Abstract
Present work deals with the development of high-speed NO2sensor based on functionalized Single Walled Carbon Nanotubes (SWNTs). To improve the sensing properties of SWNTs, SWNTs is functionalized with the enzyme N-benzyloxycarbonylglycine (Z-Gly-OH). Various parameters of the functionalization process such as time and temperature are also optimized and analysed in detail. Z-Gly-OH has created a functionalization of the 1,3-dipolar cycloaddition type, which is capable of doing modification in the properties of CNTs while maintaining the electronic properties of CNTs. The functionalization with Z-Gly-OH makes it possible to obtain amino groups on the surface of nanotubes in the absence of a solvent, while during this reaction it is possible to obtain intermediate functionalization in the form of benzyl carbamate, which can affect the sensitivity of sensors. In order to develop solid state device, Ti(8 nm)/Au(100 nm) interdigitated electrodes (IDEs) are fabricated on thermally oxidized Si substrate by using standard photolithography process. Dielectrophoresis is employed for deposition of as-functionalized SWNTs (f-SWNTs) between the IDEs. The gas sensing performance of as-developed is tested for NO2 gas as function of NO2 concentration (70 ppm to 20 ppm). As-developed gas sensor shows fast response/recovery (~ 88 s/95 s) as well as high sensitivity 27%. In order to analysed selectivity of as-developed gas sensor, the cross sensitivity has been observed for carbon monoxide (CO) and Methane (CH4). Before development of sensor, as-prepared f-SWNTs is analysed by SEM, Raman and FT-IR for its morphological and structural characteristics.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In modern era, environmental pollution becomes a major concern. As per World Health Organization (WHO) data, millions of people lost their life due inhaling of microscopic air-pollutant particles. The small quantity of mentioned pollutant particles is capable to make serious impact on our lungs, heart and brain [1]. Due to operation of combustion machineries and equipment, the harmful gases such as nitrogen dioxide (NO2) and nitrogen oxide (NO) are mixing in the environment. Such type of toxic gases is spoiling the eco-system of earth and continuously making bad impact on human as well as wild-life [2]. The monitoring of toxic gases such as NO2 and NO is become an urgent task. Apart from eco-system of environment, monitoring of NO2 in exhaled air from our body is also has importance in medical field. It has been reported that monitoring the NO2 in exhaled air from human body can detect the early stage of lungs disease such as chronic obstructive pulmonary disease (COPD) [3].
In addition, NO2 is used for the production of nitric and sulfuric acids and other applications [4]. The annual turnover of nitric acid in Russia alone is 71 million tons per year, which demonstrates the volumes of NO2 used only as raw materials [5]. In addition, nitrogen dioxide is also a waste gas obtained as a result of other industries, in particular, in the field of oil production [6]. The main disadvantage of nitric oxide is its extremely strong toxicity, which is why nitric oxide is dangerous to the environment, since it poisons the environment, and dangerous to humans, especially to the respiratory and nervous systems [7].
The discussed issues and importance of NO2 monitoring motivate us to work on NO2 detector. Nowadays, large number of techniques have been analysed for developing NO2 gas sensor [8, 9]. Despite the existence of known solutions, this field is constantly developing due to the invention of new concepts, usage of new materials, which leads to the miniaturization of devices and the ability to do more accurate analysis of the environment composition or the substance under study [9]. So, in the field of research related to the detection of NO2 gas, the use of carbon nanomaterials, in particular, carbon nanotubes (CNTs), has undeniable advantages and in general is a good prospect [10].
Since discovery of CNTs, It has received rapid growth and wide distribution among the scientific community. Due to exceptional properties of CNTs such as high conductivity, high thermal stability and large surface area, CNTs, still, has an increasing relevance. CNTs can be used in various applications, in particular, as a conductive polymer material, sensing element in detectors, high-strength material etc. [11,12,13,14].
The main advantage of CNTs in the field of gas sensors is the ability to detect molecules at gas concentrations equal to 1 ppb [15]. In addition, a significant advantage of carbon nanotubes is that the gas sensors based on CNTs, in general, is a low cost product. The disadvantages in CNTs based gas sensor include the lack of selectivity, slow response and recovery [10]. The limitations over the CNTs based sensors can be improved with the help of surface modifications [10]. Currently, the following research areas are relevant to the gas sensors based on CNTs:
-
increasing the selectivity of CNTs to certain substances;
-
creation of the most sensitive CNT composites;
-
creation of sensor arrays for wide-profile detection.
As for sensor arrays, there has been a various amount of publications. These arrays can overcome the most important drawback of CNTs (a lack of selectivity). In this case CNTs with different type of functionalizations are used. That allows the sensor detect different gases in a different way and differentiate them [16].
At the moment, quite a lot of CNT functionalizations have been developed that allow creating durable composites [17]. Nevertheless, in the articles using modified CNTs for gas sensors, a rather limited range of modifying substances is used, which needs to be expanded, in particular, for the task of increasing the selectivity of these sensors [10].
In general, non-covalent and covalent functionalization is utilized for manipulation the surface properties of CNTs. Such type of functionalization is further divided into destruction of the crystal lattice ("sidewall") of CNTs and by the mechanism of 1,3 dipolar cycloaddition [18]. Researchers also developed the functionalization process for tip of CNTs as well as endohedral functionalization, but these types of functionalizations are not performed for making gas sensors [19].
The difference between non-covalent functionalization and covalent functionalization is that in the first case, functionalization occurs without the formation of any chemical bonds; in the second case, depending on the type (with the destruction of the crystal lattice or by the mechanism of 1,3 dipolar cycloaddition), either complete destruction of the crystal lattice occurs at the site of defect localization or at the ends of CNTs (with deterioration of the conductivity of CNTs, since the creation of defects is performed artificially using strong acids), or partial transformation of CNT double bonds into single ones with the addition of some chemical group/functionalizing substance [19]. The result of covalent functionalization is usually the formation of ketones, carboxyl, carbonyl or hydroxyl groups, which can later be subjected to secondary modification [20]. At the same time, in the case of the 1,3 dipolar cycloaddition reaction, it is possible, to directly obtain amino groups on the CNT surface by the mechanism of the Prato reaction [21].
A significant disadvantage of non-covalent functionalization is the limitation of further interactions with CNTs due to weak Van der Waals forces between CNTs and the functionalizing substance (for example, further functionalization of CNTs), while there is no such restriction with covalent functionalization [22]. It is also worth noting that when large molecules of a functionalizing substance are attached (in the case of non-covalent functionalization), the problem of exceeding the Debye length may arise, so that, on the contrary, the sensitivity of sensors will deteriorate or the usage of CNTs will be pointless, since they will not participate in the detection process [23]. Hence, the reaction of 1,3 dipolar cycloaddition is the most preferable option for the modification of CNTs, since it does not significantly impair the physical properties of CNTs, while it creates strong bonds with the functional group.
In the scientific literature there are many methods of CNT functionalization by cycloaddition, for example, reaction with azomycin ylides, with nitrenes, with carbenes, reactions with zwitter ions, Bingel reaction and addition of imines [24].
Many of these reactions are used to produce various functional groups that may have a high reactivity to a particular class of chemicals. For example, carboxyl groups react well to ammonia and amino groups, and amino groups, being bases, can act as proton acceptors and react with acids, etc. [20].
In addition, functionalized CNTs have the ability to polymerize depending on the type of functional group and the spatial structure of the carbon skeleton [20]. Much attention in research is paid to amino groups, because substances containing this group polymerize well and, moreover, may have good conductivity [20]. Thus, polyaniline is often used as a functionalizing substance [25]. CNTs functionalized by amino groups also have the ability to be embedded in polymer matrices to improve the properties of composite materials [20].
In addition to being used in polymer chemistry, such CNTs have been found excellent as sensitive gas sensors, for example, NO2 gas sensors [26].
Among the existing sensors on CNTs with various modifications, CNTs functionalized by amino groups stand out especially because they have high sensitivity to NO2 gas [27].
The effectiveness of this functionalization was noticed for the purpose of creating sensors due to the fact that carbon in the state of sp2 hybridization participates in the formation of a bond and spends an unpaired electron on the formation of a covalent bond; hence the nanotube loses charge carriers during functionalization. The increased sensitivity of the sensor is apparently associated with a decrease in the number of free electrons in the crystal lattice and, consequently, the predominance of acceptor characteristics. Thus, NO2, having one unpaired electron, acts as a donor of the latter and, being in the vicinity of the CNT, gives away a free electron adsorbed on the surface of the CNT, which in turn increases the conductivity of the entire nanotube [28].
Thus, the production of amino groups on the surface of carbon nanotubes is an urgent task in the field of polymer chemistry, as well as in the task of manufacturing sensors. Particularly acute is the need to create waste-free methods of CNT functionalization, after which no further purification of the resulting raw materials from a solvent or catalyst is required [29].
As mentioned above, the amidation of CNTs by 1,3 dipolar cycloaddition is carried out by the Prato reaction mechanism. This reaction is based on the interaction of CNT with the result of the interaction of an amino acid and paraformaldehyde, which during the reaction is depolymerized into gaseous formaldehyde (or in the liquid phase, depending on the presence of a solvent) [30]. After heating this mixture, an ylide (oxazolidinone [21]) is formed, which interacts with CNT. The resulting amount of oxazolidinone formed during the reaction significantly affects the result of functionalization [21]. In such type of functionalization, temperature is a key parameter [31]; the reaction temperature should not exceed 210 °C due to the possibility of decomposition of oxazolidinone at higher temperatures [21]. It is also possible to pre-process the initial reagents, in particular, paraformaldehyde, for example, using an agate mortar [32]. This treatment allows increasing the degree of homogeneity of the mixture and speed up the reaction time [21].
There are various approaches to perform this functionalization. Thus, it would be nice to note the functionalization with a α-amino acid derivative in a dimethylformamide solution, which was carried out with C60 fullerenes [31]. Functionalization was carried out for 5 days at a temperature of 130 °C. As the experiment in the reference [33] showed, the functionalization can be reduced to 30 h (the functionalization of nanofibers was carried out) compared to the functionalization in dimethylformamide, which was performed within 120 h. Also, it should be noted that there is no solvent in the reaction, there are no by-products, which is an additional advantage.
As a result of functionalization, two functional groups can be formed – benzyl carbamate and pyrollidine (see Fig. 1a and b) [34].
A distinctive feature of amidated CNTs is the good solubility and stability of CNT solutions (about 2 weeks) in polar solvents such as water, ethanol, acetone, chloroform, dichloromethane etc. [35].
As per design of sensors based on CNTs, mainly, capacitive and resistive sensors are developed. For development of such type of gas sensor, various types of CNTs such as vertical aligned, horizontally aligned/randomly arranged are used [36]. It is worth noting that resistive sensors on CNTs manufactured using dielectrophoresis are one of the concepts that can allow creating highly sensitive sensor arrays for large-format detection when using CNTs functionalized with various substances [37].
The present work is devoted to the research and development of a high-speed NO2 gas sensor based on f-SWNTs. The SWNTs is functionalized with the Z-Gly-OH by using a one-pot functionalization method. The 1,3 dipolar cycloaddition by was chosen as the functionalization method, since this functionalization makes it possible to simplify the process of obtaining amino groups on the CNT surface by performing functionalization in the absence of a solvent, which in the future can also be used to obtain composite materials on an industrial scale. The solid state device is fabricated by deposition of as-developed f-SWNTs between the gold IDEs. The gold IDEs were fabricated by standard photolithography technique on SiO2/Si substrate and f-SWNTs is deposited between the IDEs by using well-known dielectrophoresis technique. Prior to development of solid state device, CNTs (pre and post functionalization process) is analysed by SEM and Raman spectroscopy.
The functionalization results were verified by using IR spectroscopy, also the stability of the CNTs in polar solvents such as water was analyzed. Several approaches to the functionalization were studied and the solution with the largest number of amino groups was selected.
2 Experimental section
SWNTs with 95% purity (length = 5-30 µm, diameter = 1-2 nm) is bought from Chengdu Organic Chemicals Co. Ltd., Chinese Academy of Sciences. The enzyme N-benzyloxycarbonylglycine (Z-Gly-OH) (purity > 98%) is purchased from Alfa Aesar. And paraformaldehyde (purity = 95%) is bought from Loba Chemie Pvt. Ltd.
Functionalization of SWNTs was performed in the absence of solvent by a one-pot functionalization process [36]. In general 30 mg of granulated paraformaldehyde were grounded in an agate mortar to a homogeneous powder state to fasten the process. The resulting powder was mixed with N-benzyloxycarbonylglycine Z-Gly-OH (5 mg) and single-walled carbon nanotubes (5 mg) in ethanol. The solution was then slowly evaporated and heated in the oven at a temperature of 210 °C for 3 h.
Studies of as-developed f-SWNTs were carried out on the Nicolet iS50 IR spectrometer using the ATR prefix. The morphological and structural quality of as-developed f-SWNTs was investigated by SEM and Raman spectroscopy.
Interdigitated electrodes and parallel comb-like electrodes with a 108-nm-thick Au(100 nm) /Ti(8 nm) metallization layer on 90 nm SiO2 made by standard lithography process were used for the gas sensing. The gap between the electrodes was 5 µm.
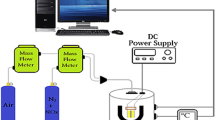
Solid state device was made dielectrophoresis techniques (see Fig. 1c). Dielectrophoresis was performed at the 5 Vpp, 900 kHz for 1 min for each sample. A drop of 2 µm was placed onto the electrodes. Sensor sensitivity measurements were performed with the exposure of gas sensors to NO2 gas as function of concentration (20 ppm–70 ppm). Experimental set-up for measuring the characteristics of sensors is shown in Fig. 1d. The desired concentration was achieved by mixing of air in NO2 gas flows.
Response curve was calculated as:
where \({R}_{\mathrm{initial}}\)—resistance of a gas sensor before the NO2 exposure, R—resistance of a gas sensor during the NO2 exposure.
The response/recovery time is crucial parameters for developing detectors for the desired application. The response/recovery times are defined as the time required for absorption and desorption of the NO2 on, or from the sensor surface to reach the saturation or to reduce the conductivity back to the baseline conductance.
3 Result and discussion
Figure 2a and b show the SEM images of SWNTs for pre and post functionalization process. Figure 2a shows the uniform distribution CNTs with fine diameter. It has been clearly visualized in the SEM image of Fig. 2b that some islands are constructed on sample. Islands are constructed due to agglomeration of CNTs in functionalization process. The structural quality of pristine CNTs as well as post functionalization has been analysed by Raman spectroscopy. Figure 2c shows the Raman spectra for pristine CNTs (in red) and post functionalize process (in black). The important peaks have been observed on 150 cm−1, 1290 cm−1 and 1560 cm−1. The intense peak around 150 cm−1 represents the RBM mode associated with SWNTs and it verifies the existence of SWNTs. The intense peak on 1560 cm−1 shows the G-band while the weak peak on 1290 cm−1 represents the D-band. The high intensity of G-band and low intensity of D-band verifies the high structural quality of CNTs. In case of f-SWNTs, the intensity of G-band has been reduced, sharply. It is possible due to thin coating of functionalization material on the surface of SWNTs. The creation of defects during the functionalization steps can also reduce the intensity of G-band. We have analysed the various method for functionalization of CNTs with Z-Gly-OH and the process gave better functionalization has been employed for development of gas sensor. It has been observed that the pre-crushing of paraformaldehyde and Z-Gly-OH in a mortar, increased the solubility and stability of as-obtained solution of f-SWNTs in water. Also the better dispersion gives the use of a formaldehyde solution, instead of dry paraformaldehyde. Due to the reaction between the paraformaldehyde and Z-Gly-OH a dipole is formed, the scheme of dipole formation can be seen in Fig. 2d. As-prepared dipole is found instable in nature; to provide stability to dipole prepared material was further reacted with acetonitrile (see Fig. 2e). The overall reaction made C4H8N2COOCH2Ph. In final step, as-prepared material was heated with SWNTs at 200 °C for 3 h. The resulting substance was analyzed by a FT-801 IR Fourier spectrometer (see Fig. 2f). In Fig. 2f, valence oscillations corresponding to peaks N–H are observed in the 3200–3164 cm−1 zone. This bond is also recorded in the 1628 cm−1 zone. In the 1448 cm−1 zone, there is a peak corresponding to fluctuations in the C–N–C bond. The N–H group is observed in the 3200–3164 cm−1 zone. Peaks 3003 and 2943 cm−1 corresponds to the vibrations of carbon atoms in the state of sp2 and sp3 hybridization, respectively.
a and b shows the SEM images for morphology of SWNTs and as-prepared f-SWNTs, respectively, c shows the Raman spectra for CNTs for pre (in red) and post (in black) functionalization process. d shows the Formation of a 1,3 dipole. The reaction between 1,3 dipole and acetonitrile can be seen in e and f shows the FT-IR spectra for as-prepared f-SWNTs (Color figure online)
In the zone from 1600 to 1375 cm−1 there are peaks corresponding to the pulsation vibrations of the aromatic ring in the molecule in the structure of the carbon chain, designated as R1. There is also a C–O–C group in the carbon chain, which corresponds to the peaks of deformation vibrations in the 1212 cm−1 zone [38].
The majority of peaks present in FT-IR spectra are well matched with existing data [40], which verifies the functionalization of CNTs with amino functional groups. The further analysis of as-prepared nanomaterials has been done by XRD and FT-IR (see Fig. 4 and 5 of Supplementary data file). XRD data for pre- and post-functionalized SWNTs has been verified that the functionalization process made very less defect on surface of SWNTs.
It has been observed that the resulting solutions have good dispersion, unlike pure non-functionalized CNTs, and the degree of dispersion increases with the increment in N–H groups on the surface of the crystal lattice of CNTs. Also, the obtained dispersion had sufficient stability; complete settling of CNTs in solutions occurs within 1 day, however, if the solutions are slightly shaken, the CNTs will be mixed evenly in the solution again. Figure 3 shows the sensing response of as-developed f-SWNTs based device. The sensing performance has been analyzed for NO2 gas as a function of carrier concentration of gas (70 ppm to 20 ppm). To analyze the sensing performance of as-developed device, the value of resistance has been recorded in real time while the flow of NO2 turned ON/OFF for repetitive cycles. Figure 3a shows the change in resistance in real time for multiple cycles as function of concentration of NO2 gas. It has been clearly observable in Fig. 3a the resistance of device reduced sharply with turning ON flow of NO2 gas. The value of device resistance is returned to its previous state with stopping of NO2 supply. The sensing performance of as-developed device has been recorded for 20 ppm, 30 ppm and 70 ppm concentration of NO2 gas. Initially the base resistance of device is recorded around 221 KΩ and, when the NO2 flow with 70 ppm concentration supplied to sensing chamber, the resistance of device rapidly reduced to 163 KΩ. When the NO2 supply inside the chamber disconnected, the resistance of device again reached to it base value (221 KΩ). Later, the sensing performance was recorded for 50 ppm and 20 ppm of NO2 gas supply and the pattern in change in resistance value was found same for all concentration of NO2. It has been observed that change in resistance was highly dependent on concentration of NO2 gas. The change in resistance has been found as 58 KΩ, 42 KΩ and 26 KΩ for 70 ppm, 50 ppm and 20 ppm of NO2 gas flow, respectively. As seen in Fig. 3a, the sensing performance has been recorded for multiple cycles and all cycles show quite stable behavior. The other important sensing parameters such as response and recovery time have also been observed. As-developed sensor shows very fast response time. The response time of sensor has been found as 88 s, 130 s and 115 s for 70 ppm, 50 ppm and 20 ppm concentration of NO2 gas. In case of gas sensor long recovery time is always matter of concern. In case of present work, the recovery time has been found around 600 s for 70 ppm NO2 gas while the recovery was performed by naturally in air. The recovery time was abruptly improved by illumination of sensor with a UV lamp. With illumination of UV-light, the recovery time has been found in the range of 95 to 120 s. The sensitivity plot for as-developed sensor can be seen in Fig. 3b. As-developed sensor shows very high sensitivity for all tested concentration of NO2. The values of sensitivity has been found as 26.58%, 19.3% and 10.54% for NO2 gas concentration 70 ppm, 50 ppm and 20 ppm, respectively. In our previous reported work, the sensing performance of pristine SWNTs as well as PEI-functionalized SWNTs had been observed [39]. It has been observed that pristine SWNTs based gas sensor shows response time around 300 s while PEI- functionalized SWNTs shows the response time 240 s for 50 ppm of NO2 gas concentration. In present case, the response time has been reduced by 2.3 times and 1.85 times as compare to pristine SWNTs and PEI- functionalized SWNTs based sensor, respectively [39]. In general, SWNTs is known as p-type semiconductor. When the interaction between SWNTs and NO2 molecules is occurred, NO2 molecules absorbed the electrons from SWNTs. In the result of this phenomenon, concentration of majority carriers increased in SWNTs; hence resistance of device decreased sharply. When the molecules are detached from SWNTs, resistance of device increased again [40, 41]. In case of functionalization of SWNTs with an amino functional group, the acceptor property of SWNTs increased sharply. Also, the amino group provides the pathway for the transportation of gas molecules [42]. These are the possible reasons to get the fast response and high sensitivity in f-SWNTs-based gas sensor. The NO2 sensing performance of as-developed sensor has been compared with existing data (see Table 1) and it has been observed that as-developed sensor shows very fast response with acceptable sensitivity.
The selectivity of the as-developed sensor has been verified by observing the cross-sensitivity with CO and CH4. In both cases, the value of resistance has been recorded for various concentrations of CH4 and CO [See Fig. 1a and b of Supplementary data file]. In the case of CH4, a small shift in resistance has been noticed but the values of change in resistance are found as same for 30 sccm to 70 sccm of CH4 flow. In the case of CO, no proper response has been found. In both cases, signals have been found very noisy. The value of the base resistance of the device has been found to increase in both cases. Another important point has been noticed that the as-developed f-SWNTS based gas sensor showed very slow recovery for CH4 as well as CO. In order to find out lower and higher limit of NO2 gas detection, we examined as-developed f-SWNTs-based gas sensor with maximum range of our gas sensing setup i.e. from 5 to 150 ppm. Surprisingly, the response of f-SWNTs based NO2 sensor has been found very high for 5 ppm as well as 150 ppm of NO2 gas. The sharp change in resistance has been clearly observable for 5 ppm as well as 150 ppm of NO2 exposure. As-developed device shows very fast response/recovery time (around 1 s) for 5 ppm as well as 150 ppm of NO2 gas. The values of sensitivity have been estimated as 43.5% and 51% for 5 ppm and 150 ppm of NO2 gas, respectively [See Fig. 2a and b of Supplementary data file]. The results verified that as-developed f-SWNTs based device can detect NO2 in broad range of concentration.
4 Conclusion
In present work, a high speed NO2 gas sensor with high sensitivity has been developed. As-developed gas sensor is worked on the principle of the chemiresistive and operable on room temperature. The solid state device was successfully fabricated based on Z-Gly-OH functionalized SWNTs. The sensing performance of as-developed device has been analyzed as function of concentration of NO2 gas (20 ppm–70 ppm). The fastest response time and sensitivity has been found as 88 s and 26.58% for 70 ppm NO2 gas, respectively. As-developed f-SWNTs based gas sensor shows fast and better response than pristine as well as PEI-functionalized SWNTs.
Prior to development of device, SWNTs was successfully functionalized with amino group using solvent free, one pot functionalization process. The prepared f-SWNTs was analyzed for their morphological and structural properties by SEM and Raman spectroscopy. The attachment of amino functional group on the surface of SWNTs was verified by FT-IR transmittance analysis. The device has been prepared by utilizing standard photolithography and DEP process.
Data availability
There is no any data need to be presented. This is normal research article which is carried out in the University. All data generated or analyzed during this study are included in this article.
References
P. Mehrotra, Biosensors and their applications: a review. J. Oral Biol. Craniofac. Res. 6(2), 153 (2016)
C.C. Conrad, K.G. Hilchey, A review of citizen science and community-basedenvironmental monitoring: issues and opportunities. Environ. Monit. Assess 176, 273 (2011)
Y. Bar-Cohen, Biomimetics—using nature to inspire human innovation. Bioinspir. Biomim. 1, 1 (2006)
«Sulfuric acid production in Russia 2016–2025», Statista Research Department, 2021
"Mapкeтингoвoe иccлeдoвaниe pынкa aзoтнoй киcлoты"-TK Solution aнaлиз pынкoв. Бизнec плaниpoвaниe, https://tk-solutions.ru/russia-rynok-azotnoy-kisloty-has to be translated!
I. Ialongo, N. Stepanova, J. Hakkarainen, H. Virta, D. Gritsenko, Satellite-based estimates of nitrogen oxide and methane emissions from gas flaring and oil production activities in Sakha Republic Russia. Atmos. Environ. 10, 11 (2021)
Alexandra Amaducci, John W. Downs, «Nitrogen Dioxide Toxicity», StatPearls, January 2022
S. Kumar, V. Pavelyev, P. Mishra, N. Tripathi, A review on chemiresistive gas sensors based on Carbon Nanotubes: Device and Technology transformation. Sens. Actuators A 283, 174 (2018)
J. Wang, W. Zhang, R. Cao, Y. Xiangyu, H. Lai, Analysis of nitrogen dioxide in environment. Adv. Biosci. Biotechnol. 7(6), 278 (2016)
V. Schroeder, S. Savagatrup, M. He, S. Lin, T.M. Swager, Carbon nanotube chemical sensors. Chem Rev. 119(1), 599 (2019)
S. Iijima, Helical microtubules of graphitic carbon. Nature 354, 56 (1991)
N. Sharma, P. Sharma, C. Patel, Synthesis, properties and applications of carbon nanotubes review article. Mater. Sci. Eng. 6(2), 61 (2019)
V.N. Popov, Carbon nanotubes: properties and application. Mater. Sci. Eng. 43(3), 61 (2004)
N. Tripathi, P. Mishra, B. Joshi, S.S. Islam, Precise control over physical characteristics of Carbon Nanotubes by differential variation of Argon flow rate during Chemical Vapor Deposition processing: a systematic study on growth kinetics. Mater. Sci. Semicond. Process. 35, 207 (2015)
M. Fujioka, H. Watanabe, Y. Martin, M. Nakano, Member, IEEE and J. Suehiro, Separation and enrichment of semiconducting carbon nanotubes and its application to highly sensitive carbon nanotube gas sensor. In: IEEE nanotechnology materials and devices conference, Jeju, pp. 18–21 (2011)
U. Yaqoob, M.I. Younis, Chemical gas sensors: recent developments, challenges, and the potential of machine learning: a review. Sensors 21(8), 2877 (2021)
B.I. Kharisov, O.V. Kharissova, Coordination and organometallic compounds in the functionalization of carbon nanotubes. J. Coord. Chem. 67, 23–24 (2014)
S. Banerjee, T. Hemraj-Benny, S.S. Wong, Covalent surface chemistry of single-walled carbon nanotube. Adv. Mater. 17(1), 17 (2005)
R. Araujo, M.C. Paiva, M.F. Proenca, C.J.R. Silva, Functionalization of carbon nanofibres by 1,3-dipolar cycloaddition reactions and its effect on composite properties. Compos. Sci. Technol. 67, 806 (2006)
Francis Avilés, Juan V. Cauich-Rodríguez, Patricio Toro-Estay, Mehrdad Yazdani-Pedram, Héctor Aguilar-Bolados, Improving Carbon Nanotube/Polymer Interactions in Nanocomposites. In: Carbon nanotube-reinforced polymers, 1st ed., Chapter: 5, 2017, pp. 83–115.
R. Araújo, F.M. Fernandes, M.F. Proença, C.J.R. Silva, M.C. Paiva, The 1,3-dipolar cycloaddition reaction in the functionalization of carbon nanofibers. J. Nanosci. Nanotechnol. 7, 3441 (2007)
A.D. Crescenzo, V. Ettorre, A. Fontana, Non-covalent and reversible functionalization of carbon nanotubes. Beilstein J. Nanotechnol 5, 1675 (2014)
Andrey V. Dobrynin, 1.05-Solutions of Charged Polymers, In: Polymer science: a comprehensive reference, University of Connecticut, Storrs, Volume 1, 2012, pp. 81–132
H.-C. Wu, X. Chang, L. Liu, F. Zhao, Y. Zhao, Chemistry of carbon nanotubes in biomedical applications. J. Mater. Chem. 20(6), 1036 (2010)
D. He, C. Zeng, C. Xu, N. Cheng, H. Li, S. Mu, M. Pan, Polyaniline-functionalized carbon nanotube supported platinum catalysts. Langmuir 27(9), 5167 (2011)
I. Sayagoa, H. Santos, M.C. Horrillo, M. Aleixandre, M.J. Fernández, E. Terrado, I. Tacchini, R. Aroz, W.K. Maser, A.M. Benito, M.T. Martínez, J. Gutiérrez, E. Munoz, Carbon nanotube networks as gas sensors for NO2 detection. Talanta 77, 758 (2008)
K. Timsorn, C. Wongchoosuk, Adsorption of NO2, HCN, HCHO and CO on pristine and amine functionalized boron nitride nanotubes by self-consistent charge density functional tight-binding method. Mater. Res. Express 7, 5 (2020)
Y. Li, M. Hodak, W. Lu, J. Bernholc, Mechanisms of NH3 and NO2 detection in carbon-nanotube-based sensors: an ab initio investigation. Carbon 101, 177 (2016)
R.F. Araújo, M.C. Paiva, M.F. Proenca, C.J.R. Silva, Functionalization of carbon nanofibers by 1,3 - dipolar cycloaddition reactions and its effect on composite properties. Compos. Sci. Technol. 67, 806 (2006)
M. Maggini, G. Scorrano, M. Prato, Addition of azomethine ylides to C60: synthesis, characterization, and functionalization of fullerene pyrrolidines. J. Am. Chem. Soc. 115, 9798 (1993)
M.C. Paiva, F. Simon, R.M. Novais, T. Ferreira, M.F. Proenca, W. Xu, Controlled functionalization of carbon nanotubes by a solvent-free multicomponent approach. ACS Nano 4, 7379 (2010)
M.C. Paiva, R.M. Novais, R.F. Araujo, K.K. Pederson, M.F. Proença, C. Silva, C. Costa, S. Lanceros-Méndez, Organic functionalization of carbon nanofibers for composite applications. Polym. Compos. 31(3), 369 (2010)
D. Tasis, N. Tagmatarchis, V. Georgakilas, M. Prato, Soluble carbon nanotubes. Chem. Eur. J. 9, 4000 (2003)
M.C. Paiva, F. Simon, R.M. Novais, T. Ferreira, M.F. Proenca, W. Xu, Controlled functionalization of carbon nanotubes by a solvent-free multicomponent approach. NANO 4, 7379 (2010)
K. Chun, I. Moon, J. Han, S. Do, J. Lee, S. Jeon, Highly water-soluble multi-walled carbon nanotubes aminefunctionalized by supercritical water oxidation. Nanoscale 5(21), 10171 (2013)
W. Yun, J.T.W. Yeow, A review of carbon nanotubes-based gas sensors. J. Sens. 2009, 1 (2009)
W. Li, F. Hennrich, B.S. Flavel, S. Dehm, M. Kappes, R. Krupke, Principles of carbon nanotube dielectrophoresis. Nano Res. 14, 2188 (2021)
O. Eren, N. Ucar, A. Onen, N. Kizildag, I. Karacan, Synergistic effect of polyaniline, nanosilver, and carbon nanotube mixtures on the structure and properties of polyacrylonitrile composite nanofiber. J. Compos. Mater. 50, 15 (2015)
S. Kumar, V. Pavelyev, P. Mishra, N. Tripathi, Thin film chemiresistive gas sensor on single-walled carbon nanotubes-functionalized with polyethylenimine (PEI) for NO2 gas sensing. Bull. Mater. Sci. 43, 1 (2020)
D. Kumar, I. Kumar, P. Chaturvedi, A. Chouksey, R.P. Tandon, P.K. Chaudhury, Study of simultaneous reversible and irreversible adsorption on single-walled carbon nanotube gas sensor. Mater. Chem. Phys. 177, 276 (2016)
G.P. Evans, D.J. Buckley, N.T. Skipper, I.P. Parkin, Single-walled carbon nanotube composite inks for printed gas sensors: enhanced detection of NO2, NH3 EtOH and acetone. RSC Adv. 4(93): 51395 (2014)
W. Liu, L. Xu, K. Sheng, C. Chen, X. Zhou, B. Dong, X. Bai, S. Zhang, G. Lu, H. Song, APTES-functionalized thin-walled porous WO3 nanotubes for highly selective sensing of NO2 in a polluted environment. J. Mater. Chem. A 6, 10976 (2018)
S. Liu, B. Yu, H. Zhang, T. Fei, T. Zhang, Enhancing NO2 gas sensing performances at room temperature based on reduced graphene oxide-ZnO nanoparticles hybrids. Sens. Actuators B 202, 272 (2014)
M. Naik, Y.-S. Lee, A. Qurashi, Chemically grafted aminated carbon nanotubes and l-lysine in ultramodified conditions for carbon dioxide storage. ACS Omega 3(9), 10442 (2018)
P.-G. Su, C.-T. Lee, C.-Y. Chou, K.-H. Cheng, Y.-S. Chuang, Fabrication of flexible NO2 sensors by layer-by-layer self-assembly of multi-walled carbon nanotubes and their gas sensing properties. Sens. Actuators B 139(2), 488 (2009)
Acknowledgements
The work was carried out with the support of funds from the Samara University Development Program for 2021–2030 as part of the Priority 2030 program. The material investigation was supported by Minsvyaz RF and RVC JSC (Grant LRC «Trusted Sensor Systems» No 009/20 from 10.04.2020, uniqueidentifier 0000000007119P190002).
Funding
The work was carried out with the support of funds from the Samara University Development Program for 2021–2030 as part of the Priority 2030 program.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design material. Material preparation and investigation, data collection and analysis were performed by AG, MG. KT, VP and DA. The original manuscript is written by AG, MG and NT. The manuscript is critically examined by the PM and VP. Materials characterization was supported by VP and NAD. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
Authors don’t have any conflict of interests.
Informed consent
There is not any consent for this article to be informed. This is normal research which is carried out in the University.
Research involving human and animals participants
This research does not involve any human and animal participant.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gorshkova, A., Gorshkov, M., Tripathi, N. et al. Enhancement in NO2 sensing properties of SWNTs: A detailed analysis on functionalization of SWNTs with Z-Gly-OH. J Mater Sci: Mater Electron 34, 102 (2023). https://doi.org/10.1007/s10854-022-09551-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10854-022-09551-5