Abstract
The SrWO4 + x wt.%Li2WO4(0 ≤ x ≤ 1.5) ceramics with low dielectric constant and high quality factor (Q×f) were fabricated. The impacts of Li2WO4 on sintering, structure, and microwave performance were also investigated. The results exhibit that the appropriate amount of Li2WO4 addition can lower sintering temperature and improve the densification of ceramics. All ceramic specimens adopt a tetragonal scheelite structure and can be sintered below 950 °C. The relative density and polyhedral deformation of SrO8 determine the dielectric properties of the sintered ceramics. The 1.0-wt% Li2WO4-added SrWO4 ceramic sample sintered at 875 °C for 2 h reveals satisfactory characteristics with Q×f = 88,893 GHz, εr = 8.4, and τf = −48.7 ppm/°C. Moreover, the ceramic material is well compatible with Ag electrodes. These findings demonstrate that the as-prepared SrWO4-based materials have great potential for low-temperature co-fired ceramics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The development of microelectronics technology makes the device or component tends to miniaturization, high integration, fast transmission rate, and high reliability. The demands of high frequency, fast propagation speed, dense wiring, and low cost are put forward for the performance of packaging materials. It also requires better quality and stability of the packaging process [1, 2]. Electronic devices based on microwave dielectric ceramics (MWDCs) are the key components for 5G base stations. Along these lines, microwave ceramics have been extensively investigated as the preferred dielectric material for microwave device applications [3, 4]. Low-temperature co-fired ceramic (LTCC) technology is a highly efficient and uncomplicated way of packaging passive electronic devices, which can meet the requirements of miniaturization, high integration, and multi-functionality [5,6,7,8]. It also realizes multi-layer stacking of components and is co-fired with an internal electrode with high conductivity. In practical applications, LTCC substrate materials not only have low εr, favorable quality factor, and near-zero τf but also can satisfy the needs of fast signal propagation, excellent high-frequency characteristics, and good temperature stability [9, 10], which are desirable for many industries, including aerospace, military, communication, and automotive electronics [11, 12]. Owing to its importance, the development of microwave dielectric ceramics with excellent performance and low sintering temperature (lower than melting point of Ag, 961 °C) have been a subject of intensive research in recent times [13].
In prior research, many microwave dielectric ceramics with excellent performance have been reported. Common low-temperature microwave dielectric ceramic systems mainly include borate, phosphate, tungstate, molybdate, and vanadate, such as Mg3B2O6 [14], LiCaBO3 [15], KSrPO4 [16], Li0.16Cu0.92MoO4 [17], CaMoO4 [18], Ba2V2O7 [19], and Ba3Mg(V2O7)2 [20]. Among them, the tungstate system has outstanding performance and is an ideal choice for LTCC materials. For example, the SrWO4 sintered at 1150 °C has an exceptional performance: εr = 8.1, Q×f = 56,000 GHz, and τf = −55 ppm/°C. Nevertheless, its firing temperature is more than 950 °C, which cannot meet the requirement for LTCC materials. Popularly, three approaches are available to reduce the sintering temperature. The first one is to prepare powder with high surface activity by a chemical process and the second one is to use raw powder with tiny particles. However, the above two methods are costly, complicated, and not conducive to batch production. The most effective and cheapest way is to add sintering additives in ceramics to realize liquid-phase sintering [21] or form a solid solution. Lithium-based compounds are often selected as sintering aids. Xi et al. [8] obtained excellent properties (Q×f = 38,093 GHz, εr = 2.9, τf = −2.2 ppm/°C) by adding 0.3 mol% Li2O to CuO–ZnO–B2O3 ceramics sintered at 785 °C. Other material systems using Li2CO3 as sintering aids were reported as follows: Ba3V2O8 + 8 wt% Li2CO3 [22] (Q×f = 33,000 GHz, εr = 13.1, τf = + 13 ppm/°C), Mg3(VO4)2-0.5Ba3(VO4)2+0.065 wt% Li2CO3 [23] (Q×f = 74,000 GHz, εr = 13, τf = −6 ppm/°C), and Sr2V2O7 + 3 mol% Li2CO3 [24] (Q×f = 73,800 GHz, εr = 9.9, τf = −28.8 ppm/°C). In addition, LiF is also a common sintering aid, for instance, LiInO2 + 3 wt% LiF [25] (Q×f = 52,500 GHz, εr = 13.6, τf = + 18.1 ppm/°C) and CaMgSi2O6 + 2 wt% LiF [26] (Q×f = 64,800 GHz, εr = 7.5, τf = −34 ppm/°C).
As we know, Li2WO4 was chosen because it not only contains the same element (W) as the base material, which can avoid the formation of a second phase in the end but also has excellent properties (Q×f = 62,000 GHz, εr = 5.5, τf = −146 ppm/°C) and low firing temperature (640 °C) [27], which makes it easy to realize the purpose of this work. Therefore, in this work, Li2WO4 was added to SrWO4 to achieve firing at low temperatures and superior dielectric performance. The effects of Li2WO4 on sintering, structure, microstructure, and microwave performance were analyzed carefully. Besides, the chemical compatibility between the ceramics and Ag electrodes was explored.
2 Experimental procedures
Tungstate ceramics were fabricated by the solid-state reaction method. The reagent-grade powders of SrCO3 (≥ 99%, Xilong Scientific Co., Ltd), WO3 (≥ 99.95%, Ganzhou Xinzhen New Material Co., Ltd), and Li2CO3 (≥ 98%, Xilong Scientific Co., Ltd) were applied as starting materials. WO3 was placed in a drying dish at room temperature, SrCO3 and Li2CO3 were dried in a 150 °C oven for 24 h, and the raw materials were accurately weighted according to the chemical formula: SrWO4 and Li2WO4, respectively. Powders were planetary milled for 12 h in a ball mill jar with ZrO2 balls and alcohol as the grinding medium. After drying at 100 °C, SrWO4 and Li2WO4 powders were pre-sintered at 900 °C and 500 °C for 2 h, respectively. The two powders were mixed based on the design composition SrWO4 + x wt.%Li2WO4(0 ≤ x ≤ 1.5) and re-milled for 12 h. After adding 7-wt% PVA for granulation, the powders were pressed into ceramic sheets of 12 mm in diameter and 6 mm in height with a pressure of 100 MPa and sintered at 825–925 °C for 3 h to obtain ceramics.
The crystalline phase identification of the ceramic samples was ascertained by X-ray diffraction (Bruker D8 Advance, Germany) with Cu Ka radiation in the 2θ range of 20–80°. The bulk density was measured by the Archimedes method. A field emission scanning electron microscope (Quanta, FEG450, America) equipped with energy-dispersive spectroscopy (EDS) was used to observe the microscopic morphology of ceramic samples. Raman studies were performed using LabRAM HR Evolution (HORIBA, France) Raman spectroscopy. The dielectric properties were measured by Vector Network Analyzer (N5230C, Agilent Technologies, America) with the TE01δ mode dielectric resonator. The resonant frequency temperature coefficient (τf) was tested by the parallel plate method. The value of τf is obtained from the change of resonant frequency at 25 °C and 75 °C based on the following equation:
3 Results and discussion
Figure 1a–d displays the XRD profile, SEM image, relative density, and microwave dielectric characteristics of SrWO4 ceramics at various sintering conditions. The prepared ceramic sample is pure-phase SrWO4 as seen in Fig. 1a. In Fig. 1b, SrWO4 ceramics sintered at 1000 °C adopt uniform and dense microstructure with an average grain size of 12.6 μm. With the increment of firing temperature, the dielectric permittivity and Q⋅f value first rise and then reduce, which is following the change of relative density, as depicted in Fig. 1c–d. The best dielectric properties are obtained at a temperature of 1000 °C, and the results are comparable to those reported in the literature [28]. However, the sintering temperature (>950 °C) is too high to satisfy the requirement for LTCC materials, and the subsequent work is concentrated on reducing the sintering temperature and improving the microwave dielectric performance as well as exploring the co-firing compatibility of ceramics and silver electrodes.
To reduce the sintering temperature, Li2WO4 was introduced into the SrWO4 ceramics, and the relative density of SrWO4 + x wt% Li2WO4(0 < x ≤ 1.5) after sintering is shown in Fig. 2a. The relative density tends to start increasing and then decreasing with the rise of the firing temperature for all samples. The additions of Li2WO4 significantly improve the sintering densification. The specific reasons are as follows: during the sintering process, the solid-phase particles are wetted and compacted by the liquid phase caused by Li2WO4 and then slip and rearrange, making the samples dense. The optimal sintering temperature and optimum additive amount of Li2WO4 are 875 °C and 1.0 wt%, respectively.
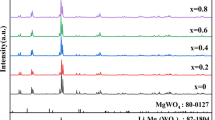
Figure 2b shows the XRD patterns of SrWO4 + x wt% Li2WO4(0 < x ≤ 1.5) ceramics sintered at 875 °C. All diffraction peaks for ceramic samples could be indexed by SrWO4 (PDF#85–0587) with a tetragonal structure and I41/a space group, without heterogeneous phases [29]. This suggests that Li2WO4 is not chemically reacted with SrWO4 and only exists as a liquid phase during the sintering process [30]. The liquid phase promotes powder particles rearrangement, diffusion mass transfer, and densification [31,32,33].
Figure 3a–g exhibits the SEM image of SrWO4 + x wt% Li2WO4(0 < x ≤ 1.5) ceramics. For the fixed sintering temperature of 875 °C, unevenly growing grains and more holes are observed in the sample with x = 0.5, indicating that the sample has a lower density. When the content of Li2WO4 increases to 1.0 wt%, the grain size decreases, the pores significantly reduce, and a relatively uniform compact microstructure is obtained. With further increasing Li2WO4 content to 1.5 wt%, however, the heterogeneous microstructure accompanied by an abnormal grain growth is formed, which is due to the presence of excess liquid phase, deteriorating sintering capability [34]. This is consistent with the change in relative density in Fig. 2a. At x = 1.0, the average grain size monotonously grows from 3.2 to 5.5 μm as the firing temperature changes from 825 to 925 °C. The most uniformly compact structure can be achieved at 875 °C seen in Fig. 3b. High temperature is not conducive to sintering and compaction, as shown in Fig. 3f–g. The above changes in microstructure are consistent with the changes in relative density as shown in Fig. 2a.
Figure 4 displays the dielectric properties of SrWO4 + x wt% Li2WO4(0 < x ≤ 1.5) ceramics at various temperatures. In general, the external factors affecting the dielectric properties include densification, grain boundaries, and secondary phase. It is found that all samples sintered at 875 °C have the ultimate dielectric constant, Q×f value and τf absolute value. The best Q×f value (88,893 GHz, f = 10.5 GHz) is obtained when x = 1.0. Combining Figs. 2 and 3, the increase in Q×f value is due to its uniform grain microstructure, high relative density, and high crystallinity because of the same crystal phases. In addition, the εr increases first and then decreases with increasing temperature, which is similar to the relative density variation. The high densification or lower porosity would result in higher permittivity [35]. The τf value changes remarkably with the addition of Li2WO4. Generally, τf is correlated with the phase composition and the additive content. From Fig. 4a and c, the absolute value of τf decreases with the reduction of εr, which is consistent with the result reported by Chen et al. [36].
The vibrational properties of the ceramics are characterized by Raman spectroscopy, as shown in Fig. 5. All ceramic samples have similar profiles, indicating that the Li2WO4 does not change its vibrational modes. Nine distinct vibrations are detected in all specimens. Modes 1 to 4 are the types of motion and the rigid molecular unit of Sr2+, which are the translational type of the external mode. Modes 5 to 9 represent the internal modes, which correspond to the vibration of [WO4]2 [29]. The relationships between the FWHM (Full width at half maximum) of mode 1 and the Q×f values are presented in Fig. 6. The decline of the FWHM value corresponds to the decrease in the damping coefficient, which leads to the increase of Q×f value [37, 38].
Moreover, the dielectric losses at microwave frequency are influenced by their structural properties and can be evaluated by the packing fraction, which is available from the below equation [39]:
where Z is the number of formula units per cell and Z = 4 for the tetragonal scheelite SrWO4. The association between the Q×f value and the packing fraction is demonstrated in Fig. 6. High packing fractions constrain the space for atoms to move in the lattice by impeding non-harmonic vibrations, resulting in lower intrinsic losses [40]. Therefore, the ceramic doped with 1.0-wt% Li2WO4 possesses the largest Q×f, as seen in Fig. 6.
To evaluate whether there is a chemical reaction between SrWO4-based ceramics with the silver electrode, the SrWO4 + 1.0 wt% Li2WO4 powders are chosen to be co-fired with 20-wt% Ag powders. The co-firing results are presented in Fig. 7. Only two phases, SrWO4 and Ag, are identified in the XRD pattern. In the BSE micrograph, the brighter particles are identified as Ag, which is in agreement with the EDS analysis result. These results confirm that SrWO4 + x wt% Li2WO4(0 < x ≤ 1.5) ceramics do not react with Ag electrode.
Compared with other Li2WO4-added systems, as listed in Table 1, the SrWO4 + 0.1 wt% Li2WO4 ceramics have suitable processing temperatures, low permittivity, and great Q×f value, which are well matched to the requirements of LTCC materials.
4 Conclusion
SrWO4 ceramics with low dielectric permittivity and favorable Q×f values are obtained by adding Li2WO4. A suitable amount of Li2WO4 could improve the sintering behavior and reduce the temperature of SrWO4 ceramics from 1000 to 875 °C as well as enhance the microwave dielectric properties. All ceramic samples do not form a second phase and exhibit a negative τf value. The relative densities and polyhedral deformation have a strong influence on the dielectric properties. The SrWO4 + 1.0 wt% Li2WO4 sample sintered at 875 °C has the optimum performance: εr = 8.4, Q×f = 88,893 GHz, and τf = −48.7 ppm/°C. Furthermore, the ceramic is compatible with the Ag electrode, indicating that the SrWO4 + x wt% Li2WO4(0 < x ≤ 1.5) ceramics with excellent performance are expected to be applied to LTCC substrate materials.
Data availability
Our datasets of the paper are available from the corresponding author on reasonable request.
References
J. Xi, G.H. Chen, F. Liu, F. Shang, J. Xu, C. Zhou, C. Yuan, Synthesis, microstructure and characterization of ultra-low permittivity CuO-ZnO-B2O3-Li2O glass/Al2O3 composites for ULTCC application. Ceram. Int. 45, 24431–24436 (2019)
H. Zhu, Y. Wang, Y. Dong, S. Ta, Q. Zhang, Microwave properties of BaMo1-xWxO4 ceramics and its chemical stability on electrode metals. Ceram. Int. 46, 9872–9877 (2020)
K. Liu, X. Wang, P. Gao, X. Wei, Y. Xiao, S. Deng, X. Qu, G. He, Q. Li, L. Deng, X. Chen, H. Zhou, Novel CaLn4Mo3O16 (Ln = La, Nd, and Sm) ceramics: sintering behaviour, phase structure and microwave dielectric performance. Ceram. Int. 48, 27360–27368 (2022)
X. Wang, J. Lv, Y. Xu, L. Zhang, Y. Shen, H. Zhou, D. Zhou, K. Song, H. Guo, F. Shi, Dielectric responses and structure-property relationships of Ca1-xBaxWO4 composite microwave dielectric ceramics. J. Alloys Compd. 925, 166669 (2022)
C.C. Xia, D.H. Jiang, G.H. Chen, Y. Luo, B. Li, C.L. Yuan, C.R. Zhou, Microwave dielectric ceramic of LiZnPO4 for LTCC applications. J. Mater. Sci. Mater. Electron. 28, 12026–12031 (2017)
G.H. Chen, C.C. Xia, J.S. Chen, Improved microwave dielectric properties for CaTi0.55(Al0.5Nb0.5)0.45O3 ceramics with low firing temperature by B2O3 addition. J. Mater. Sci. Mater. Electron. 29, 509–513 (2018)
H. Mao, X. Chen, F. Wang, W. Zhang, Effects of alkaline earth oxides on the densification and microwave properties of low-temperature fired BaO-Al2O3-SiO2 glass-ceramic/Al2O3 composites. J. Mater. Sci. 54, 12371–12380 (2019)
J. Xi, F. Shang, F. Liu, J. Xu, G. Chen, A facile preparation of temperature-stable borate ultra-low permittivity microwave ceramics for LTCC applications. Ceram. Int. 46, 19650–19653 (2020)
J. Xi, B.B. Lu, J.J. Chen, G. Chen, F. Shang, J. Xu, C. Zhou, C. Yuan, Ultralow sintering temperature and permittivity with excellent thermal stability in novel borate glass-ceramics. J. Non-Cryst Solids 521, 119527 (2019)
M.K. Zitani, T. Ebadzadeh, S. Banijamali, R. Riahifar, C. Rüssel, S.K. Abkenar, H. Ren, High quality factor microwave dielectric diopside glass-ceramics for the low temperature co-fired ceramic (LTCC) applications. J. Non-Cryst Solids 487, 65–71 (2018)
S. Li, C. Li, M. Mao, K. Song, Y. Iqbal, A. Khesro, S.S. Faouri, Z. Lu, B. Liu, S. Sun, D. Wang, High Q×f values of Zn-Ni co-modified LiMg0.9Zn0.1-xNixPO4 microwave dielectric ceramics for 5G/6G LTCC modules. J. Eur. Ceram. Soc. 42, 5684–5690 (2022)
Q. Zhang, L. Xu, X. Tang, H. Zhang, Y. Zhou, Y. Jing, Y. Li, Y. Liu, H. Su, Structural characteristics and microwave dielectric properties of Zn1-xBixVxW1-xO4-based ceramics for LTCC applications. J. Eur. Ceram. Soc. 42, 5684–5690 (2022)
Z. An, J. Lv, X. Wang, Y. Xu, L. Zhang, F. Shi, H. Guo, D. Zhou, B. Liu, K. Song, Effects of LiF additive on crystal structures, lattice vibrational characteristics and dielectric properties of CaWO4 microwave dielectric ceramics for LTCC applications, Ceram. Int. (2022) S0272884222022933
U. Došler, M.M. Kržmanc, D. Suvorov, The synthesis and microwave dielectric properties of Mg3B2O6 and Mg2B2O5 ceramics. J. Eur. Ceram. Soc. 30, 413–418 (2010)
Y.P. Liu, Y.N. Wang, Y.M. Li, B. Jianjiang, Low temperature sintering and microwave dielectric properties of LiMBO3 (M = Ca, Sr) ceramics, Ceram. Int. 42, 6475–6479 (2016)
J. Bao, J.L. Du, L.T. Liu, H. Wu, Y. Zhou, Z. Yue, A new type of microwave dielectric ceramic based on K2O-SrO-P2O5 composition with high quality factor and low sintering temperature. Ceram. Int. 48, 784–794 (2022)
X.Y. Lyu, Z.X. Li, J.J. Jin, Y. Xue, C. Yu, L. Ren, M. Zhang, H. Zhou, Sintering behavior, structure, and microwave properties of novel Li2xCu1-xMoO4 ceramics, Ceram. Int. (2022) S0272884222007143
G.-K. Choi, J.-R. Kim, S.H. Yoon, K.S. Hong, Microwave dielectric properties of scheelite (A = Ca, Sr, Ba) and wolframite (A = Mg, Zn, Mn) AMoO4 compounds. J. Eur. Ceram. Soc. 27, 3063–3067 (2007)
M.-R. Joung, J.-S. Kim, M.-E. Song, S. Nahm, J.-H. Paik, Formation process and microwave dielectric properties of the R2V2O7 (R = Ba, Sr, and Ca) ceramics. J. Am. Ceram. Soc. 92, 3092–3094 (2009)
R. Naveenraj, E.K. Suresh, D. Johnson, R. Ravendran, Preparation and microwave dielectric properties of Ba3A(V2O7)2 (A = Mg, Zn) ceramics for ULTCC applications, Eur. J. Inorg. Chem. 2019 (2019)
R.Z. Zuo, J. Zhang, J. Song, Y. Xu, Liquid-phase sintering, microstructural evolution, and microwave dielectric properties of Li2Mg3SnO6-LiF ceramics. J. Am. Ceram. Soc. 101, 569–576 (2018)
Y. Deng, P. Yao, B. Li, A novel ultra-low temperature sintered Li2CO3 doped Ba3V2O8 microwave ceramics. Mater. Lett. 285, 129125 (2021)
H. Ogawa, A. Yokoi, R. Umemura, A. Kan, Microwave dielectric properties of Mg3(VO4)2-xBa3(VO4)2 ceramics for LTCC with near zero temperature coefficient of resonant frequency. J. Eur. Ceram. Soc. 27, 3099–3104 (2007)
M.-R. Joung, J.-S. Kim, M.-E. Song, S. Nahm, J.-H. Paik, Microstructure and microwave dielectric properties of the Li2CO3-added Sr2V2O7 ceramics. J. Am. Ceram. Soc. 93, 2132–2135 (2010)
J. Chen, W. Fang, Y. Tang, jie Li, L. Fang, Effects of LiF addition on the densification and microwave dielectric properties of LiInO2 ceramics. Ceram. Int. 47, 28960–28967 (2021)
Y. Lai, H. Su, G. Wang, T. Xiaoli, X. Huang, X. Liang, H. Zhang, J. Li, K. Huang, X. Renshaw, Wang, Low-temperature sintering of microwave ceramics with high Qf values through LiF addition. J. Am. Ceram. Soc. 102, 1893–1903 (2018)
D. Zhou, C.A. Randall, L.-X. Pang, H. Wang, J. Guo, G.-Q. Zhang, X.-G. Wu, L. Shui, X. Yao, Microwave dielectric properties of Li2WO4 ceramic with ultra-low sintering temperature. J. Am. Ceram. Soc. 94, 348–350 (2011)
S.H. Yoon, D.-W. Kim, S.-Y. Cho, K.S. Hong, Investigation of the relations between structure and microwave dielectric properties of divalent metal tungstate compounds. J. Eur. Ceram. Soc. 26, 2051–2054 (2006)
Y. Peng, J. Li, E.-C. Xiao, J. Wang, Z. Qi, Z. Yue, X. Dong, Y. Chen, G. Chen, F. Shi, Lattice vibrational characteristics, dielectric properties and structure-property relationships of (1-x)SrWO4-xTiO2 composite ceramics. Mater. Chem. Phys. 258, 123889 (2021)
S.J. Kim, E.S. Kim, Microwave dielectric properties of 0.85CaWO4-0.15SmNbO4 ceramics with sintering additives. Ceram. Int. 35, 137–141 (2009)
Y. Yang, Y. Wang, J. Zheng, N. Dai, R. Li, H. Wu, B. Wu, Microwave dielectric properties of ultra-low loss Li2Mg4Zr0.95(Mg1/3Ta2/3)0.05O7 ceramics sintered at low temperature by LiF addition. J. Alloys Compd. 786, 867–872 (2019)
B. Tao, W. Wang, H. Liu, T. Du, H. Wu, C. Xing, D. Wang, Y. Zhang, Low-temperature sintering LiF-doped Li4Mg3[Ti0.6(Mg1/3Nb2/3)0.4]2O9 microwave dielectric ceramics for LTCC applications. Ceram. Int. 47, 2584–2590 (2021)
Z. Liang, J. Li, J. Wu, Y. Yang, B. Lu, Y. Zhang, H. Zhang, Enhanced microstructure and dielectric properties of low-temperature sintered MgO-xwt%LiF ceramics for high-frequency applications. Ceram. Int. 48, 2704–2709 (2022)
C. Zhang, Y. Chen, X. Li, H. Ren, G. Wang, X. Dong, Effect of LiF addition on sintering behavior and dielectric breakdown mechanism of MgO-based microwave dielectric ceramics. J. Materiomics 7, 478–487 (2021)
C. Pei, Y. Li, C. Hou, B. Xie, G. Yao, W. Ren, Z. Ren, P. Liu, Sintering behavior and microwave dielectric properties of V5+ substituted Li3Mg2SbO6 ceramics. J. Mater. Sci. Mater. Electron. 30, 14495–14499 (2019)
Y.-C. Chen, H.-M. You, K.-C. Chang, Influence of Li2WO4 aid and sintering temperature on microstructures and microwave dielectric properties of Zn2SnO4 ceramics. Ceram. Int. 41, 5257–5262 (2015)
C. Cai, X. Chen, H. Li, J. Xiao, C. Zhong, S. Zhang, Microwave dielectric properties of Ca1-xSrxMgSi2O6 ceramics. Ceram. Int. 46, 27679–27685 (2020)
C. Yin, Z. Yu, L. Shu, L. Liu, L. Yang, C. Li, Improvements on sintering behavior and microwave dielectric properties of Li4WO5 ceramics through MgO modification. Ceram. Int. 47, 2802–2808 (2021)
M. Xiao, Q. Gu, Z. Zhou, P. Zhang, Study of the microwave dielectric properties of (La1-xSmx)NbO4 (x = 0-0.10) ceramics via bond valence and packing fraction. J. Am. Ceram. Soc. 100, 3952–3960 (2017)
W. Luo, L. Li, S. Yu, J. Qiao, Z. Sun, B. Zhang, M. Du, Novel wodginite structured MnO-SnO2-Ta2O5 microwave dielectric ceramic. J. Alloys Compd. 742, 72–77 (2018)
H. Zhou, J. Huang, X. Tan, N. Wang, G. Fan, X. Chen, Compatibility with silver electrode and microwave dielectric properties of low firing CaWO4-2Li2WO4 ceramics. Mater. Res. Bull. 89, 150–153 (2017)
Z. Wang, L. Song, J. Bian, Low temperature sintering and microwave dielectric properties of Li2TiO3-Li2WO4 composite ceramics. Ceram. Int. 39, 9767–9772 (2013)
Funding
No funding was received for the research reported in the article.
Author information
Authors and Affiliations
Contributions
BH contributed to experimental scheme and writing and original draft preparation; TX performed experimental validation and data curation; FS modified the manuscript. GC conceived and designed the conception of the study, provided resources, and revised the manuscript. All the authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, B., Chen, G., Xia, T. et al. Microwave dielectric properties of Li2WO4-added SrWO4 ceramics for LTCC applications. J Mater Sci: Mater Electron 33, 21925–21934 (2022). https://doi.org/10.1007/s10854-022-08980-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-022-08980-6