Abstract
Hetero-structured semiconductor composite as an ideal multifunctional photocatalyst has significant advantage for the improvement of photocatalytic efficiency. Here, core–shell hetero-structured α-Fe2O3@Carbon (α-Fe2O3@C) composite including of α-Fe2O3 nanoparticles (NPs) inner-core and amorphous carbon outer-shell was successfully synthesized via a simple hydrothermal method. The experiment results indicate that the present core–shell structure has a remarkable impact on the interfacial synergies. The possible photocatalytic mechanism of core–shell α-Fe2O3@C composite is investigated in detail. Thanks to the following advantages: the core–shell structure provides more heterogeneous interfaces, so the incident light would be reflected multiply and enhanced the light absorption capacity. In addition, the heterogeneous interface synergistic effect between amorphous carbon and α-Fe2O3 as the bridge gives rise to a rapid approach to transport photo-induced carriers and thus enhances the electronic transfer capacity. It is anticipated that the core–shell nanostructure provides a neoteric mentality for designing superior photocatalysts with outstanding advantageous features applicable to, among others, hydrogen generation, green energy, water splitting, etc.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Nowadays, water purification and reuse have been proved to be an easy or eco-friendly fix to moderate shortages of clean water caused by excessive use of pesticides, organic dyes, detergents, [1, 2] et al. Photocatalysis technologies based on semiconductor oxides have become an optional and effective approach to reducing environmental pollution and efficient utilization of green energy [3,4,5,6]. It is well known that the typical photocatalytic reaction can be succeeded along with the generation of electrons in the conduction band (CB) and holes in the VB (valence band) under visible irradiation [7, 8]. Furthermore, the activity of photocatalytic reaction is influenced by the light intensity, intrinsic energy gap, absorption edge and surface structure including defects, surface area of photocatalyst [9,10,11,12]. One of the foremost mechanisms is the activity of photoelectrons can affect the reaction efficiency [13, 14]. However, the high recombination rate of photoelectrons and holes of single semiconductor oxides limits the photocatalytic performance and leads to low photocatalytic efficiency [15, 16]. Numerous strategies have been explored to enhance the photocatalytic activity such as reducing the recombination rate by forming heterojunction, optimization in conductivity by doping with conductive material for expediting the electronic transfer capacity [17,18,19]. Compared to the single photocatalyst, core–shell hetero-structured semiconductor oxide composite photocatalyst in which semiconductor oxides acts as the core and conductive material acts as the shell has attracted extensive consideration due to their unique performances with salient advantageous features such as electronic transfer capacity and photoelectric synergistic effect [20, 21].
In this context, α-Fe2O3 as an environmentally and friendly n-type semiconductor has been considered as one of promising candidates for photocatalytic applications, drug delivery platform and bio-chemical sensors which due to its narrow energy gap and excellent chemical stability [22,23,24,25]. However, improving the photocatalytic efficiency of α-Fe2O3 to fulfill practical application remains an enormous challenge, because of low diffusion length and high electron–hole pairs recombination led to the bottleneck of poor quantum yield and visible-light trapping [26]. One of the keys to overcome these conditions is lowering the recombination rate by forming core–shell hetero-structure, enhancement in conductivity by combining with carbonaceous material for improving the charge transfer ability [27]. Inspired by this observation, among the most correlational research of α-Fe2O3 the main focus appears to construct α-Fe2O3-based hetero-structured materials in different forms. Constructing a core–shell structure, which is designed based on α-Fe2O3 core and carbonaceous materials shell, is an intentional approach to optimize the excellence of the interface synergistic effect. Moreover, this core–shell hetero-structured designing mentality would minimize the electron and hole spatial overlap [21]. The combination approach of core–shell heterojunction solves the low diffusion length and decreases the electron–hole recombination, and expedites the electron transfer while enhancing photocatalytic performance and stability.
In this work, we report a sample and effective strategy to solve the aforementioned bottleneck of single α-Fe2O3 in the photocatalytic field. The core–shell structure of amorphous carbon-encapsulated α-Fe2O3 composite was synthesized via a one-step hydrothermal method. The experiments prove that this core–shell structured α-Fe2O3@C nanocomposite is the superior photocatalyst for photocatalytic degradation of rhodamine B (RhB). The possible photocatalytic mechanism of as-synthesized α-Fe2O3@C composite was investigated in detail. The interface synergistic effects of core–shell structure between amorphous carbon and α-Fe2O3 are likely to enhance the electronic transfer capacity and the photocatalytic activity. Our findings provide a neoteric mentality for designing a novel photocatalyst and enrich a potential application in the photoelectric energy conversion.
2 Experimental
2.1 Synthesis of the α-Fe2O3 NPs precursor
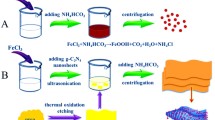
The α-Fe2O3 NPs precursor were synthesized via a simple hydrothermal method. In the specific experimental process: 0.6 g of ferric chloride and 1 g of sodium acetate were dissolved in 120 mL of deionized water. And the mixed solution was stirred until dissolved to form a stable solution. After dissolving completely, the stirred suspension was poured into an autoclave with a capacity of 120 mL and heated at 250 °C for 5 h. After the completion of the reaction and allowed cool to room temperature. Finally, the reaction products were centrifuged and separated from the black precipitation with deionized water and anhydrous ethanol.
2.2 Generation of core–shell structured α-Fe2O3@C nanocomposite
0.25 g of the as-prepared α-Fe2O3 NPs was dispersed in 60 mL of ethanol solution. Then, 0.26 g ferric chloride and 0.16 g terephthalic acid were added to the above-mentioned ethanol solution and the dispersion was uniform by ultrasonic for 10 min. The mixed solution was poured into a beaker and stirred in a water bath at 70 °C for 30 min. The precipitates were separated by centrifugation and washed with deionized water and anhydrous ethanol, respectively. 0.12 g of mentioned above α-Fe2O3 dispersed into 50 mL of deionized water, and glucose (C6H12O6·H2O, 0.1 mol, 0.2 mol, 0.3 mol) with different mole ratios of α-Fe2O3were added into the mixed solution. After uniform ultrasonic dispersion, the products were transferred to autoclave with a capacity and heated at 220 °C for 12 h. The precipitate was separated by centrifugation and washed three times with deionized water and anhydrous ethanol. The products generated with different mole ratios glucose (0.1 mol, 0.2 mol, 0.3 mol) were denoted hereinafter as S1, S2 and S3, respectively.
2.3 Characterization techniques
The structural characterization of samples was characterized by the X-ray diffraction (XRD) configuration using a powder diffractometer (Bruker AXS, Karlsruhe, Germany). The morphology and micro-structural of as-prepared samples were examined using scanning electron microscopy (SEM, JSM-7001F, JEOL, Japan) and transmission electron microscopy (TEM, 2100F; JEOL, Japan), separately. UV–VIS spectra were tested by the spectrophotometer (3100 UV–VIS-NIR, Shimadzu, Kyoto, Japan). The Raman spectrum was recorded by a spectrometer (Raman-11, Nanophoton Corporation, Japan) at room temperature.
2.4 Photoelectrochemical measurements and photocatalytic activity
The photoelectrochemical (PEC) is tested via using an electrochemical workstation (IM6, Zahner, Karlsruhe, Germany) and the 500 W Xe light was used as the source of visible light. First, 1 mg as-prepared samples were mixed with 10 mL Nafion and 10 mL ethanol, the mixed was smeared on 1 cm2 square FTO glass and then the samples were dried at 60 °C for 3 h in air. The above dried glass was used as the working electrode, Ag/AgCl electrode and a Pt wire were employed as the reference electrodes and counter, respectively. Then, Na2SO4 solution (0.2 mol/L) was employed as the electrolyte. The photocurrent property of the photoanode was carried out in each 60 s respectively.
The concentration variation of RhB solution was displayed from the spectrum strength of the absorption for the UV–Vis light irradiation. 100 mL of the RhB solution, with an initial concentration of 10 mg/L was deposited in a container. The mixed liquor was stirred in a dark environment for 10 min to ensure equilibrium of adsorption desorption. The maximum absorbance of extracting solution was analyzed by the spectrophotometer, while the variations of the absorption maximum at different intervals was tested. The concentration of RhB aqueous was decided by measuring intensity of the maximum absorbance wavelength of the suspension liquid. The degradation percentage (\(\eta\)) of the solutions could be calculated by follows [28]:
The rate of dye degradation is quantified with the first order exponential fit. Where the Co is the initial RhB dye concentration, Ct the dye concentration after an illumination time t (min). The variation of the RhB relative concentrations (Ct/C0, and -Ln (Ct/C0)) as a function of irradiation time.
3 Results and discussions
The morphology and micro-structure of α-Fe2O3 and the as-prepared α-Fe2O3@C samples (S1-S3) were conducted through SEM and TEM. As shown in Fig. 1a, α-Fe2O3 has a spherical shape with a uniform diameter of around 20 nm, and the appearance of the single α-Fe2O3 consists in the state of particle aggregation. The results show that with the increase of content of glucose, the diameter of α-Fe2O3 @C sample was increased. When the α-Fe2O3 @C sample generated with the mole ratios of glucose of 0.2 mol and 0.3 mol, and that leads to the aggregation performance degradation of α-Fe2O3 @C nanocomposites. This is mainly due to the small pore size of amorphous carbon material, and the carbon shell inhibits the agglomeration of α-Fe2O3@C NPs [29, 30]. In order to investigate the specific micro-structure of α-Fe2O3@C NPs, the TEM and HRTEM images of S2 (as shown in Fig. 1e, f) prove that core–shell like structure composites with obvious contrast between the black and gray parts can be observed, demonstrating that the shell is amorphous carbon material. As displayed in Fig. 1f, the HRTEM of α-Fe2O3@C S2 indicates the changes in diameter were from 20 nm for core spheres to 24 nm for core–shell composite. It can be proved that the amorphous carbon is successfully coated on the surface of α-Fe2O3 NPs with the thicknesses of about 4 nm. The HRTEM also shows lattice spacing of core is about 0.35 nm, which corresponds to the (012) plane of α-Fe2O3. It can also find that the outer carbon shell exhibits amorphous nature. Based on the above results, the as-prepared α-Fe2O3@C nanocomposites are the core shell structure with α-Fe2O3 NPs packaged with amorphous carbon, obviously.
Figure 2 displays the XRD patterns and Raman spectra of α-Fe2O3 and as-prepared α-Fe2O3@C samples (S1-S3). It can be found that the XRD patterns centered at 24.14°, 33.14°, 35.62°, 40.84°, 49.46 o, 54.04 o, 57.56 o, 62.44 o, 63.95 o, 71.85° and 75.39 o are assigned as (012), (104), (110), (113), (024), (116), (018), (214), (300), (101) and (200) crystal planes of typical rhombohedral α-Fe2O3 (JCPDS card No. 33-0664), respectively. Moreover, the diffraction peaks of both α-Fe2O3@C samples (S1-S3) are indexed by the rhombohedral α-Fe2O3 phase without any obvious peaks of other phases. Dramatically, no distinct diffraction peaks of amorphous carbon phase are observed in the α-Fe2O3@C nanocomposites. The peaks corresponding to amorphous carbon could be too weak to be clearly observed [31]. Researchers were able to use Raman spectroscopy to look at the change of the vibrational energy of the bond and then characterize the variety rules of the structure of carbon materials [32, 33].
Figure 3 illustrates the Raman spectra of α-Fe2O3 and α-Fe2O3@C samples (S1-S3). Obviously, it can find that the different typical Raman spectrum of as-prepared α-Fe2O3@C samples S1-S3. The peaks corresponding to samples (S1-S3) at around 1341 cm−1 and 1595 cm−1 corresponds to the D band and G band, respectively. It can see that the D band and G band relate to the disorder induced in the carbon structure and the vibration of sp2 carbon vibration, respectively. On the contrary, no characteristic peaks of carbon were detected in α-Fe2O3. The Raman results indicate amorphization of graphite including more topological defects, disorders and vacancies in the carbon shell of the α-Fe2O3@C sample [34]. On the basis of above analysis, the α-Fe2O3@C nanocomposite with core–shell structure is an ordered assembly system in which α-Fe2O3 NPs are covered with carbon materials via chemical bonds or other interactions [35], and the outer carbon shell also retained amorphous nature.
Photocurrent measurement normally is used for inquiring the separation efficiency of photo-induced carriers (electrons or holes) [36, 37]. Photocurrents of the as-prepared single α-Fe2O3 and α-Fe2O3@C samples (S1-S3) were gauged for studying the electronic interaction between α-Fe2O3 and carbon materials, and the current density versus potential curves were recorded via circularly turn on and off the light irradiation, as shown in Fig. 4. Dramatically, all α-Fe2O3@C samples exhibit higher transient photocurrent when compared with single α-Fe2O3, and the photocurrent of the α-Fe2O3@C S2 reached 0.25 mA/cm2 and was about two times as high as that of single α-Fe2O3. It evidences that the core–shell complex structure could effectively restrain current carrier recombination. Moreover, outer shell carbon as a protective material could inhibits the photo-corrosion of α-Fe2O3 under visible-light illumination. In addition, it can be found that the transient photocurrent value of α-Fe2O3@C displays a rising and falling variation trend, which meant that the additive amount of glucose in α-Fe2O3@C samples is closely related to surface carrier concentration. Notably, compared to the other samples, the α-Fe2O3@C S2 also presents higher photocurrents owing to optimal additive amount of glucose or the layer thickness of outer carbon shell. Here, the core–shell structure offers a large interface between the α-Fe2O3 core and the carbon shell. The core–shell structure contains many heterojunction interfaces and the surface states raised by the larger core–shell interface [38]. It creates the good possibility for leaping into the conduction band edge [39]. Thus, the balance of synergistic and the charge separation leads to enhanced transient photocurrent efficiency.
In order to study the photo absorption ability and optical properties of as-prepared α-Fe2O3 and α-Fe2O3@C, Fig. 5 illustrates the light absorption of pure α-Fe2O3 and α-Fe2O3@C S2 nanocomposite. The absorption peak of α-Fe2O3@C S2 is 460 nm, which is similar to the single α-Fe2O3 except for about 15 nm red shift. The construction of α-Fe2O3@C core–shell structure may leads to a red-shift, which is conductive to the light utilization and photoelectric conversion efficiency [40]. This phenomenon could be attributed to the following reasons: the synergistic effect for light absorption was generated in the α-Fe2O3@C core–shell structure. In other words, when a large interface contact area existed between carbon materials and α-Fe2O3 NPs, the interfacial scattering effect of visible light was enhanced. As a result, the transmission of the visible photon in the α-Fe2O3@C nanocomposite was increased via longer optical path length [41], which leaded to a significantly enhanced light absorption in the visible region. Meanwhile, these interface and surface states of the α-Fe2O3@C core–shell structure accompanied with carbon defect states would result in the composites obvious to the visible light [42]. The results indicate that α-Fe2O3@C exhibits a semiconductor character with an indirect band gap. Band-gap energies (Eg) are evaluated from the absorption edges of the reflectance spectra, as shown in the insets of Fig. 5. It is observed that band gap of α-Fe2O3@C (2.25 eV) is smaller than that of α-Fe2O3 (2.36 eV). The observed narrowing of the band gap in α-Fe2O3@C nanocomposite can be considered to be attributable to the increased hybridization of the energy levels by the surface defects formed of amorphous carbon materials, shifting the valence and conduction bands and subsequently decreasing the band gap [43]. It can also be considered to attribuatable to oxygen vacancies leading to lowered about 0.11 eV. Therefore, the core–shell heterojunction of α-Fe2O3@C boosted the separation and utilization of photo-induced carriers and improved the photocatalytic performance [44].
To study the photocatalytic activity of as-prepared α-Fe2O3 and α-Fe2O3@C NPs, the degradation experiment for RhB solution was carried out as shown in Fig. 6. For comparison, the control experiment was carried out under in dark environment and without photocatalyst under the UV–vis light irradiation, respectively. As shown in Fig. 6a, it can be found that RhB reaches the adsorption desorption equilibrium under dark conditions within 10 min. In the absence of photocatalyst, there is no appreciable degradation of RhB, significantly. The degradation curves of RhB under the UV–vis light irradiation are shown in Fig. 6b. It can be found that crystal core-amorphous shell structured α-Fe2O3@C samples exhibit excellent photocatalytic degradation performance, leading to the significant improved performance compared to pure α-Fe2O3. After 80 min of reaction, the degradation of RhB rates reach 38%, 81%, 99.8% and 67% for α-Fe2O3 and α-Fe2O3@C (S1-S3), respectively. Obviously, the photocatalytic performance of the α-Fe2O3@C S2 nanocomposite is the most optimal. The experimental results are similar to that of photocurrent measurement.
According to Fig. 7a, the photocatalytic activity of α-Fe2O3@C is much higher than that of single α-Fe2O3, and the distinct rate constant (k, min−1) of α-Fe2O3@C S2 reaches the maximum of 0.024 min−1. Notably, the order of rate constant is S2 > S1 > S3 > α-Fe2O3. Moreover, the degradation stability performance is also a key factor for the α-Fe2O3@C. The degradation curves of RhB for three cycles is shown in Fig. 7b, it can be seen that the photocatalytic performance of the α-Fe2O3@C S2 nanocomposite remained almost unchanged. The circulation experiments proved that core–shell α-Fe2O3@C structure provide a pathway to have the most efficient photocatalytic stability and reproducibility. The photocatalytic performances of as-prepared samples were evaluated by the degradation of different kinds of dyes Phenol, Methylbenzene (MB), Methyl Orange (MO), and Congo Red (CR) as shown in Fig. 7c. Moreover, compared with several common core–shell photocatalysts for 80 min degradation of RhB as shown in Table 1, it’s obvious to observe that α-Fe2O3@C has an excellent photocatalytic performance in the cationic dyes. From Fig. 7d, the signals of DMPO-•OH were readily detected with the α-Fe2O3 and α-Fe2O3@C system, confirming the formation of •OH radicals.
Based on the above analysis, the schematic diagram of proposed photocatalytic mechanism of α-Fe2O3@C nanocomposite is illustrated in Fig. 8. First, the core–shell structured composite formed when amorphous carbon materials are coated on the surface of α-Fe2O3 NPs. Under irradiation, more pathways provided a faster interfacial charge carrier transfer attributed to reasonable core–shell structure [48, 49]. Second, when the α-Fe2O3 absorbs energy to produce photogenerated electron–hole pair, the free electrons are excited from the VB of α-Fe2O3 into its conduction band in a specific photocatalytic reaction (see Fig. 8a). The interface transfer process contains holes and excited electrons and its deactivation by recombination, identifies the efficiency of the photocatalyst [50, 51]. In particular, the excited electrons can transfer from α-Fe2O3 to carbon materials through the interface between core–shell structure more easily. Thus, both processes make for electron separation and stabilization, hindering the electron hole pair recombination process (Fig. 8b). In the end, the electron–hole pairs are divided effectively, the holes are trapped by H2O to form hydroxyl radicals (·OH). And the reserved electrons in the CB of α-Fe2O3 reacted with O2 to produce radical dot ·O2−, which was involved in the degradation of organic substrates [52]. Thus, the electrons transferred in CB can reduce the absorbed O2 to create active ·O2− [53]. And so beyond that, the produced ·O2− liveness are not only can degrade pollutants, but also a provenience of ·OH radicals and photodegradation rate is perfected. As a consequence, the more radical ·OH radical and remaining active ·O2− are assigned to the photodegradation of RhB pollutants rapidly [54, 55]. Therefore, the core–shell heterojunction of α-Fe2O3@C boosted the separation and utilization of photo-induced carriers and improved the photocatalytic performance [56, 57].
4 Conclusions
To make full use of the interfacial synergies and separation efficiency of electron–hole pair, a strategy of core–shell structure designing is proposed to effectively enhance the photocatalytic property. By regulating and controlling the content of carbon, the core–shell structured α-Fe2O3@C composite including of inner-core α-Fe2O3 NPs and outer-shell amorphous carbon is successfully prepared via a simple hydrothermal method. Compared with the pure α-Fe2O3, it can be proved that the as-prepared α-Fe2O3@C nanocomposite exhibits improved and effective photocatalytic activity for the degradation of RhB. Owing to the core–shell structure endowing more pathways for the charge carrier transfer and the interfacial synergies mechanism. As a result, the photogenerated electron–hole pairs are separated effectively between the core–shell interface, and the efficient separation of electron and hole is the primary motivation for enhancement of the photocatalytic performance. This study provides a neoteric mentality for the fabrication of novel hetero-structure for photocatalysis or other green energy applications.
Data availability
All data generated or analyzed during this study are included in this published article.
References
X. Liu et al., Noble metal-metal oxide nanohybrids with tailored nanostructures for efficient solar energy conversion, photocatalysis and environmental remediation. Energy Environ Sci. 10, 402–434 (2017)
V.J. Babu et al., Review of one-dimensional and two-dimensional nanostructured materials for hydrogen generation. Phys. Chem. Chem. Phys. 17, 2960–2986 (2015)
A. Fujishima, K. Honda, Electrochemical photolysis of water at a semiconductor electrode. Nature 238(5358), 37–38 (1972)
X. Chen et al., Semiconductor-based photocatalytic hydrogen generation. Chem. Rev. 110(11), 6503–6570 (2010)
M.Q. Wen et al., Synthesis of MoS2/g-C3N4 nanocomposites with enhanced visible-light photocatalytic activity for the removal of nitric oxide (NO). Opt. Express 24, 10205–10212 (2016)
Y. Ye et al., Theoretical and experimental investigation of highly photocatalytic performance of CuInZnS nanoporous structure for removing the NO gas. J. Catal. 357, 100–107 (2018)
J. Liu et al., Metal-free efficient photocatalyst for stable visible water splitting via a two-electron pathway. Science 347(6225), 970–974 (2015)
Y.-K. Peng, S.C.E. Tsan, Facet-dependent photocatalysis of nanosize semiconductive metal oxides and progress of their characterization. Nano Today 18, 15–34 (2018)
L. Li et al., Sulfur-doped covalent triazine-based frameworks for enhanced photocatalytic hydrogen evolution from water under visible light. J. Mater. Chem. A 4(32), 12402–12406 (2016)
A. Kudo, Y. Misek, Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 38(1), 253–278 (2009)
X. Dong, F.X. Cheng, Recent development in exfoliated two-dimensional g-C3N4 nanosheets for photocatalytic applications. J. Mater. Chem. A 3(47), 23642–23652 (2015)
L. Jiang et al., Doping of graphitic carbon nitride for photocatalysis: a reveiw. Appl. Catal. 217, 388–406 (2017)
P. Xu et al., Use of iron oxide nanomaterials in wastewater treatment: a review. Sci. Total Environ. 424, 1–10 (2012)
J. Liu et al., Self-assembling TiO2 nanorods on large graphene oxide sheets at a two-phase interface and their anti-recombination in photocatalytic applications. Adv. Funct. Mater. 20(23), 4175–4181 (2010)
D.-N. Bui et al., Relationship between the electrochemical behavior of multiwalled carbon nanotubes (MWNTs) loaded with CuO and the photocatalytic activity of Eosin Y-MWNTs-CuO system. Appl. Surf. Sci. 266, 288–293 (2013)
J. Tian et al., Recent progress in design, synthesis, and applications of one-dimensional TiO2 nanostructured surface heterostructures: a review. Chem. Soc. Rev. 43(20), 6920–6937 (2014)
Y. Wang et al., Polymeric graphitic carbon nitride as a heterogenous organocatalyst: from photochemistry to multipurpose catalysis to sustainable chemistry. Angew. Chem. Int. Ed. 51(1), 68–89 (2012)
J.L. White et al., Light-driven heterogenous reduction of carbon dioxide: photocatalysts and photoelectrodes. Chem. Rev. 115(23), 12888–12935 (2015)
C. Wang et al., Recent progress in visible light photocatalytic conversion of carbon dioxide. J. Mater. Chem. A. 7(3), 865–887 (2019)
A.S. Al-Hussaini et al., New semiconducting core-shell nanocomposites. Compos Interface 27(4), 385–399 (2020)
Z. Li et al., Design of highly stable and selective core/yolk–shell nanocatalysts-A review. Appl Catal B-Environ 188, 324–341 (2016)
J. Wang et al., Exceptional photocatalytic activities for CO2 conversion on Al-O bridged g-C3N4/α-Fe2O3 Z-scheme nanocomposites and mechanism insight with isotopes. Appl. Catal. B 221, 459–466 (2018)
B. Sun et al., Magnetic Fe2O3/mesoporous black TiO2 hollow sphere heterojunctions with wide-spectrum response and magnetic separation. Appl. Catal. B 221, 235–242 (2018)
P. George et al., All-optical nanoscale heating and thermometry with resonant dielectric nanoparticles for controllable drug release in living cells. Laser Photonics Rev. 14(3), 1900082 (2020)
L.K. Li et al., Optical fiber optofluidic bio-chemical sensors: a review. Laser Photonics Rev. 15(7), 2000526 (2021)
N. Wang et al., Rational design and synthesis of SnO2 encapsulated alpha-Fe2O3 nanocubes as a robust and stable photo-fenton catalyst. Appl. Catal. B 210, 23–33 (2017)
M. Mishra, D.-M. Chun, α-Fe2O3 as a photocatalytic material: a review. Appl. Catal. A Gen. 498, 126–141 (2015)
K.S. Ranjith et al., Alignment, morphology and defect control of vertically aligned ZnO nanorod array: competition between “Surfactant” and “Stabilizer” roles of the amine species and its photocatalytic properties. Cryst. Growth Des. 14(6), 2873–2879 (2014)
Z.W. Zhang et al., A spontaneous dissolution approach to carbon coated TiO2 hollow composite spheres with enhanced visible photocatalytic performance. Appl. Surf. Sci. 286, 344–350 (2013)
S. Shanmugam et al., Synthesis and characterization of TiO2@C core-shell composite nanoparticles and evaluation of their photocatalytic activities. Chem. Mater. 18(9), 2275–2282 (2006)
J. Guo et al., Interdispersed amorphous MnOx-carbon nanocomposites with superior electrochemical performance as lithium-storage material. Adv Funct Mater 22(4), 803–811 (2012)
J. Liu et al., Facile synthesis of carbon-doped mesoporous anatase TiO2 for the enhanced visible-light driven photocatalysis. Chem. Commun. 50(90), 13971–13974 (2014)
D.S. Knight, W.B. White, Characterization of diamond films by Raman spectroscopy. J. Mater Res. 4, 385–393 (1989)
J. Lu et al., One-pot synthesis of fluorescent carbon nanoribbons, nanoparticles, and graphene by the exfoliation of graphite in ionic liquids. ACS Nano 3(8), 2367–2375 (2009)
M.S. Dresselhaus et al., Perspectives on carbon nanotubes and graphene Raman spectroscopy. Nano Lett. 10(3), 751–758 (2010)
W. Zhen et al., The enhancement of CdS photocatalytic activity for water splitting via anti-photocorrosion by coating Ni2P shell and removing nascent formed oxygen with artificial gill. Appl. Catal. B 221, 1243–1257 (2018)
P. Wang et al., Shell thickness engineering significantly boosts the photocatalytic H2 evolution efficiency of CdS/CdSe core/shell quantum dots. ACS Appl. Mater. Interfaces 9(41), 35712–35720 (2017)
B. Cheng et al., Effects of interface States on photoexcited carriers in ZnO/Zn2SnO4 type-ii radial heterostructure nanowires. ACS Appl. Mater. Interfaces 6(6), 4057–4062 (2014)
S.C. Rai et al., Piezo-phototronic effect enhanced UV/Visible photodetector based on fully wide band gap type-ii ZnO/ZnS Core/Shell nanowire array. ACS Nano 9(6), 6419–6427 (2015)
M. Kim et al., Efficient visible light-induced H2 production by Au@CdS/TiO2 nanofibers: synergistic effect of core-shell structured Au@CdS and densely packed TiO2 nanoparticles. Appl. Catal. B 166, 423–431 (2015)
C.Z. Luo et al., Preparation of 3D reticulated ZnO/CNF/NiO heteroarchitecture for high-performance photocatalysis. Appl. Catal. B 116, 217–223 (2015)
K. Zhang et al., Nanoconfined Nickel@Carbon core-shell cocatalyst promoting highly efficient visible-light photocatalytic H2 production. Small 14(18), 1801705 (2018)
X. Lü et al., A general preparation strategy for hybrid TiO2 hierarchical spheres and their enhanced solar energy utilization efficiency. Adv. Mater. 22(33), 3719–3722 (2010)
Y. Zhuang et al., Template free preparation of TiO2/C core-shell hollow sphere for high performance photocatalysis. J. Alloy. Compd. 662, 84–88 (2016)
Y. Guo et al., Synthesis of magnetic core–shell carbon dot@MFe2O4 (M= Mn, Zn and Cu) hybrid materials and their catalytic properties. Mater. Chem. A 4(11), 4044–4055 (2016)
Y. Niu et al., Structures and photocatalytic activity of α-Fe2O3@TiO2 core-shell nanoparticles. Solid State Commun. 345, 114683 (2022)
Q. Tian et al., Tube-like ternary α-Fe2O3@SnO2@Cu2O sandwich heterostructures: synthesis and enhanced photocatalytic properties. ACS Appl. Mater. Interfaces. 6(15), 13088–13097 (2014)
J. Yu et al., Photogenerated electron reservoir in hetero-p-n CuO-ZnO nanocomposite device for visible-light-driven photocatalytic reduction of aqueous Cr (VI). J. Mater. Chem. A 3(3), 1199–1207 (2015)
N. Li et al., Precisely controlled fabrication of magnetic 3D Fe2O3@ZnO core-shell photocatalyst with enhanced activity: ciprofloxacin degradation and mechanism insight. Chem. Eng. J. 308, 377–3853 (2017)
C. Wang et al., Controlled formation of TiO2/MoS2 core-shell heterostructures with enhanced visible-light photocatalytic activities. Part. Part. Syst. Charact. 33(4), 221–227 (2016)
S. Adhikari et al., Construction of heterojunction photoelectrode via atomic layer deposition of Fe2O3 on Bi2WO6 for highly efficient photoelectrochemical sensing and degradation of tetracycline. Appl. Catal. B: Environ. 24, 11–24 (2019)
T. Guo et al., A novel alpha-Fe2O3@g-C3N4 catalyst: Synthesis derived from Fe-based MOF and its superior photo-Fenton performance. Appl. Surf. Sci. 469, 331–339 (2019)
L. Zhu et al., Hierarchical ZnO decorated with CeO2 nanoparticles as the direct Z-Scheme heterojunction for enhanced photocatalytic activity. ACS Appl. Mater. Interfaces 10(46), 39679–396879 (2018)
Y. He et al., Remarkably enhanced visible-light photocatalytic hydrogen evolution and antibiotic degradation over g-C3N4 nanosheets decorated by using nickel phosphide and gold nanoparticles as cocatalysts. Appl. Surf. Sci. 517, 146187 (2020)
X. Liu et al., Efficient Fe3O4-C3N4-Ag2MoO4 ternary photocatalyst: synthesis, outstanding light harvesting, and superior hydroxyl radical productivity for boosted photocatalytic performance. Appl. Catal. A 568, 54–63 (2018)
H. Huang et al., Efficient activation of persulfate by a magnetic recyclable rape straw biochar catalyst for the degradation of tetracycline hydrochloride in water. Sci. Total Environ. 758, 143957 (2021)
T. Guo et al., Efficient persulfate activation by hematite nanocrystals for degradation of organic pollutants under visible light irradiation: Facet-dependent catalytic performance and degradation mechanism. Appl. Catal. B: Environ. 286, 119883 (2021)
Acknowledgements
This work was supported by the Special Talents in Shanxi Province (Grant No. 201901D211074), National Natural Science Foundation of China (Grant No. 21671147)
Funding
Special Talents in Shanxi Province,201901D211074,Jianle Xu,National Natural Science Foundation of China,21671147,Shuang Wang
Author information
Authors and Affiliations
Contributions
JX contributed to conception and experiment design. JX and QY contributed to experiment operation, acquisition of data, analysis, and interpretation of data. PL and SW contributed to acquisition of data and drafting the article. JX and XZ contributed to revising article critically for important intellectual content. JX contributed to analysis and interpretation of data. CP contributed to experiment design.
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare no conflict of interest.
Research involving human and animal participants
This article does not contain any studies with human participants or animals performed by any of the authors. In this experiment, we did not collect any samples of human and animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, J., Yao, Q., Li, P. et al. Construction of amorphous carbon-coated alpha-Fe2O3 core–shell nanostructure for efficient photocatalytic performance. J Mater Sci: Mater Electron 33, 22549–22559 (2022). https://doi.org/10.1007/s10854-022-09033-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-022-09033-8