Abstract
In this article, we have studied the structural, magnetic, optical, and photocatalytic properties of Bi1−xCaxFe1−xNixO3 multiferroics synthesized by sol–gel method. X-ray diffraction predicted the superposition of two structural phases (rhombohedral-R3c + orthorhombic-Pnma) for (x = 0.05, 0.10) samples. Correspondingly, drastic variations in the Raman modes were detected at low and high wavenumbers with increasing the Ca–Ni content. The larger change in the magnetic parameters at room temperature was identified for Bi0.90Ca0.10Fe0.90Ni0.10O3 nanoparticles caused by the formation of a new orthorhombic phase. The XPS spectroscopy study supported the magnetic enhancement in x = 0.10 samples due to increasing concentration of Fe2+, Ni2+ ions, and oxygen vacancies. The shift of ESR pattern with doping agrees with the magnetization values. The energy band gap values were altered by increasing the Ca–Ni content in BiFeO3 samples, from 2.22 to 2.05 eV. The photocatalytic activity of Ca–Ni doped BiFeO3 photocatalyst showed higher degradation rate (− 0.035/min) of Methylene blue (MB) in comparison to pure BiFeO3.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Multiferroicity is the nature of existence of more than two ferroic orders out of the following viz., ferroelectric, ferromagnetic, and ferro elastic in a single phase of material. Such types of materials are called multiferroic materials [1,2,3]. Recently, multiferroics are giving rise to the possibilities for electric-field control of magnetization in such a way that these materials do have the potential applications for spintronics, data storage, and high-frequency magnetic devices [4, 5]. The grand challenges in multiferroics are to design it with enhanced magnetic, ferroelectric, and optical properties. Bismuth ferrite (BiFeO3) (BFO) is a novel single phase multiferroic compound, having ferroelectric transition temperature TC ~ 1103 K and antiferromagnetic transition temperature TN ~ 643 K [6, 7]. BFO is characterized by strong coupling feature between its ferroelectric and antiferromagnetic ordering. The multiferroic materials are an integral need for multiple device applications to serve in the premises of spintronics, LEDs, and magnetic recording systems [8, 9]. Currently, BiFeO3 has also become popular as a source of best photocatalyst under UV–visible range and piezo-catalyst due to its lower band gap which is near about 2.1 eV, its stability and electrically polarized nature [10,11,12]. Besides all these characteristics, industrial applications of BiFeO3 are still very less due to its large charge (e− and h+) recombination rate within lattices of particles [11,12,13]. To find out the best result, several strategies have been reported by previous articles like single or co-doping of rare earth elements, formation of composite heterostructure, and by using different synthesis methods [14,15,16]. Single or co-doping of rare earth elements with BiFeO3 is widely treated as a best way to boost the magnetic, optical, and photocatalytic performance [17, 18]. The dopant-elements work as the charge trapping sites within the lattice of BiFeO3, it improves the movement of charges from one carrier to another carrier and reduces their recombination rate [19,20,21]. Usually, the needful substitution of dopants, in limited concentration, can influence the modification of structural, magnetic, optical, and photocatalytic nature of BiFeO3. The semiconductor BiFeO3 nanoparticles, when excited by light under different UV–visible wavelengths, the lower band gap actively participates in photocatalytic activities [22,23,24]. The activated ions and super radicals present in photocatalyst helps in decontamination of organic, inorganic, bacterial, and microbial pollutants from contaminated water [25, 26]. As a result of this, huge work has been carried out in the field of Bi-based photocatalytic materials for the application of purification of water in recent decades [27,28,29]. Presently, this work focuses on the observed effects of co-doping of Ca–Ni on the structural, magnetic, optical, and photocatalytic properties of BiFeO3 nanoparticles synthesized via the sol–gel technique that have been explored in detail here. Rietveld refinement of the XRD pattern result indicates that, with increasing Ca–Ni amount in BiFeO3 from x = 5–10%, the R3c phases decrease while Pnma phase increases correspondingly. The TEM images show that the particle size of the considered samples is in the nano-range, which is observed to decrease as Ca and Ni content rises in BiFeO3. The Raman modes conform to the result of XRD and show structural variation from R3c phase to Pnma phase. The M-H loop and ESR results suggest the presence of strong ferromagnetic nature in Ca-Ni doped BiFeO3 in comparison to pristine BFO. XPS study supported the magnetization enhancement in x = 0.10 samples due to increasing concentration of Fe2+, Ni2+ ions, and oxygen vacancies. The optical properties are analysed by using UV–visible absorption spectra which confirm the alteration of band gap of Bi1−xCaxFe1−xNixO3 nanoparticles from 2.2 to 2.05 eV with Ca–Ni content. The change in band gap improves the photocatalytic activity, also the appreciable MB dye degradation 98.36% in 160 min irradiation under UV-light is observed using Bi1−xCaxFe1−xNixO3 (x = 0.10) photocatalyst.
2 Experimental details
2.1 Photocatalyst synthesis
In the synthesis of Bi1−xCaxFe1−xNixO3 nanoparticles, the chemical reagents Bi(NO3)3.5H2O (≥ 98%), Fe(NO3)3.9H2O (≥ 98%), Ca(NO3)2 (≥ 98%), Ni(NO3)2.6H2O (≥ 99.9%); Tartaric Acid (≥ 99.0%) and Nitric acid were used. All the chemical (Sigma) reagents used were of analytic grade and do not require additional purification treatment. Bi1−xCaxFe1−xNixO3 (x = 0.0, 0.05, 0.10) multiferroic nanoparticles (NPs) were synthesized by sol–gel method using chelating reagent tartaric acid [18]. The schematic flow-chart of the synthesis of Bi1−xCaxFe1−xNixO3 nanoparticles is shown in the Fig. 1. The calculated amount of Bi(NO3)3.5H2O mixed in deionized water and the transparent solution was obtained by adding the 15 ml of dilute nitric acid. After this the calculated amount of Fe(NO3)3.9H2O were added. For the complete combustion of the nitrates stoichiometric amount of tartaric acid was added to the transparent solution. The transparent light yellow coloured solution was stirred upon hot plate at 60 °C for 24 h. This transparent mixture solution was kept on 60–80 °C with continuous stirring till fluffy gel was obtained. The dried samples were calcined at 600 °C for 2 h in an air ambience. After the calcination, sample was ground with the help of mortal pastel to get the powder form of material for further characterization. The similar process was used in all Bi1−xCaxFe1−xNixO3 (x = 0.05, 0.10) samples, only the calculated amount of dopants, calcium nitrate and nickel nitrate were added in the precursor solution. All other synthesis conditions were kept unchanged.
2.2 Characterizations
The prepared samples were characterized structurally by using Shimadzu 600 XRD. TECNAI 200 kV TEM Fei along with Electron Optics was used in order to analyse the morphology of the samples. The backscattering configuration was used while performing Raman spectroscopy with LabRAM HR spectrometer. The magnetic resonance spectra were gathered by adopting JES FA200 CW ESR Spectrometer, using X-band gun diode. The measurements for magnetism of the samples were identified with the assistance of Lakeshore VSM 7410. The optical and photocatalytic properties of the synthesized samples were analysed by using LABMAN-900S UV–visible spectrometer. To find out the photocatalytic degradation of MB dye, a 250 W Hg bulb was kept in water circulating jar placed in quartz container (500 ml) at constant temperature (below 10 °C) with continuous stirring. For observation of dye diminishment with time under UV–Visible light, equal amount of samples were collected and after centrifugal process, all samples were passed through UV–visible absorbance spectra to find out their MB dye degradation efficiency comparative data.
3 Result and discussion
3.1 X-ray diffraction, SEM and TEM analysis
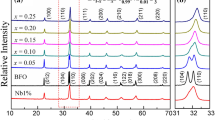
The X-ray diffraction peaks of the sol–gel synthesized BiFeO3 (BFO), Bi0.95Ca0.05Fe0.95Ni0.05O3, Bi0.90Ca0.10Fe0.90Ni0.10O3, and Bi0.85Ca0.15Fe0.85Ni0.15O3 samples are displayed in Fig. 2. All peaks in pure BFO diffraction pattern can be labelled with the R3c space group as per the distorted rhombohedral structure (JCPDS file no. 71–2494) [18]. The diffraction pattern shifted towards higher angles leading to the unit cell contraction in BFO when the Ca-Ni were partially doped on Bi and Fe site correspondingly. The intense, sharp nature diffraction peaks are indicating the samples’ high crystalline properties. The nonappearance of any other peak indicated the phase purity of the synthesized x = 0.00, 0.05, & 0.10 samples within the XRD limitations. However, XRD pattern of Bi0.85Ca0.15Fe0.85Ni0.15O3 shows the large impurity phase corresponding to ferrite.
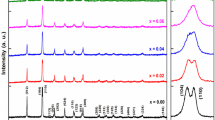
Phase formation and crystal structure of the pure and Ca–Ni substituted BiFeO3 nanoparticles were further analized by Rietveld refinement of their XRD patterns adopting FULLPROF software as shown in Fig. 3a–c. The Rietveld analysis showed pure BFO exhibited rhombohedral structure with R3c space, while co-doped samples showed co-existence of rhombohedral and orthorhombic structure with R3c and Pnma space group, respectively. Although, with increasing Ca–Ni amount in pure BiFeO3 from x = 5–10%, the presence of R3c decreases while Pnma phase fraction progressively increases as shown in Table 1. Moreover, increasing Ca–Ni concentration leads to the evolution of peaks related to the Pnma phase. Such severe changes in XRD pattern indicates that the Ca–Ni co-doped at Bi-site and Fe- site of BFO correspondingly, suppress and merges the sharp diffraction peak (110) and (104) related to R3c phase in the doped samples in order to get single diffraction peak related to Pnma phase. This is confirmed from Rietveld analysis. It also indicates that co-dopant change the crystalline nature of solid solution to nanocrystalline via gradual enhancement in the broadening of diffraction peaks with increasing doping. From Rietveld analysis, the lattice parameter values, fraction values of phases, atomic position coordinate values, and R-factors of both phases were also obtained and are summarized in Table 1. The observed and calculated XRD patterns showed compatible results. The values of scale-factor, unit cell parameters (a, b, c), elemental positions (x, y, z), half-width values (U, V, W), thermal and background parameters were changed, whereas the elemental occupancy remained same during whole refinement processes. The peak fitting and background correction have been performed by adopting Pseudo-Voigt function with linear interpolation method correspondingly [30,31,32]. The estimated structural refinement results were summarized in Table 1. The average crystallite size of the synthesized nanocrystalline Bi1−xCaxFe1−xNixO3 samples was found with the assistance of Scherrer’s formula.
where symbol D represents crystalline size, symbols K and λ denotes the shape-factor (0.9) and wavelength of Cu kα XRD radiation (1.54 Å), respectively. The \({\upbeta}_{\mathrm{hkl}}\) refers to Full Width at Half Maxima (FWHM) parameters used during Rietveld refinement of XRD patterns. In accord to Rietveld method, the different parameters to the FWHM from diffraction patterns can be identified using the equation,
Here U, V, W represent the shape parameters of the peaks. IG for isotropic size effect and DST represents strain—coefficient. The crystallite size and their lattice parameters for the Bi1−xNaxFe1−xCoxO3 samples are presented in Table 1. The decrease in the crystalline size was observed with higher Ca–Ni doping in pure BiFeO3 nanocrystals. This could be achieved because of the ion size disparity between the host and the doped ions leads to lattice strain. This lattice strain also introduced local structural distortion causing acceleration in the nucleation rate, leading to a reduction in crystallite size. The TEM and HRTEM micrographs of Ca–Ni doped BiFeO3 nanoparticles are represented in Fig. 3e, f and Fig. S2. It shows a spherical particle with a nanosized of about 50–90 nm range for pure BFO sample and 10–30 nm range for 10% (Ca–Ni) doped sample. The TEM result shows that the size of nanoparticles is in the nano-range and reduced as Ca–Ni content rises in BFO. The reduction in nanoparticles size due to Ca–Ni doping in BiFeO3 occurs due to presence of lattice induction through ionic radius mismatches. The lattice strains increase the nucleation rate and help in limiting the growth of nanoparticles.
Figure 4a–c represent the SEM micrographs revealing the morphology of the Bi1−xCaxFe1−xNixO3 samples. According to these micrographs, the synthesized samples have dense morphology, their grain shapes are spherical, and their sizes are in between 20 and 125 nm. SEM images also demonstrate the decrease in grain size and increased agglomeration with increasing the Ca-Ni content. Grain growth in Bi1−xCaxFe1−xNixO3 (x = 0.05 and 0.10) nanocrystalline samples is inversely proportional to the rate of nucleation. When rate of nucleation is accelerated by lattice strain in the system, the particle growth decreases.
The Bi, Ca, Fe, Ni, and O elemental mapping of the synthesized samples have been also completed using the EDS in SEM attachment. The elemental mapping of the Bi1−xCaxFe1−xNixO3 (x = 0.00, 0.05, and 0.10) samples represented in the supplementary information Fig. S1. The chemical composition of pristine BiFeO3 nanoparticles shows evident signals for Bi, Fe, and O consenting with the nominal ratio, 1: 1: 3, approximately. Moreover, weak signals of Ca and Ni in x = 0.05 and x = 0.10 compositions have been found in EDS spectra. The measured quantitative atomic percent values are 20.20% Bi, 19.00% Fe, and 60.80% O for BiFeO3 sample; 21.8% Bi, 0.8% Ca, 19.20% Fe, 0.2% Ni, and 58.00% O for x = 0.05 sample and 23.2% Bi, 3.2% Ca, 18.24% Fe, 2.8% Ni, and 52.56% O for x = 0.25 sample. This indicates that Ca–Ni ions have conveniently been incorporated into the host lattice. Elemental mapping of the x = 0.05 and 0.10 samples showed, Ca and Ni are uniformly distributed in the mapping micrograph. No other phase of the Ca and Ni is observed in the mapping micrographs.
3.2 Raman spectroscopy
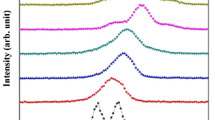
The Raman scattering spectra of Bi1−xCaxFe1−xNixO3 nanoparticles is displayed in Fig. 5a. The peak fitted Raman spectra of BiFeO3 samples is shown in Fig. 5b and for Bi0.95Ca0.05Fe0.95Ni0.05O3, and Bi0.90Ca0.10Fe0.90Ni0.10O3 samples are displayed in Fig. 6a, b, respectively. BFO having rhombohedral R3c symmetry possess 13 optical-phonon active modes (4A1 + 9E) in accord to well-known group theory [33]. As exhibited in Fig. 5a, all the key active modes that emerged in the pure BFO shows well agreement with the mode of the rhombohedral distorted (R3c) perovskite structure. The observed low frequency modes have been consigned to Bi–O vibration, whereas observed high-frequency Raman modes are linked to the stretching and bending of Fe–O [34]. The measured spectrum was fitted by performing decomposition of the curve into discrete Raman active component with a Lorentz type curve and associated peak positions are summarized in Table 2. Although, doping of Ca–Ni has resulted into few variations in Raman modes of BFO. The first is a clear decrease in the intensity of E(TO2) mode, gradual enhancement in intensity of A1 (TO1) mode, merging of E(TO2) and A1(TO1) mode. In addition, modes above 400 cm−1 that are responsible majorly for variation in magnetic behaviour are boosted for x = 0.10 samples. Such severe changes in Raman mode’s behaviour strongly suggest structural transformation from parent R3c phase to another Pnma phase.
Moreover, a broad hump in Raman spectra were observed in between 800 and 1500 cm−1, which deconvoluted further into discrete Raman modes identified as two phonons coupling in all samples. The presences of these two phonon modes were linked to the firm spin–lattice interaction between adjoining magnetic sublattices of BFO. These modes represent second over tons of E-modes present between 450 and 700 cm−1 and directly related to the magnetic properties of the BiFeO3 nanoparticles [35].
3.3 Magnetic measurements (VSM and ESR)
The room temperature magnetic hysteresis loops (M–H curve) of pure BiFeO3 (BFO), Bi0.95Ca0.05Fe0.95Ni0.05O3, and Bi0.90Ca0.10Fe0.90Ni0.10O3 samples are exhibited in Fig. 7a, the magnified view is shown in Fig. 7b. The M–H curve of pure BFO shows antiferromagnetic (AFM) behaviour with retentivity Mr = 0.005 emu/g and maximum magnetization MS = 0.11 emu/g at 15 kOe. While the hysteresis curve of x = 0.05 and 0.10 samples show ferromagnetic behaviour with Mr = 0.0018 emu/g and 0.0078 emu/g and maximum magnetization Ms = 0.37 emu/g and 2.94 emu/g at 15kOe, respectively. Despite the antiferromagnetic nature of BFO, Ca–Ni co-doped samples show ferromagnetic hysteresis loop with enhanced maximum magnetization even at higher magnetic fields (15 kOe). Besides, on comparing magnetization values of pure BFO with co-doped samples, a sensible increase was observed in Mr and MS of co-doped samples.
To further analyse the magnetic properties of synthesized Ca–Ni substituted BiFeO3 samples, ESR measurement technique has been executed at room temperature, as exhibited in Fig. 7c. The ESR spectra reveals the value of three important factors, asymmetry parameters (Pasy = 1-hU/hL, where hU and hL denote the absorption peak height), g-factor [\(g=hv/\mu\)BHres, where h (Plank constant) is 6.621 × 10–34 Joule-second, \(\mu\)B (Bohr magneton) = 9.2740 × 10–24 JT−1, and Hres is Resonance field values of different samples] and ΔBp-p (single width). The magnetic properties of co-doped samples were associated with all these three parameters [36]. The ESR spectra examination calculated g-value (2.05, 2.29, and 2.2), line width (1098, 1160, and 970 Gauss), and asymmetry parameter (0.22, 0.3, and 0.17) with x = 0.0, 0.05, and 0.10 samples, respectively. The degree spin canting values were estimated with the formula D = (Δg/g) Jsuper (where symbol D denotes the vector parameters, Δg for deviation value with respect to 2, and Jsuper is a constant value for super exchange coefficient values). The calculated g-factor value was found exceeding 2, specifying the weak ferromagnetic properties in all the samples. The area of the absorption peak, related to the number of excited spins. The area of the absorbance peak increases with increasing the Ca–Ni content, confirm the higher magnetization. The ESR spectra showing the line broadening and shift in resonance peak is attributed to the presence of non-homogenous local magnetic field triggered by the co-existence of Fe–Ni ions in the system.
3.4 X-ray photoelectron spectroscopy (XPS)
To perform analysis of magnetic behaviour, the XPS of the Ca–Ni doped BFO were examined. The XPS narrow scan spectrum of Bi 4f, Fe 2p, Ni 2p, and O1 s are displayed in Fig. 8. The two major characteristic peaks of bismuth centred at 164.1 eV and 158.8 eV, as displayed in Fig. 8a, is attributed to Bi 4f5/2 and Bi 4f7/2 correspondingly, which are mainly recognized as a signal from Bi–O bonds [36,37,38,39,40]. The visibility of the two peaks confirms the Bi3+ oxidation state in the samples. Spin–orbit intense energy of the Bi 4f doublet is 5.30 eV, which is analogous to the theoretical Spin–orbit splitting energy value of 5.31 eV [37]. Two fitted subpeaks positioned at 158.7 and 164 eV are attributed to Bi (4f7/2)–O and Bi (4f5/2)–O bonds, whereas other subpeaks positioned at 159.2 and 164.5 eV could be associated to Bi–O–Fe bonds in oxygen octahedron or relaxed Bi phase, possibly created due to cation defects and oxygen vacancies [38, 39].
The 2p1/2 and 2p3/2 splitting of Fe 2p core level is shown in Fig. 8b. Broadening of Fe 2p region can be associated to unequal charging of the surface, due to particle’s small size [36,37,38,39,40]. The coexistence of two valence state (Fe2+ and Fe3+) was affirmed from the Gaussian deconvolution of the Fe spectra. With well-known energy difference of around 8 eV between 2p3/2 and 2p1/2 duplet, Fe2p region was fitted with Fe2+ and Fe3+ oxidation states [40]. The peaks at 710.50; 725.16 eV for x = 0.05 and 710.56, 725.38 eV for x = 0.10 can be attributed to Fe3+ state of Fe2p3/2 and Fe2p1/2, whereas the peak at 709.10,723.40 eV for x = 0.05 and 709.30, 723.28 eV for x = 0.10 is credited to Fe2+ in Fe2p1/2 and Fe2p3/2, respectively. In addition to that, the two-satellite peak was observed at position 718.18 eV, 731.85 eV for x = 0.05 and 718.07 eV, 731.33 eV for x = 0.10 samples, caused by spin–orbit coupling. The fitting determined the ratio of Fe2+: Fe3+ as 0.35 and 0.48 (in Fe2p3/2), 0.74 and 0.84 (in Fe2p1/2) for x = 0.05, and 0.10 samples, respectively, indicating towards increase in quantity of Fe2+ ions with increasing Ca–Ni co-doping. The appearance of Fe2+ ions contributed a vital part in the boosting of double exchange interaction between Fe2+ and Fe3+ ions through oxygen, that follow a drastic change in BFO magnetic behaviour from antiferromagnetic to ferromagnetic nature on Ca–Ni co-substitution [41]. The surface peaks situated at 712.7 eV, were observed along with it for x = 0.05 and x = 0.10 samples that arose owing to reduction in coordination number of Fe3+ ions on the surface.
Figure 8c exhibits the XPS spectra of Ni for x = 0.05 and x = 0.10 samples. In the spectra, the well separated Ni 2p1/2 and Ni 2p3/2 peaks were observed that are further deconvoluted into four peaks with help of peak fit software. The fitted peaks positioned at 873 and 854.5 are associated to Ni2+ ions for x = 0.05 sample, whilst the peaks positioned at 855.9 and 861.7 are linked to the Fe3+ ions and satellite peaks. Similar peaks position for Ni2+, Ni3+ ions, and satellite peaks were observed for x = 0.10 sample, a slight shift of peak position of the spectra towards lower binding energy. This shift may be owing to increasing distortion in the structure of BFO on increasing co-doping concentration. Hence, XPS analysis predicts about the co-existence of Ni2+ and Ni3+ ions in co-doped samples with Ni2+/Ni3+ ratio of 1.31 and 2.21 for x = 0.05 and 0.10 samples, correspondingly. It indicates about higher concentration of Ni2+ ions in x = 0.10 samples that are responsible for magnetic enhancement.
Figure 8d exhibits the O1s asymmetric spectra of x = 0.05 and 0.10 samples. The broad spectra is clearly observable and therefore deconvoluted by fitting three Gaussian peaks. The deconvoluted peaks at a low binding (L.B.) energy 529.4 cm−1 (x = 0.05) and 529.7 cm−1 (x = 0.10) attributes to lattice oxygen at normal sites. Whereas other two deconvoluted peaks at middle binding (M.B.) energy 531 cm−1 (x = 0.05) and 530.8 cm−1 (x = 0.10) and at high binding (H.B.) energy 532.6 cm−1 (x = 0.05) and 532.2 cm−1 (x = 0.10) are accredited to surface or defect corelated oxygen such as O−, O2− [42]. Although no significant change in the position of deconvoluted peaks occurs with doping, the region under the peak changed significantly as we increased dopant concentration in BFO from x = 0.05 to 0.10. The ratio of the region that comes under the curve of the two peaks (the region of the peak with low binding energy/region of the peak with medium binding energy) can be important because this ratio is precisely related with the concentration of oxygen vacancies. A comparatively large area contribution of the M.B. energy peak for x = 0.10 sturdily advise the occurrence of oxygen vacancies in x = 0.10 in comparison to x = 0.05 sample.
3.5 UV–Visible spectroscopy
The optical properties of nanoparticles depend on their electronic characteristics and bandgaps. These optical properties of pristine BiFeO3, Bi0.95Ca0.05Fe0.95Ni0.05O3, and Bi0.90Ca0.10Fe0.90Ni0.10O3 samples were analysed by UV–Visible absorption spectra displayed in Fig. 9a. The energy bandgap (Eg) was calculated by employing ‘Tauc’s relation’
where A refers to a constant, hv refers to photon energy, Eg represents band gap, and α denotes absorption coefficient [41, 42]. The band gap is calculated through the use of Tauc’s plot, i.e. (αhv)2 vs hv plot of pristine BiFeO3, Bi0.95Ca0.05Fe0.95Ni0.05O3, and Bi0.90Ca0.10Fe0.90Ni0.10O3 samples as displayed in Fig. 9b–d. The band gap of pure BiFeO3 is 2.22 eV although after substituting Ca–Ni for x = 0.05 and x = 0.10 the band gap is diminished from 2.22 to 2.17 eV and 2.2 to 2.05 eV, respectively, displayed in Fig. 9b–d. The substitution of Ca–Ni, results to charge imbalance which indicates the existence of oxygen vacancies in prepared samples. The variation in angle of Fe–O–Fe towards 180° also affects their electronic structure and creates impurity bands. This impurity band enhances charge transfer between the carrier by alteration of energy bandgap [43, 44]. The substitution of Ca–Ni in pristine BFO shows lower energy band gap values, which resulted in improved optical properties of these samples.
3.6 Photocatalytic activity
Photocatalytic effect on MB dye degradation was examined by using BiFeO3, Bi0.95Ca0.05Fe0.95Ni0.05O3, and Bi0.90Ca0.10Fe0.90Ni0.10O3 nano-catalyst under 250Watt mercury vapour lamp. Figure 10a–c shows change in absorbance peak of MB dye in presence of UV–Visible light at different time-intervals. The 10PPM Concentration of MB dye and 0.02 gm mass of photocatalyst was used in photocatalytic reaction. Dye degradation efficiency of Ca–Ni substituted catalysts was calculated by C/Co vs irradiation time graph as given in Fig. 10d, where C and Co refer to final and initial concentration of MB dye during photocatalytic reaction [45, 46]. Graphical analysis revealed that the Bi0.90Ca0.10Fe0.90Ni0.10O3 shows maximum MB degradation, i.e. 98.36% as compare to BiFeO3 (96.1%) and Bi0.95Ca0.05Fe0.95Ni0.05O3 (95.1%) after equal time observation of 160 min. The rate constant for degradation of the MB dye is determined via usage of Langmuir–Hinshelwood (L–H Model) [47, 48]. L–H model expression is represented by equation given by
where r refers to degradation rate, Kr refers to rate constant, K refers to equilibrium constant, and C refers to reaction constant. KC is considered as negligible due to less value of C.
So,
where k (min−1) represents the first order rate constant. At t = 0 and C = C0. After entering the values of t and C0; ln(C/C0) = kt. The maximum k value is achieved as − 0.035 min−1 for the Bi0.90Ca0.10Fe0.90Ni0.10O3 photocatalyst. Substitution of Ca–Ni in pure BiFeO3 leads to the increment of oxygen vacancies. Due to presence of more free carriers and reduced charge recombination rate, the photocatalytic properties are enhanced in synthesized samples. The interaction of light encouraged electrons and holes on the surface of Ca–Ni substituted BiFeO3 photocatalysts. This absorption process of electron–holes on the surface of photocatalyst leads to redox process where O2 decreases and superoxide anion radical increases, and oxidation process produces hydroxyl radical (OH.) that participate in the decontamination process of MB dye [47]. This process facilitates the transferring of the electrons and holes to the surface of the photocatalyst. This photocatalytic reaction results into the decontamination of the MB dye and purification of water.
3.7 Mechanism of dye degradation
The photocatalytic dye degradation mechanism has been shown in Fig. 11 with advance oxidation process (AOP). When UV–Visible light was irradiated on the surface of Bi1−xCaxFe1−xNixO3 nanomaterials, an excited electron (e−) moves from VB to CB and produces photoinduced holes (h+) on the VB sites. Under continuous charge-separation process, e− and h+ migrated on the surface of photocatalyst and perform redox reaction with sorbed radicals. The photogenerated holes (h+) respond with H2O and produce hydroxyl (OH−) radicals, whereas electrons (e−) react with oxygen (O2) to form superoxide (O2.−) anion radicals. These superoxide radicals (O2.−) combine with holes to form OH. which is decomposed to OH− radicals. Lastly, the OH. participates in oxidation process with MB dye and after reaction CO2 and H2O are obtained [49]. The complete photocatalytic mechanism is expressed in the following equations.
This improved photocatalytic activity due to increases charge carrier transfer and decreases charge recombination rate [50, 51]. The Bi0.90Ca0.10Fe0.90Ni0.10O3 photocatalyst was found as the best dye degradation nanomaterial for degradation of MB Dye. To illustrate the mechanism of photodegradation, the trapping experiment provided the precise results to find the role and responsibility of active species [52]. The scavenger radicals helped to find out further explanation of mechanism of photocatalytic MB dye diminishment from water in the presence of UV–visible light.
To imitate the mechanism of photocatalytic reaction for the degradation of MB dye, the experiment was performed in the presence as well as absence of active species scavengers’ reagents. There were two trapping reagents; ethylene diamine tetra-acetic acid (EDTA) and Isopropanol (IPA) were used as O .-2 and OH. trapping reagents, respectively. In 10 ppm MB dye solution, a fixed amount of photocatalyst was added with same amount of scavenger reagents EDTA or IPA [50]. The whole photocatalytic reaction was performed under UV irradiation at constant temperature. The samples were collected after each 10 min intervals and their absorption spectra was found with the help of UV–Visible Spectroscopy as shown in Fig. 12. The absorption graph confirmed that the photocatalytic activity BiFeO3 and Bi0.90Ca0.10Fe0.90Ni0.10O3 catalysts decreased in the existence of EDTA and IPA. The kinetics graphs as shown in Fig. 13) also confirmed that the MB dye degradation rate of BiFeO3 was reduced from 95.2 to 71.33% and 1.41% in the presence of EDTA and IPA, respectively. Similarly, the MB diminishment efficiency for Bi0.90Ca0.10Fe0.90Ni0.10O3 catalysts also reduced from 98.38 to 48.68% and 29.2% with EDTA and IPA trapping reagents. The results showed that holes and superoxide oxygen radicals were mainly responsible for MB dye degradation [52, 53].
4 Conclusion
Bi1−xCaxFe1−xNixO3 (x = 0.0, 0.05, 0.10) multiferroic nanoparticles were synthesized by a well-known synthesis method named sol–gel using tartaric acid chelating reagent. Rietveld analysis confirms pure BFO exhibited rhombohedral structure with R3c space, whilst co-doped samples show co-existence of rhombohedral and orthorhombic phases with R3c and Pnma space group, respectively. Raman spectra shows reduction in intensity of E(TO2) mode, gradual enhancement in intensity of A1(TO1) mode, merging of E(TO2) and A1(TO1) mode. Additional modes above 400 cm−1 involved in changes in magnetic behaviour and these modes are boosted for x = 0.10 samples. Changes in Raman mode’s behaviour strongly suggest structural transformation from R3c to another space group Pnma. The M–H curve of pure BFO shows antiferromagnetic (AFM) behaviour with retentivity Mr = 0.005 emu/g and net magnetization MS = 0.11 emu/g at 15 kOe. Whilst the hysteresis curve of x = 0.05 and 0.10 samples show ferromagnetic behaviour with Mr = 0.0018 emu/g and 0.0078 emu/g and maximum magnetization Ms = 0.37 emu/g and 2.94 emu/g at 15kOe, respectively. XPS result indicates the increase of Fe2+ ions with increasing Ca–Ni co-doping. The existence of Fe2+ ions contribute significantly in boosting of double exchange interaction between Fe2+ and Fe3+ ions through oxygen, followed by a drastic change in BiFeO3 magnetic behaviour from antiferromagnetic to ferromagnetic nature on Ca–Ni co-substitution. ESR spectra parameters along with the XPS results confirm the increase of ferromagnetic properties in Ca-Ni substituted samples. UV–visible outcomes specify the energy band gap values reduced after substituting Ca-Ni in host BiFeO3 from 2.2 to 2.17 eV for Bi0.95Ca0.05Fe0.95Ni0.05O3 and 2.2 eV to 2.05 eV for Bi0.90Ca0.10Fe0.90Ni0.10O3 samples. The photocatalytic result demonstrated that the Bi0.90Ca0.10Fe0.90Ni0.10O3 photocatalyst shows maximum MB dye degradation 98.36% in 160 min irradiation under UV-light. The efficient photodegradation of Ca–Ni substituted BiFeO3 is due to the more photo induced charge separation and reduction of charge recombination rate.
References
N.A. Spaldin, R. Ramesh, Advances in magnetoelectric multiferroics. Nat. Mater. 18, 203–212 (2019). https://doi.org/10.1038/s41563-018-0275-2
J.F. Scott, Applications of magnetoelectrics. J. Mater. Chem. 22, 4567–4574 (2012). https://doi.org/10.1039/C2JM16137K
G. Catalan, J.F. Scott, Physics and applications of bismuth ferrite. Adv. Mater 21, 2463–2485 (2009). https://doi.org/10.1002/adma.200802849
Y. Li, M.-S. Cao, D.-W. Wang, J. Yuan, High-efficiency and dynamic stable electromagnetic wave attenuation for La doped bismuth ferrite at elevated temperature and gigahertz frequency. RSC Adv. 5, 77184–77191 (2015). https://doi.org/10.1039/C5RA15458H
B. Sun, Y. Liu, W. Zhao, P. Chen, Magnetic-field and white-light controlled resistive switching behaviours in Ag/[BiFeO3/γ Fe2O3]/FTO device. RSC Adv. 5, 13513–13518 (2015). https://doi.org/10.1039/C4RA14605K
S. Das, S. Rana, S. MdMursalin, P. Rana, A. Sen, Sonochemically prepared nanosized BiFeO3 as novel SO2 sensor. Sens. Actuat. B-Chem. 218, 122–127 (2015). https://doi.org/10.1021/ac9812429
D. Tiwari, D.J. Fermin, T.K. Chaudhuri, A. Ray, Solution processed bismuth ferrite thin films for all-oxide solar photovoltaics. J. Phys. Chem. C 119, 5872–5877 (2015). https://doi.org/10.1021/jp512821a
S. Acharya, S. Martha, P.C. Sahoo, K. Parida, Glimpses towards the modification of perovskite with graphene-analogous materials in photocatalytic applications. Inorg. Chem. Front. 2, 807–823 (2015). https://doi.org/10.1039/C5QI00124B
A. Rogov, M. Irondelle, F. Ramos-Gomez, J. Bode, D. Staedler, S. Passemard, S. Courvoisier, Y. Yamamoto, F. Waharte, D. Ciepielewski, P. Rideau, S. Gerber-Lemaire, F. Alves, J. Salamero, L. Bonacina, J.-P. Wolf, Simultaneous multi-harmonic imaging of nanoparticles in tissues for increased selectivity. ACS Photonics 2, 1416–1422 (2015). https://doi.org/10.1021/acsphotonics.5b00289
F. Mushtaq, X. Chen, M. Hoop, H. Torlakcik, E. Pellicer, J. Sort, C. Gattinoni, B.J. Nelson, S. Pané, Piezoelectrically enhanced photocatalysis with BiFeO3 nanostructures for efficient water remediation. Iscience 4, 236–246 (2018). https://doi.org/10.1016/j.isci.2018.06.003
M.E. Castillo, V.V. Shvartsman, D. Gobeljic, Y. Gao, J. Landers, H. Wende, D.C. Lupascu, Effect of particle size on ferroelectric and magnetic properties of BiFeO3 nanopowders. Nanotechnology 24, 355701 (2013). https://doi.org/10.1088/0957-4484/24/35/355701
T.J. Park, G.C. Papaefthymiou, A.J. Viescas, A.R. Moodenbaugh, S.S. Wong, Size-dependent magnetic properties of single-crystalline multiferroic BiFeO3 nanoparticles. Nano Lett. 7, 766–772 (2007). https://doi.org/10.1021/nl063039w
X. Bai, M. Bugnet, C. Frontera, P. Gemeiner, J. Guillot, D. Lenoble, I.C. Infante, Crystal growth mechanisms of BiFeO3 nanoparticles. Inorg. Chem. 58(17), 11364–11371 (2019). https://doi.org/10.1021/acs.inorgchem.9b00461
P.S.V. Mocherla, C. Karthik, R. Ubic, M.S. Ramachandra Rao, C. Sudakar, Tunable bandgap in BiFeO3 nanoparticles: the role of microstrain and oxygen defects. Appl. Phys. Lett. 103, 10220910 (2013). https://doi.org/10.1063/1.4813539
P. Suresh, S. Srinath, Effect of synthesis route on the multiferroic properties of BiFeO3: a comparative study between solid state and solgel methods. J. Alloys Compd. 649, 843–850 (2015). https://doi.org/10.1016/j.jallcom.2015.07.152
J.P. Zhou, R.J. Xiao, Y.X. Zhang, Z. Shi, G.Q. Zhu, Novel behaviors of single-crystalline BiFeO3 nanorods hydrothermally synthesized under magnetic field. J. Mater. Chem. 3, 6924–6931 (2015). https://doi.org/10.1039/C5TC00747J
B. Liu, B. Hu, Z. Du, Hydrothermal synthesis and magnetic properties of single-crystalline BiFeO3 nanowires. Chem. Commun. 47, 8166–8168 (2011). https://doi.org/10.1039/C1CC11896J
S. Chauhan, M. Kumar, S. Chhoker, S.C. Katyal, A comparative study on the structural, vibrational, dielectric, magnetic and optical properties of microcrystalline, nanocrystalline BiFeO3 and core-shell structured BiFeO3@SiO2 nanocomposites. J. Alloy. Compd. 666, 454–467 (2016). https://doi.org/10.1016/j.jallcom.2016.01.116
Q. Zhang, D. Sando, V. Nagarajan, Chemical route derived bismuth ferrite thin films and nanomaterials. J. Mater. Chem. C 4, 4092–4124 (2016). https://doi.org/10.1039/C6TC00243A
D.V. Karpinsky, I.O. Troyanchuk, M.V. Bushinsky et al., Crystal structure and magnetic properties of Bi1−x Ca xFe1−xMn(Ti)xO3 ceramics across the phase boundary. J Mater. Sci. 51, 10506–10514 (2016). https://doi.org/10.1007/s10853-016-0271-3
F. Lin, Q. Yu, L. Deng et al., Effect of La/Cr codoping on structural transformation, leakage, dielectric and magnetic properties of BiFeO3 ceramics. J. Mater. Sci. 52, 7118–7129 (2017). https://doi.org/10.1007/s10853-017-0947-3
S. Bharathkumar, M. Sakar, K. Rohith, S. Balakumar, Versatility of electrospinning on the fabrication of fibrous mat and mesh nanostructures of bismuth ferrite (BiFeO3) and their magnetic, photocatalytic activities. Phys. Chem. Chem. Phys. 17, 17745–17754 (2015). https://doi.org/10.1039/C5CP01640A
X. Yang, G. Xu, Z. Ren, X. Wei, C. Chao, S. Gong, G. Shen, G. Han, The hydrothermal synthesis and formation mechanism of single-crystalline perovskite BiFeO3 microplates with dominant (012) facets. Cry. Eng. Comm. 16, 4176–4182 (2014). https://doi.org/10.1039/C3CE42488J
M.R. Hoffmann, W. Choi, D.W. Bahnemann, Environmental applications of semiconductor photocatalysis. Chem. Rev. 95, 69–96 (1995). https://doi.org/10.1021/cr00033a004
H. Zhou, Y. Qu, T. Zeid, X. Duan, Towards highly efficient photocatalysts using semiconductor nanoarchitectures. Energy Environ. Sci. 5, 6732–6743 (2012). https://doi.org/10.1039/C2EE03447F
P. Caroline, G. Vinitha, D. Joseph, A review on the visible light active BiFeO3 nanostructures as suitable photocatalyst in the degradation of different textile dyes. Environ. Nanotechnol. Monitor. Manag. 7, 110–120 (2017). https://doi.org/10.1016/j.enmm.2017.02.001
F. Gao, X.Y. Chen, K.B. Yin, S. Dong, Z.F. Ren, F. Yuan, T. Yu, Z.G. Zou, J.-M. Liu, Visible-light photocatalytic properties of weak magnetic BiFeO3 nanoparticles. Adv. Mater. 19, 2889–2892 (2007). https://doi.org/10.1002/adma.200602377
J. Kang, Y. Tang, M. Wang, C. Jin, J. Liu, S. Li, Z. Li, J. Zhu, The enhanced peroxymonosulfate-assisted photocatalytic degradation of tetracycline under visible light by g-C3N4/Na-BiVO4 heterojunction catalyst and its mechanism. J. Environ. Chem. Eng. 9, 105524 (2021). https://doi.org/10.1016/j.jece.2021.105524
M. Sakar, S. Balakumar, P. Saravanan, S. Bharathkumar, Compliments of confinements: substitution and dimension induced magnetic origin and bandbending mediated photocatalytic enhancements in Bi1-xDyxFeO3 particulate and fiber nanostructures. Nanoscale 7, 10667–10679 (2015). https://doi.org/10.1039/C5NR01079A
S.M. Selbach, T. Tybell, M.A. Einarsrud, T. Grande, Size-dependent properties of multiferroic BiFeO3 nanoparticles. Chem. Mater. 19, 6478–6484 (2007). https://doi.org/10.1021/cm071827w
K. Fujii, H. Kato, K. Omoto, M. Yashima, J. Chen, X. Xing, Experimental visualization of the Bi–O covalency in ferroelectric bismuth ferrite (BiFeO3) by synchrotron X-ray powder diffraction analysis. Phys. Chem. Chem. Phys. 15, 6779–6782 (2013). https://doi.org/10.1039/C3CP50236H
J.A. Alonso, M.J. Martínez-Lope, J.L. Garcıa-Muñnoz, M.T. Fernández-Díaz, A structural and magnetic study of the defect perovskite LaNiO2:5 from high-resolution neutron diffraction data. J. Phys. Condens. Matter 9, 6417–6426 (1997). https://doi.org/10.1088/0953-8984/9/30/010
J. Bielecki, P. Svedlindh, D.T. Tibebu, S. Cai, S.-G. Eriksson, L. Böorjesson, C.S. Knee, Structural and magnetic properties of isovalently substituted multiferroic BiFeO3. Phy. Rev. B 86, 184422 (2012). https://doi.org/10.1103/PhysRevB.86.184422
P. Hermet, M. Goffinet, J. Kreisel, Ph. Ghosez, Raman and infrared spectra of multiferroic bismuth ferrite from first principles. Phys. Rev. B 75, 220102 (2007). https://doi.org/10.1103/PhysRevB.75.220102
H. Feng, The role of Coulomb and exchange interaction on the Dzyaloshinskii-Moriya interaction (DMI) in BiFeO3. J. Mag. Mag. Mater. 322, 1765–1769 (2010). https://doi.org/10.1016/j.jmmm.2009.12.025
S. Chauhan, M. Kumar, S. Chhoker, S.C. Katyal, M. Jewariya, B.N. Suma, G. Kunte, Structural modification and enhanced magnetic properties with two-phonon in Ca-Co codoped BiFeO3 nanoparticles. Ceram. Int. 41, 14306–14314 (2015). https://doi.org/10.1016/j.ceramint.2015.07.062
S. Chauhan, M. Kumar, A. Yousuf, P. Rathi, M. Sahni, S. Singh, Effect of Na/Co co-substituted on structural, magnetic, optical and photocatalytic properties of BiFeO3 nanoparticles. Mater. Chem. Phys. 263, 124402 (2021). https://doi.org/10.1016/j.matchemphys.2021.124402
S. Chauhan, B. Tripathi, M. Kumar, M. Sahni, R.C. Singh, S. Singh, Influence of Na substitution on structural, magnetic, optical and photocatalytic properties of bismuth ferrite nanoparticles. J. Mater. Sci. 31(22), 20191–20209 (2020). https://doi.org/10.1007/s10854-020-04540-y
Z. Quan, W. Liu, H. Hu, S. Xu, B. Sebo, G. Fang, M. Li, X. Zhao, Microstructure, electrical and magnetic properties of Ce-doped BiFeO3 thin films. J. Appl. Phys. 104, 084106 (2008). https://doi.org/10.1063/1.3000478
A. Tamilselvan, S. Balakumar, M. Sakar, C. Nayek, P. Murugavel, K.S. Kumar, Role of oxygen vacancy and Fe–O–Fe bond angle in compositional, magnetic, and dielectric relaxation on Eu-substituted BiFeO3 nanoparticles. Dalton Trans. 43, 5731–5738 (2014). https://doi.org/10.1039/C3DT52260A
R. Pisarev, A. Moskvin, A. Kalashnikova, T. Rasing, Chemical pressure effect on optical properties in multiferroic bulk BiFeO3. Phys. Rev. B 79, 235128 (2009). https://doi.org/10.1063/1.4757589
H. Zhang, W. Liu, P. Wu, X. Hai, M. Guo, X. Xi, J. Gao, X. Wang, F. Guo, X. Xu, C. Wang, G. Liu, W. Chu, S. Wang, Novel behaviors of multiferroic properties in Na-doped BiFeO3 nanoparticles. Nanoscale 6, 10831–10838 (2014). https://doi.org/10.1039/c4nr02557a
S. Chauhan, M. Kumar, H. Pandey, S. Chhoker, S.C. Katyal, Ca–Li substitution driven structural, dynamics of electron density, magnetic and optical properties of BiFeO3 nanoparticles. J. Alloys Compd. 811, 151965 (2019). https://doi.org/10.1016/j.jallcom.2019.151965
Y. Zhang, Y. Yang, Z. Dong, J. Shen, Q. Song, X. Wang, W. Mao, Y. Pu, X. Li, Enhanced photocatalytic activity of Ba doped BiFeO3 by turning morphologies and band gap. J Mater Sci: Mater Electron. 31, 15007–15012 (2020). https://doi.org/10.1007/s10854-020-04064-5
Y. Zhang, Z. Wang, J. Zhu, X. He, H. Xue, S. Li, X.A. Li et al., Ferroelectric polarization effect on the photocatalytic activity of Bi0.9Ca0.1FeO3/CdS S-scheme nanocomposites. J. Environ. Sci. 124, 310–318 (2022). https://doi.org/10.1016/j.jes.2021.09.021
S. Li, Y. Tang, M. Wang, J. Kang, C. Jin, J. Liu, Z. Li, J. Zhu, NiO/g-C3N4 2D/2D heterojunction catalyst as efficient peroxymonosulfate activators toward tetracycline degradation: characterization, performance and mechanism. J. Alloy. Compd. 880, 160547 (2021). https://doi.org/10.1016/j.jallcom.2021.160547
Z. Hu, D. Chen, S. Wang, N. Zhang, L. Qin, Y. Huang, Facile synthesis of Sm-doped BiFeO3 nanoparticles for enhanced visible light photocatalytic performance. Mater. Sci. Eng. B 220, 1–12 (2017). https://doi.org/10.1016/j.mseb.2017.03.005
S. Irfan, Y. Shen, S. Rizwan, H.C. Wang, S.B. Khan, C.W. Nan, Band-gap engineering and enhanced photocatalytic activity of Sm and Mn doped BiFeO3 nanoparticles. J. Am. Ceram. Soc. 100, 31–40 (2017). https://doi.org/10.1111/jace.14487
E. Moradi, H. Farajnejad Ghadi, M. Rabbani, R. Rahimi, Microwave-assisted synthesized and characterization of BiFeO3 (CTAB/PEG/PVA) nanocomposites by the auto-combustion method with efficient visible-light photocatalytic dye degradation. J. Mater. Sci. 32, 8237–8248 (2021). https://doi.org/10.1007/s10854-020-05202-9
S. Irfan, L. Li, A.S. Saleemi, C.W. Nan, Enhanced photocatalytic activity of La3+ and Se4+ co-doped bismuth ferrite nanostructures. J. Mater. Chem. A 5(22), 11143–11151 (2017). https://doi.org/10.1039/C7TA01847A
S.M. Lam, Z.H. Jaffari, J.C. Sin, Hydrothermal synthesis of coral-like palladium-doped BiFeO3 nanocomposites with enhanced photocatalytic and magnetic properties. Mater. Lett. 224, 1–4 (2018). https://doi.org/10.1016/j.matlet.2018.04.058
S. Bharathkumar, M. Sakar, S. Balakumar, Egg white-mediated synthesis of BiFeO3 cubes and their enhanced photocatalytic degradation properties under solar irradiation. J. Mater. Sci. (2022). https://doi.org/10.1007/s10854-022-08213-w
A. Indriyani, Y. Yoki, R. Yunarti, D. Apriandanu, S. Rizki, One-pot green fabrication of BiFeO3 nanoparticles via Abelmoschus esculentus L. leaves extracts for photocatalytic dye degradation. Appl. Surface Sci. 563, 150113 (2021). https://doi.org/10.1016/j.apsusc.2021.150113
Acknowledgments
Sunil Chauhan offers a warm gratitude to Science and Engineering Research Board (SERB), File Number TAR/2019/000210, Govt. of India to accomplish the current research work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. All co-authors have seen and agree with the contents of the manuscript. All co-authors certify that the submission is original work and is not under review at any other publication.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tripathi, B., Chauhan, S., Kumar, M. et al. Structural, magnetic, optical, and photocatalytic properties of Ca–Ni doped BiFeO3 nanoparticles. J Mater Sci: Mater Electron 33, 16856–16873 (2022). https://doi.org/10.1007/s10854-022-08555-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-022-08555-5