Abstract
Fast and effective detection of glucose has important significance in clinical medicine and the diagnosis of diabetes. Electrochemical non-enzymatic glucose sensor has received extensive attention to detect glucose in the recent years due to its simple operation and low cost. In this paper, Cu–CdIn2O4 nanoparticles decorated on nickel foam electrode as a sensitive non-enzymatic electrochemical sensor for glucose detection are synthesized by a one-step non-aqueous sol–gel method. Different Cu-doping levels are designed as performance comparison analysis. The electrocatalytic performance of the Cu–CdIn2O4 nanoparticles/Ni foam electrodes towards glucose oxidation is evaluated by cyclic voltammetry and current time. The results show that 15% Cu–CdIn2O4/Ni foam electrode shows higher sensitivity, as well as an excellent anti-interference and long-term stability towards glucose detection compared with 10% and 20% Cu–CdIn2O4/Ni foam electrodes. Hence, 15% Cu–CdIn2O4/Ni foam can be regarded as an efficient and a promising sensing material for glucose detection. Such satisfactory performance is not only attributed to the synergistic effect of Cd, In, and Cu, but also benefits from the uniform distribution of 15% Cu–CdIn2O4 nanoparticles on the Ni foam, which provides more reactive sites for the electrochemical catalytic reaction.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Glucose is an energy source and an intermediate product of metabolism for living cells in the human body. Glucose is easily absorbed into the blood to maintain the body's functional activities, but once the levels are above 7 mmol/L it can lead to diabetes. Diabetes is a serious metabolic disease that poses a great threat to human health and produces a series of complications. Therefore, it is of great significance to use an appropriate way to real-time monitor glucose level for human health protection and disease prevention. Currently, various methods such as fluorescent [1], optical [2], acoustic [3], and electrochemical methods [4] have been widely used to detect glucose, among which electrochemical glucose sensors [5, 6] including enzymatic glucose sensor and non-enzymatic glucose sensor [7] have received extensive interest and achieved great success due to their ease of operation, stable, fast, accurate, and reliable test results [8, 9]. Enzymatic glucose sensors usually use glucose oxidase (GOx) or glucose dehydrogenase (GDH) as biological catalysts to accelerate the oxidation of glucose, so as to achieve the purpose of detection. Although the enzymatic glucose sensor has exhibited some merits such as high sensitivity and good selectivity, its application is limited to a certain extent due to high price, easy to be affected by the surrounding environment, and poor long-term stability. To tackle the issues, more efforts have been devoted on the development of non-enzymatic glucose sensor with low cost, ease of operation, high sensitivity, and good stability. Different from the enzymatic glucose sensors, the non-enzymatic glucose sensors use inorganic materials as catalysts to realize the oxidation of glucose in neutral and alkaline environment. To date, various nanomaterials such as noble metals [10], metal nitrides [11,12,13], metal oxides [14,15,16], metal sulfides [17,18,19,20], metal alloys [21, 22], and metal hydrate [23] have been extensively developed for non-enzymatic glucose sensors. Among them, metal oxides based non-enzymatic glucose sensors are the most widely studied due to their low cost, excellent electrocatalytic activities, and superior stability [24]. Especially, the bimetallic oxides with the structure AB2O4 such as MnCo2O4 [25], CuGa2O4 [26], NiCo2O4 [27] CuCo2O4 [28], and NiGa2O4 [29] have been considered as promising catalysts for glucose oxidation due to the synergistic effect of bimetals.
CdIn2O4 is a typical n-type bimetallic oxide [30] with metal-like high conductivity, bimetallic synergy, and excellent electrical properties, which can be used as a ideal catalyst for glucose oxidation. To the best of our knowledge, CdIn2O4 nanomaterials have been rarely reported for electrochemical analysis. More importantly, numerous reports have indicated that composite materials exhibit obvious advantages in improving performance by using their synergistic effects compared with the single component materials. At present, there are only few reports on elements doped by CdIn2O4, appropriate doping has been considered an effective method to enhance the electrochemical properties by providing electronic defects and changing the electrical conductivity. Transition metals such as Cr, Cu, Ni, Co, and Fe often act as appropriate dopants in various fields since they change the original crystal parameters and enhance the catalytic activity [31].
In this work, Cu-doped CdIn2O4 nanoparticles decorated on Ni foam are synthesized by a one-step non-aqueous sol–gel method. This novel Cu-doped CdIn2O4 nanoparticles/Ni foam electrodes can be used as a non-enzymatic sensor for electrocatalytic oxidation of glucose. Several characterization techniques and electrochemical instruments are applied to evaluate the properties of the nanomaterials. As expected, the obtained Cu-doped CdIn2O4 nanoparticles/Ni foam electrodes display excellent electrocatalytic properties, high sensitivity, and fast response time for glucose detection.
2 Experimental
2.1 Material and reagents
Indium nitrate hydrate (InN3O9. xH2O, 99.9%), Cadmium acetate (Cd(CH3CO2)2, 99.5%), benzyl alcohol (C7H8O, 99.8%), and cupric acetate (C4H6CuO4. H2O, 99.0%) are purchased from Macklin Biochemical Co., Ltd (Shanghai, China). Nickel foam (Ni foam) is supplied by Lizhiyuan Battery Materials Co., Ltd (Shanxi, China). All chemical reagents are analytical grade in this experiment and without any further purification.
2.2 Synthesis of Cu–CdIn2O4 nanoparticles decorated on Ni foam
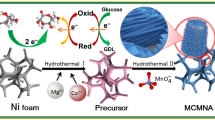
Firstly, Ni foams (1 cm × 2 cm) are cut and immersed in dilute hydrochloric acid solution for 12 h to remove impurities, and then are washed with DI water and absolute ethyl alcohol alternately. Subsequently, the treated Ni foams are dried at 60 °C for further use. The pure CdIn2O4 nanoparticles decorated on Ni foam are synthesized according to our previous report [32]. The Cu–CdIn2O4 nanoparticles decorated on Ni foam are synthesized by a one-step non-aqueous sol–gel method. The schematic illustrations of the synthesis and electrochemical glucose detection process of the Cu–CdIn2O4 nanoparticles electrode are shown in Fig. 1. Typically, 0.4512 g InN3O9·xH2O and 0.3999 g Cd(CH3CO2)2 are added to 60 mL C7H8O solvent. Then, a certain amount of C4H6CuO4. H2O with different mass ratio to pure CdIn2O4 nanoparticles (10, 15, and 20%) and the treated Ni foams are added into the above solution, followed by stirring for 12 h to get the homogeneous solution. Then the obtained solution is transferred to 100 mL Teflon-lined stainless steel autoclave and maintained at 200 °C for 48 h. After the reaction is over, the autoclave is cooled to room temperature naturally, the decorated Ni foams and resulting precipitates are washed several times by centrifugation with ethanol and DI water alternately and dried at 60 °C in air for further investigation.
2.3 Characterization
X-ray diffraction (XRD; D/Max-3B, Rigaku, Japan) with CuKα1 radiation (λ = 0.15406 nm, 40 kV, 100 mA) is applied to analyze the crystal phase of the samples. Scanning electron microscopy (SEM, Quanta 200, FEI, America) and transmission electron microscopy (TEM, JEM-2010, JEOL, Japan) are conducted to investigate the morphology of the samples. X-ray photoelectron spectroscopy (XPS, ESCALAB 250, Thermo Fisher Scientific, America) and energy-dispersive spectroscopy (EDS; Bruker Nano GMBH, Germany) are applied to determine the chemical states and the chemical compositions. The samples characterized by XRD, TEM, EDS, and XPS are powder samples shaken by ultrasonic method on Ni foam.
2.4 Electrochemical measurements
The electrochemical measurements are performed on the CHI 760 electrochemical workstation (CH Instruments Co., Shanghai, China). A conventional three-electrode system is employed, including the decorated Ni foams with Cu–CdIn2O4 nanoparticles as the working electrode, a platinum sheet as the counter electrode, and a saturated calomel electrode (SCE) as the reference electrode. Electrochemical analysis is performed in a 200 mL 3.5 M KOH electrolyte solution at room temperature, including the current time (I–t), cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS). The I–t curve represents the current changes caused by glucose redox reaction at a constant voltage of 0.45 V. CV curve is characterized in the potential range of -0.075 V to 0.675 V at a scan rate of 50 mV s−1. EIS is carried out at the equilibrium potential of open circuit, and the frequency range is selected as 0.01–105 Hz in 3.5 M KOH electrolyte solution.
3 Results and discussions
3.1 Characterization
The XRD patterns of pristine CdIn2O4, 10%, 15%, and 20% Cu-doped CdIn2O4 samples are shown in Fig. 2a. All diffraction peaks of the un-doped, 10%, and 15% Cu-doped CdIn2O4 are well attributed to the phase of CdIn2O4, and there are no other byproducts detected, which imply that the Cu ions incorporate into the lattice of CdIn2O4 without destroying the crystal structure. While for 20% Cu-doped CdIn2O4, in addition to the phase with CdIn2O4, the phase of Cd(OH)2 is also clearly observed, indicating that when the content of Cu is too high, more Cd ions in CdIn2O4 would be replaced by Cu ions, since the Cd and Cu ions have small difference in ionic radius, 0.095 and 0.073 nm, respectively. The displaced Cd ions react with hydroxy (–OH) in benzyl alcohol to generate Cd(OH)2. Furthermore, the doping of Cu changes the lattice constants of CdIn2O4. Typically, from the enlarged diffraction peak of (311) plane shown in Fig. 2b, it is understood that the position of the diffraction peak has a slight shift to a large angle with Cu doping, indicating that Cu ions incorporate into the lattice of CdIn2O4 and cause the lattice to shrink, and the interplanar spacing to decrease (according to Bragg’s law, the interplanar spacing is inversely proportional to the diffraction angle).
Figure 3 systematically performs the morphological features of the obtained Cu–CdIn2O4 nanoparticles decorated on Ni foams. The SEM images of 10%, 15%, and 20% Cu–CdIn2O4 nanoparticles decorated on Ni foams are shown in Fig. 3a, b, and c, respectively. One can observe that there are obvious differences in the morphology of the as-prepared products with the content of Cu doping. Among them, 10% and 20% Cu–CdIn2O4 products take on a coral-flower appearance composed of several thin short sticks. For 15% Cu–CdIn2O4, multiple irregular clusters resembling ice flowers particles are observed, meanwhile, the smaller morphology size of 15% Cu–CdIn2O4 possesses higher specific surface area and reactive sites compared with 10% and 20% Cu–CdIn2O4, which helps to improve the electrochemical performance and glucose detection. Figure 3d exhibits the space skeleton of 15% Cu–CdIn2O4 particles decorated on Ni foam, and the particles are tightly attached to the skeleton, which is beneficial to enhance the conductivity of the whole electrode. TEM image of 15% Cu–CdIn2O4 nanoparticles is shown in Fig. 3e, clearly displaying the dispersed particles. An HRTEM image (Fig. 3f) reveals the clear parallel fringes of (311) crystal planes of CdIn2O4 with a interplanar spacing value of 0.274 nm, which is shorter than the standard interplanar spacing value (0.277 nm) of pure CdIn2O4 phase, indicating that the Cu ions are successfully doped in CdIn2O4. Figure 3g presents a selected area of the electron diffracted pattern (SAED) with continuous rings, belonging to the (111), (220), and (311) planes of CdIn2O4. To further investigate the distribution of elements of 15% Cu–CdIn2O4 nanoparticles, EDS is also applied. The elemental mapping images (Fig. 3h–k) show that the 15% Cu–CdIn2O4 nanoparticles are composed of Cd, In, O, and Cu elements, and they are uniformly existed in the 15% Cu–CdIn2O4 nanoparticles.
SEM images: a–c 10%, 15%, and 20% Cu–CdIn2O4 decorated on Ni foams, respectively, d 15% Cu–CdIn2O4 decorated on Ni foam (low magnification). TEM image: e 15% Cu–CdIn2O4 nanoparticles. HRTEM image: f 15% Cu–CdIn2O4 nanoparticles. g SAED pattern of 15% Cu–CdIn2O4 nanoparticles. Elemental mapping of 15% Cu–CdIn2O4 nanoparticles for h Cd element, i In element, j O element, and k Cu elements
XPS measurement is adopted to determine the chemical and surface state of the prepared nanocomposites. The survey spectra (Fig. 4a) of 10%, 15%, and 20% Cu–CdIn2O4 nanocomposites exhibit the peaks corresponding to the Cd, In, O, and Cu elements, respectively. Figure 4b shows the high-resolution scans of O 1s in all the prepared samples. The O 1s peak could be decomposed into two fitted peaks (oxygen vacancy and lattice oxygen are expressed as OV and OL, respectively). It is observed that the OV peak of 15% Cu–CdIn2O4 sample shows the highest relative peak area, indicating it possesses more oxygen vacancy. The increase of OV means that more adsorbed oxygen (Ox−) exists on the surface of the sample, and it can combine with H+ in the electrolyte to form O–H, which contributes to the hydrolysis of CdIn2O4 and promotes the redox reaction. Hence, 15% Cu–CdIn2O4 shows better electrochemical glucose detection in our following discussions. Figure 4c–e shows the high-resolution scans of Cd 3d, In 3d, and Cu 2p in 15% Cu–CdIn2O4, respectively. Two characteristic peaks at 404.84 eV and 411.61 eV are assigned to the Cd 3d5/2 and Cd 3d3/2 binding energies for Cd 2+. The peaks at 444.11 eV and 451.63 eV are In 3d5/2 and In 3d3/2 of In 3d, which are attributed to lattice indium in CdIn2O4. The high-resolution scans of Cu 2p can be fitted as Cu 2p1/2 and Cu 2p3/2 peaks, and the binding energies of the two peaks are located at 952.54 eV and 932.75 eV, respectively.
3.2 Electrochemical performance of Cu–CdIn2O4 nanoparticles on Ni foams
The electrochemical impedance spectroscopy (EIS) behaviors of 10%, 15%, and 20% Cu–CdIn2O4/Ni foam electrodes are shown in Fig. 5. it can be observed that the semicircle diameter of the curve for 15% Cu–CdIn2O4/Ni foam electrode is smaller than that of 10% and 20% Cu–CdIn2O4/Ni foam electrodes at high frequency region, suggesting the fast charge-transfer rate and better electrical conductivity for 15% Cu–CdIn2O4/Ni foam electrode. Additionally, the intersection of the curves and the real axis is basically at the zero position, which indicates that there is a low internal resistance of the electrodes and better electrical conductivity for the prepared Cu–CdIn2O4 nanoparticles.
The CV curves of the prepared 10%, 15%, 20% Cu–CdIn2O4 nanoparticles on Ni foams and bare Ni foam electrodes in 200 mL 3.5 M KOH electrolyte solution with and without 1 mM glucose are shown in Fig. 6a. The current density of the 10%, 15%, 20% Cu–CdIn2O4 nanoparticles on Ni foams and bare Ni foam electrodes increases in the presence of glucose, indicating the glucose catalytic activity. The current density of bare Ni foam and CdIn2O4 nanoparticles on Ni foam electrodes in our previous work [32] is much lower than that of the Cu–CdIn2O4 electrodes, which indicates that doping a certain amount of Cu in CdIn2O4 nanoparticles on Ni foam would efficiently enhance the electrocatalytic activity. Meanwhile, the current response in 15% Cu–CdIn2O4 /Ni foam electrode is higher than that of 10% and 20% Cu–CdIn2O4/Ni foams electrodes, and the order can be expressed as 15% > 20% > 10%, suggesting that the 15% Cu–CdIn2O4/Ni foam electrode performs the best glucose-sensitive electrochemistry behavior. To further obtain the electrochemical kinetic information of electrocatalytic oxidation of glucose, the effect of scan rate on glucose oxidation at 15% Cu–CdIn2O4/Ni foam electrode is investigated in 3.5 M KOH electrolyte solution containing 1 mM glucose. As shown in Fig. 6b, the anodic peak-to-cathodic peak potential separation in the CV responses increases with the increase in the scan rate, which is attributed to the transport barriers of electrons and electrolyte ions during electrochemical processes, indicating the charge-transfer kinetic limitations [33]. Meanwhile, Fig. 6c shows the linearity between both the anodic peak current density (Ipa) and cathodic peak current density (Ipc) and the square root of the scan rate in the range from 10 to 180 mV s−1, and the linear equation is as follows. The linear relationship indicates that the electrochemical process is dominated by the diffusion-controlled process [34, 35].
a CV curves of Cu–CdIn2O4 nanoparticles/Ni foam electrodes in 3.5 M KOH electrolyte solution at a scan rate of 50 mV s−1 with and without 1 mM glucose; b CV curves of 15% Cu–CdIn2O4 nanoparticles/Ni foam electrode at different scan rates. c Linear plots of peak current density versus the square root of the scan rate
Simultaneously, the diffusion coefficient ratio between 15% Cu–CdIn2O4/Ni foam electrode and pristine CdIn2O4/Ni foam electrode [32] can be obtained by using Randles–Sevcik formula [36]:
The calculated result shows that the diffusion coefficient of 15% Cu–CdIn2O4/Ni foam electrode (DCu–Cd) is 5.47-fold that of pristine CdIn2O4 electrode (DCd), indicating that 15% Cu–CdIn2O4/Ni foam electrode displays better electrochemical performance than pristine CdIn2O4.
The amperometric sensing responses of 10%, 15%, and 20% Cu–CdIn2O4/Ni foam electrodes are investigated towards different concentration of glucose from 0.001 to 1 mM (R2 = 0.99) at a constant voltage of 0.45 V, and the glucose solution is added every 50 s in a continuously stirred 3.5 M KOH electrolyte solution. As shown in Fig. 7a, c, and e, the current response exhibits a linear stable stepwise rise with the increase of glucose concentration. However, the curves deviate from linearity in the low concentration glucose range, which may be due to the instability of the initial current of the electrodes. The sensitivity is obtained by observing the corresponding slope parameter, as shown in Fig. 7b and d, the sensitivity of 10% and 15% Cu–CdIn2O4/Ni foam electrodes is 3.810 and 5.360 mA mM−1 cm−2 in 0.01–1.0 glucose concentration range, respectively. The sensitivity of 20% Cu–CdIn2O4/Ni foam electrode in Fig. 7f shows 3.358 mA mM−1 cm−2 in the 0.1–1.0 glucose concentration range. The 15% Cu–CdIn2O4/Ni foam electrode performs the highest electrochemical sensing response towards glucose detection compared with the other two electrodes, implying the excellent electrochemical catalytic oxidation reaction at the surface of 15% Cu–CdIn2O4 nanoparticles. However, the low response of 20% Cu–CdIn2O4/Ni foam electrode to glucose may be due to the inactivity of the second phase substance for glucose detection. Detection limit of the electrodes for glucose can be calculated by the formula LOD = 3σ/S, where σ is the standard deviation of the intercept and S is the slope of the calibration plot. The detection limit of 10%, 15%, and 20% Cu–CdIn2O4/Ni foam electrodes is 3.42, 3.39, and 4.11 μM, respectively. In addition, a comparison of various non-enzymatic glucose sensors in previous works is shown in Table 1. Obviously, 15% Cu–CdIn2O4/Ni foam electrode in our work displays a higher sensitivity towards glucose detection. It may benefit from the synergistic interactions between Cu(II)/Cu(III) and Cd(II)/Cd(III), In(II)/In(III).
The amperometric I–t curves of a 10% Cu–CdIn2O4 nanoparticles/Ni foam electrode, c 15% Cu–CdIn2O4 nanoparticles/Ni foam electrode, e 20% Cu–CdIn2O4 nanoparticles/Ni foam electrode upon the addition of various concentration of glucose at 0.45 V. Corresponding calibration diagram of the response current density and glucose concentration, b 10% Cu–CdIn2O4 nanoparticles/Ni foam electrode, d 15% Cu–CdIn2O4 nanoparticles/Ni foam electrode, f 20% Cu–CdIn2O4 nanoparticles/Ni foam electrode
Figure 8a shows the enlarged section of the I–t curve of 15% Cu–CdIn2O4/Ni foam electrode at 0.02 mM glucose concentration region, which tells that the current response increases sharply and reaches a steady state within 4 s, indicating a short response time of the proposed non-enzymatic glucose sensor. Anti-interference property is an important aspect of evaluating glucose sensor performance in practical applications. Figure 8b illustrates the anti-interference property of 15% Cu–CdIn2O4/Ni foam electrode by successive injection of 50 μM glucose, 5 μM ascorbic acid (AA), 5 μM sodium chloride (NaCl), 5 μM sodium nitrate (NaNO3), 5 μM Urea, and 5 μM potassium chloride (KCI) at an interval of 50 s into 3.5 M KOH electrolyte solution under continuous stirring. The result indicates that there is a high response to glucose, on the contrary, it is difficult to observe an obvious current response to AA, NaCl, NaNO3, and KCI. Then, we continue to test the current response of all the interference species again, it can be observed that the electrode still shows the same response as the first cycle, suggesting its superior anti-interference property and excellent reproducibility, which can be reused continuously.
The long-term stability is also an important parameter for assessing sensor reliability in the practical application. Figure 9 displays the current signal and glucose sensitivity of 15% Cu–Cu–CdIn2O4/Ni foam electrode within 10 days in 3.5 M KOH electrolyte solution. It is observed that the current response values of the electrode still be maintained at more than 90% on day 10 compared with the initial value due to the electrode aging and the disappearance of unstable adsorption sites, suggesting its better electrocatalytic activity and satisfactory stability.
In order to verify the applicability of the 15% Cu–CdIn2O4/Ni foam glucose sensor, the glucose concentration is detected using the current–time method by successive step addition of the glucose to 200 mL stirred 3.5 M KOH electrolyte solution containing human serum samples. The test is executed three times, and the results are shown in Table 2. The recoveries of the 15% Cu–CdIn2O4/Ni foam non-enzymatic glucose sensor display in the range of 96.00–103.16% and the RSD is 2.11–3.56%, illustrating the potential use of as-synthesized Cu–CdIn2O4/Ni foam non-enzymatic glucose sensor in real serum sample analysis.
3.3 Glucose-sensing mechanism
The above-mentioned experimental results confirm that the 15% Cu–CdIn2O4/Ni foam electrode has apparent improvement in glucose sensitivity and its reliable utility in the detection of serum glucose in humans. As shown in Fig. 6a, b, there are obvious redox couple under alkaline conditions, which means that Cd(II)/Cd(III) and In(II)/In(III) complete the current response to glucose, and Cu(II)/Cu(III) also participates in the catalytic process, making it more responsive to glucose than pristine CdIn2O4. Combining Fig. 10, a surface depletion layer model caused by glucose is used to illustrate the proposed mechanism for the sensing behavior of Cu–CdIn2O4/Ni foam electrode. When the electrode in the KOH electrolyte solution, the Cd(II), In(II), and Cu(II) are oxidized to Cd(III), In(III), and Cu(III) in electrolyte, simultaneously, electrons are taken away from the conduction band of the Cu–CdIn2O4 and the potential barrier increase, which results in a low conductivity. When glucose is added to the electrolyte, Cd(III), In(III), and Cu(III) catalyze the glucose to gluconolactone, while Cd(III), In(III), and Cu(III) are reduced to Cd(II), In(II), and Cu(II), in turn, electrons are released come back to conduction band and increase the oxidation peak current. The detail catalytic oxidation reactions towards glucose can be described as follows:
4 Conclusion
In this work, 10%, 15%, and 20% Cu-doped CdIn2O4 nanoparticles decorated on nickel foams as the sensitive non-enzymatic electrochemical sensors are successfully synthesized via a one-step non-aqueous sol–gel method and their electrochemical performance and glucose-sensing properties are investigated. The crystal phase, morphology, and chemical composition of the nanoparticles are systematically characterized by XRD, SEM, TEM, and XPS techniques, respectively. The results of the glucose-sensitive electrochemistry behavior of Cu–CdIn2O4/Ni foam electrode indicate that Cd, In, and Cu jointly promote the electrochemical catalytic reaction in a three-electrode system (3.5 KOH as electrolyte) to achieve a highly sensitive response to glucose. Additionally, the doping content of Cu also has a great influence on the performance, among which 15% Cu–CdIn2O4/Ni foam electrode shows the best electrochemical performance towards glucose detection compared with 10% and 20% Cu–CdIn2O4/Ni foam electrodes, such as higher sensitivity (5303 μA·mM−1·cm−2), faster response time (4 s), and wide linear range (0.1–1 mM), along with remarkable selectivity, long-term stability, which could be used as an efficient and sensitive glucose monitor in the practical workplace. Such satisfactory performance of 15% Cu–CdIn2O4 sample is not only attributed to the more oxygen vacancy, the smaller particle size which possesses higher specific surface area and reactive sites compared with 10% and 20% Cu–CdIn2O4, but also benefits from the synergistic effect of Cd, In, and Cu for the electrochemical catalytic reaction.
Data availability
This work described has not been published before. All data generated or analyzed during this study are included in this published article.
References
S. Yu, L. Ding, H. Lin, W. Wu, J. Huang, A novel optical fiber glucose biosensor based on carbon quantum dots-glucose oxidase/cellulose acetate complex sensitive film. Biosensors Bioelectron. 146, 111760 (2019)
V. Rachim, W. Chung, Wearable-band type visible-near infrared optical biosensor for non-invasive blood glucose monitoring. Sensors Actuators B Chem. 286, 173–180 (2019)
J. Luo, P. Luo, M. Xie, K. Xie, B. Zhao, F. Pan, F. Zeng, D. Zhang, Z. Zheng, A new type of glucose biosensor based on surface acoustic wave resonator using Mn-doped ZnO multilayer structure. Biosensors Bioelectron. 49, 512–518 (2013)
D. Hwang, S. Lee, M. Seo, T. Chung, Recent advances in electrochemical non-enzymatic glucose sensors-a review. Anal. Chim. Acta 1033, 1–34 (2018)
B. Huang, Y. Wang, Z. Lu, H. Du, J. Ye, One pot synthesis of palladium cobalt nanoparticles over carbon nanotubes as a sensitive non-enzymatic sensor for glucose and hydrogen peroxide detection. Sensors Actuators B Chem. 252, 1016–1025 (2017)
N. Karikalan, M. Velmurugan, S. Chen, C. Karuppiah, Modern approach to the synthesis of Ni(OH)2 decorated sulfur doped carbon nanoparticles for the nonenzymatic glucose sensor. ACS Appl. Mater. Interfaces 8, 22545–22553 (2016)
S. Zaidi, J. Shin, Recent developments in nanostructure based electrochemical glucose sensors. Talanta 149, 30–42 (2016)
J. Li, H. Hu, H. Li, C. Yao, Recent developments in electrochemical sensors based on nanomaterials for determining glucose and its by product H2O2. J. Mater. Sci. 52, 10455–10469 (2017)
H. Zhu, L. Li, W. Zhou, Z. Shao, X. Chen, Advances in non-enzymatic glucose sensors based on metal oxides. J. Mater. Chem. B. 4, 7333–7349 (2016)
B. Huang, M. Wang, D. Kathiravan, A. Kurniawan, H. Zhang, W. Yang, Interfacial effect of oxygen-doped nanodiamond on CuO and micro-pyramidal silicon heterostructures for efficient non-enzymatic glucose sensor. ACS Appl. Bio Mater. 1, 1579–1586 (2018)
J. Chen, H. Yin, J. Zhou, J. Gong, L. Wang, Y. Zheng, Q. Nie, Non-enzymatic glucose sensor based on nickel nitride decorated nitrogen doped carbon spheres (Ni3N/NCS) via facile one pot nitridation process. J. Alloys Compd. 797, 922–930 (2019)
T. Liu, M. Li, P. Dong, Y. Zhang, L. Guo, Design and facile synthesis of mesoporous cobalt nitride nanosheets modified by pyrolytic carbon for the nonenzymatic glucose detection. Sens. Actuator B Chem. 255, 1983–1994 (2018)
F. Xie, X. Cao, F. Qu, A.M. Asiri, X. Sun, Cobalt nitride nanowire array as an efficient electrochemical sensor for glucose and H2O2 detection. Sensors Actuators B Chem. 255, 1254–1261 (2018)
Y. Ding, Y. Wang, L. Su, M. Bellagamba, H. Zhang, Y. Lei, Electrospun Co3O4 nanofibers for sensitive and selective glucose detection. Biosensors Bioelectron. 26, 542–548 (2010)
Q. Dong, D. Song, Y. Huang, Z. Xu, J. Chapman, W. Willis, B. Li, Y. Lei, High-temperature annealing enabled iridium oxide nanofibers for both non-enzymatic glucose and solid-state pH sensing. Electrochim. Acta 281, 117–126 (2018)
J. Xu, Y. Sun, J. Zhang, Solvothermal synthesis of Fe3O4 nanospheres for high-performance electrochemical non-enzymatic glucose sensor. Sci. Rep. 10, 16026 (2020)
J. Zhang, C. Xu, R. Zhang, X. Guo, J. Wang, X. Zhang, D. Zhang, B. Yuan, Solvothermal synthesis of cobalt tungstate microrings for enhanced nonenzymatic glucose sensor. Mater. Lett. 210, 291–294 (2018)
W. Wu, L. Wu, H. Wu, S. Wang, Y. Ding, C. Feng, Sulphides of the cobalt doped Ni7S6 type for glucose, hydrogen peroxide and nitrite sensing platform. Sensors Actuators B Chem. 250, 224–232 (2017)
C. Zhao, X. Wu, X. Zhang, P. Li, X. Qian, Facile synthesis of layered CuS/RGO/CuS nanocomposite on Cu foam for ultrasensitive nonenzymatic detection of glucose. J. Electroanal. Chem. 785, 172–179 (2017)
V. Sridhar, H. Park, Carbon encapsulated cobalt sulfide nano-particles anchored on reduced graphene oxide as high capacity anodes for sodium-ion batteries and glucose sensor. J. Alloys Compd. 764, 490–497 (2018)
P. Liu, M. Zhang, S. Xie, S. Wang, W. Cheng, F. Cheng, Non-enzymatic glucose biosensor based on palladium-copper oxide nanocomposites synthesized via galvanic replacement reaction. Sensors Actuators B Chem. 253, 552–558 (2017)
C. Wang, Y. Sun, X. Yu, D. Ma, J. Zheng, P. Dou, Z. Cao, X. Xu, Ag–Pt hollow nanoparticles anchored reduced graphene oxide composites for non-enzymatic glucose biosensor. J. Mater. Sci. Mater. Electron. 27, 9370–9378 (2016)
C. Xia, W. Ning, A novel non-enzymatic electrochemical glucose sensor modified with FeOOH nanowire. Electrochem. Commun. 12, 1581–1584 (2010)
R. Ding, L. Qi, M. Jia, H. Wang, Facile synthesis of mesoporous spinel NiCo2O4 nanostructures as highly efficient electrocatalysts for urea electro-oxidation. Nanoscale 6, 1369–1376 (2014)
Y. Zhang, L. Luo, Z. Zhang, Y. Ding, S. Liu, D. Deng, H. Zhao, Y. Chen, Synthesis of MnCo2O4 nanofibers by electrospinning and calcination: application for a highly sensitive non-enzymatic glucose sensor. J. Mater. Chem. B. 2, 529–535 (2014)
H. Yin, Y. Shi, Y. Dong, X. Chu, Synthesis of spinel-type CuGa2O4 nanoparticles as a sensitive non-enzymatic electrochemical sensor for hydrogen peroxide and glucose detection. J. Electroanal. Chem. 885, 115110 (2021)
X. Luo, M. Huang, D. He, M. Wang, Y. Zhang, P. Jiang, Porous NiCo2O4 nanoarray-integrated binder-free 3D open electrode offers a highly efficient sensing platform for enzyme-free glucose detection. Analyst 143, 2546–2554 (2018)
S. Cui, S. Gu, Y. Ding, J. Zhang, Z. Zhang, Z. Hu, Hollow mesoporous CuCo2O4 microspheres derived from metal organic framework: a novel functional materials for simultaneous H2O2 biosensing and glucose biofuel cell. Talanta 178, 788–795 (2018)
E. Kim, M. Imran, M. Akhtar, H. Shin, S. Ameen, Enticing 3D peony-like ZnGa2O4 microstructures for electrochemical detection of N,N-dimethylmethanamide chemical. J. Hazard. Mater. 404, 124069 (2021)
Y. Liu, C. Kong, X. Lu, F. Liao, F. Huang, J. Lin, One-step hydrothermal synthesis of high-performance gas-sensing crystals CdIn2O4 with octahedral shape. Cryst. Growth. Des. 12, 4104–4108 (2012)
D. Wang, D. Cai, H. Huang, B. Liu, L. Wang, Y. Liu, H. Li, Y. Wang, Q. Li, T. Wang, Non-enzymatic electrochemical glucose sensor based on NiMoO4 nanorods. Nanotechnology 26, 145501 (2015)
S. Peng, T. Lai, Y. Kong, Y. Ran, L. Su, D. Ma, X. Xiao, Y. Wang, A novel non-enzymatic glucose electrochemical sensor with high sensitivity and selectivity based on CdIn2O4 nanoparticles on 3D Ni foam substrate. Nanotechnology 32, 405502 (2021)
B. Rafiee, A. Fakhari, Electrocatalytic oxidation and determination of insulin at nickel oxide nanoparticles-multiwalled carbonnanotube modified screen printed electrode. Biosensors Bioelectron. 46, 130–135 (2013)
Y. Mu, D. Jia, Y. He, Y. Miao, H. Wu, Nano nickel oxide modified non-enzymatic glucose sensors with enhanced sensitivity through an electrochemical process strategy at high potential. Biosens. Bioelectron. 26, 2948–2952 (2011)
J. Nai, S. Wang, Y. Bai, L. Guo, Amorphous Ni(OH)2 nanoboxes: fast fabrication and enhanced sensing for glucose. Small 9, 3147–3152 (2013)
S. Liu, K. Hui, K. Hui, 1D hierarchical MnCO2O4 nanowire@MnO2 sheet core-shell arrays on graphite paper as superior electrode for asymmetric supercapacitors. Chemnanomat. 8, 593–602 (2016)
L. Wang, Y. Zhang, Y. Xie, J. Yu, H. Yang, L. Miao, Y. Song, Three-dimensional macroporous carbon/hierarchical Co3O4 nanoclusters for nonenzymatic electrochemical glucose sensor. Appl. Surf. Sci. 402, 47–52 (2017)
K. Xia, C. Yang, Y. Chen, L. Tian, Y. Su, J. Wang, L. Li, In situ fabrication of Ni(OH)2 flakes on Ni foam through electrochemical corrosion as high sensitive and stable binder-free electrode for glucose sensing. Sensors Actuators B Chem. 240, 979–987 (2017)
P. Rattanawarinchai, N. Khemasiri, N. Soyeux, S. Jessadaluk, A. Klamchuen, S. Wirunchit, A. Rangkasikorn, N. Kayunkid, D. Phromyothin, S. Rahong, J. Nukeaw, Gold nanoparticles decorated zinc oxide nanorods as electrodes for a highly sensitive non-enzymatic electrochemical glucose detection. J. Appl. Phys. 58, SDDE04 (2019)
L. Liu, Z. Wang, J. Yang, G. Liu, J. Li, L. Guo, S. Chen, Q. Guo, NiCo2O4 nanoneedle-decorated electrospun carbon nanofiber nanohybrids for sensitive non-enzymatic glucose sensors. Sensors Actuators B Chem. 258, 920–928 (2018)
J. Zhou, M. Min, Y. Liu, J. Tang, W. Tang, Layered assembly of NiMn-layered double hydroxide on graphene oxide for enhanced non-enzymatic sugars and hydrogen peroxide detection. Sensors Actuators B Chem. 260, 408–417 (2018)
P. Viswanathan, J. Park, D. Kang, J. Hong, Polydopamine-wrapped Cu/Cu(II) Nano-heterostructures: an efficient electrocatalyst for non-enzymatic glucose detection. Colloids Surf. A 580, 123689 (2019)
R. Li, X. Liu, H. Wang, Y. Wu, K.C. Chan, Z. Lu, Sandwich nanoporous framework decorated with vertical CuO nanowire arrays for electrochemical glucose sensing. Electrochim. Acta. 299, 470–478 (2019)
C. Espro, S. Marini, D. Giusi, C. Ampelli, G. Neri, Non-enzymatic screen printed sensor based on Cu2O nanocubes for glucose determination in bio-fermentation processes. J. Electroanal. Chem. 873, 114354 (2020)
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 61761047 and 41876055), the Yunnan Provincial Department of Science and Technology through the Key Project for the Science and Technology (Grant No.2017FA025), Program for Innovative Research Team (in Science and Technology) in University of Yunnan Province, and Project of the Department of Education of Yunnan Province (2022Y003).
Author information
Authors and Affiliations
Contributions
All authors contributed to the work conception and design. Investigation, material preparation, and original draft writing were performed by XT and SP. Data curation, methodology, and analysis were performed by HS, TL, and ZY. TC, XX, and YW performed the supervision, writing—review and editing. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
The work contains no libelous or unlawful statements, does not infringe on the rights of others, or contains material or instructions that might cause harm or injury.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tian, X., Peng, S., Shu, H. et al. Synthesis of Cu–CdIn2O4 nanoparticles decorated on nickel foam as a sensitive non-enzymatic electrochemical sensor for glucose detection. J Mater Sci: Mater Electron 33, 17949–17962 (2022). https://doi.org/10.1007/s10854-022-08657-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-022-08657-0