Nano Lett. 18, 4531–4540 (2018)
F. Xu, W. Xu, B. Mao, W. Shen, Y. Yu, R. Tan, W. Song, Preparation and cold welding of silver nanowire based transparent electrodes with optical transmittances >90% and sheet resistances <10 ohm/sq. J. Colloid Interface Sci. 512, 201–208 (2018)
B. Bari, J. Lee, T. Jang, P. Won, S. Ko, Kh. Alamgir, M. Arshadd, L. Guo, Simple hydrothermal synthesis of very-long and thin silver nanowires and their application in high quality transparent electrodes. Mater. Chem. A 29, 11141 (2016)
S. Na, S. Kim, Efficient and flexible ITO-free organic solar cells using highly conductive polymer anodes. Adv. Mater. 20, 4061–4067 (2018)
I. Hu, Sh. Tu, Zh. Huang, Y. Wei, Silver nanowires: synthesis technologies, growth mechanism and multifunctional applications. Mater. Sci. Eng. 223, 1–23 (2017)
M. Talukdar, E. Baker, Conductivity studies on silver oxide. Solid State Commun. 7, 309–310 (1969)
B. L’vov, Kinetics and mechanism of thermal decomposition of silver oxide. Thermochim. Acta 333, 13–19 (1999)
M.T. Molares, A.G. Balogh, T. Cornelius, R. Neumann, C. Trautmann, Fragmentation of nanowires driven by Rayleigh instability. Appl. Phys. 85, 5337–5339 (2004)
G. Haacke, New figure of merit for transparent conductors. Appl. Phys. 47, 4086 (1976)
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by MS, AE and AAA. The first draft of the manuscript was written by MS and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
This study was not supported financially by any organizations.
Human and animal rights
This article does not contain any studies involving human participants performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Saeidi, M., Eshaghi, A. & Aghaei, A.A. Electro-optical properties of silver nanowire thin film. J Mater Sci: Mater Electron 34, 110 (2023). https://doi.org/10.1007/s10854-022-09621-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10854-022-09621-8