Abstract
YFeO3+δ, Y0.8Sr0.2FeO3+δ, YFe0.8Ni0.2O3+δ, and Y0.8Sr0.2Fe0.8Ni0.2O3+δ nanoparticles have been successfully synthesized by a simple co-precipitation technique. Results obtained using thermogravimetry and differential scanning calorimetry, powder X-ray diffraction, transmission electron microscopy indicate that YFeO3, Sr-doped YFeO3, Ni-doped YFeO3, and Sr and Ni co-doped YFeO3 nanoparticles with orthorhombic structure were fabricated at 800 °C for 1 h. The obtained materials have the crystallite sizes below 30 nm and particle sizes below 40 nm. Sr and/or Ni doping led to the distortion of the YFeO3 crystal structure and thus altered the magnetic properties of the corresponding materials. The Sr-doped YFeO3, Sr and Ni co-doped YFeO3, and especially Ni-doped YFeO3 samples have significantly higher absorbance in the visible light region (~ 400–800 nm) and lower band gap than those of pure YFeO3 sample. Magnetic hysteresis loop analyses illustrate that ferromagnetic behavior of the YFeO3 nanopowders can be strongly enhanced with the addition of Sr and/or Ni. The coercivity and remanent magnetization of Sr and Ni co-doped YFeO3+δ are, respectively, around 80 and 104 times higher than those of the pure YFeO3+δ sample. The excellent optical and magnetic properties of Sr and Ni co-doped YFeO3+δ nanomaterials suggest great potential for applications related to optics and magnetism.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Yttrium iron oxide (YFeO3) is a LnFeO3 perovskite orthoferrite having an orthorhombic structure [1,2,3]. Orthoferrite yttrium (o-YFeO3) has been attracting much interest in both fundamental research and applied science [4, 5]. The structure and properties of o-YFeO3 materials depend on many factors such as particle shape and size, crystallite size, preparation method and especially, on the nature and concentration of the dopants in o-YFeO3 crystal lattice [6,7,8]. The partial doping at the site of Y3+ or Fe3+ in the lattice of YFeO3 can lead to multiple oxidation states and crystal defects, and thus, to changes in the properties of the materials [9,10,11,12]. The perovskite-type structure can be discriminated by the Goldschmidt tolerance factor (t), which can be calculated following the Eq. (1) [13,14,15,16,17].
where \(r_{A}\),\(r_{B}\) and \(r_{O}\) are the radii of large cation A, small cation B and anion O2−, respectively. For t = 1, perovskite ABO3 has an ideal cubic structure. In cases where 0.75 < t < 0.9, the structure of ABO3 is orthorhombic. For o-YFeO3 with the radii of Y3+ = 0.094, Fe3+ = 0.065, and O2− = 0.126 nm, we have t = 0.82 [8]. In all cases of t ≠ 1, the crystal lattice is distorted, the B–O–B bond angle is no longer 180° but bent, and the B–O bond lengths are different in different directions. The ABO3 structure is modified, resulting in the alteration of its electrical, magnetic, and optical properties [18,19,20,21].
Besides, according to Goldschmidt’s rules, in order to form a homogenous solid solution at a temperature largely different from the melting points of the components, the difference between the ionic radii of the cation and its dopant (Δr) should not exceed 15%, i.e., Δr/r ≤ 15% (r is the radius of the smaller cation) [10]. Indeed, according to [10], Ba-doped YFeO3 nanomaterials were successfully synthesized by the sol–gel method, followed by calcination of the precursor at 750 °C for 1 h. The results showed that the substitution of Ba2+ in the crystal lattice of YFeO3 only happened with x = 0.1. For x > 0.1, besides the orthorhombic phase YFeO3, XRD peaks of Y2O3, BaO, and BaY2O4 were also observed. The doping of Ba2+ in YFeO3 increased the crystallite size from 30 ± 2 nm (for YFeO3) to 55 ± 5 nm (for Y0.9Ba0.1FeO3). Barium-doped yttrium orthoferrite with x = 0.1 underwent qualitative transformation from a soft to a hard magnetic material, with Hc increased from 70 to 1000 Oe [22]. The structural defects, leading to changes in the magnetic properties of Ba-doped YFeO3 nanomaterials, can be explained by the large difference between the radii of the cations Y3+ (0.094 nm) and Ba2+ (0.134 nm) (Δr = 0.04 nm). Even with a small Δr (Δr ≠ 0), Mn-doped YFeO3 orthoferrite also underwent significant changes in the structure and properties [11]. The YFe1-xMnxO3 series (x = 0, 0.05, 0.1, 0.15, and 0.2) orthorhombic nanomaterials were prepared by the citric acid sol–gel method and annealing of the precursor at 800 °C for 2 h [11], showing the domination of YFeO3 hexagonal structure when the calcination temperature (T°) < 800 °C or x > 0.2. When the Mn concentration in the YFeO3 orthorhombic crystal lattice increased, particle size increased from 50 to 90 nm and saturation magnetization (Ms) increased from 0.015 to 0.08 emu g−1 for YFeO3 and YFe0.8Mn0.2O3, respectively. The partial replacement of Y3+ or Fe3+ by another metal cation also affected the optical properties of the YFeO3 material. Indeed, the doping of Sm3+ in YFeO3 orthorhombic structure decreased Hc and absorbance in the visible range (~ 400–600 nm), while increased Ms (emu g−1) and Eg (eV) [12]. In this work, Y1-xSmxFeO3 (x = 0, 0.05, 0.1, and 0.15) orthorhombic materials of 300–1200 µm were synthesized by the citric acid sol–gel method and annealing of the precursor at 900 °C for 8 h.
In our previous publication [23], YFe1-xNixO3 nanomaterials were prepared by a simple co-precipitation method with KOH 5% solution as the precipitating agent. Single phase orthorhombics YFe1-xNixO3 (x = 0, 0.1, 0.15, 0.2, and 0.25) were formed after annealing the precipitates at 800 °C for 1 h. NiO and Y2O3 appeared as impurities when T° > 800 °C or x > 0.25. The substitution of Ni with an yttrium iron oxide lattice also increased the crystallite size and magnetic characteristics of the materials. However, to the best of our knowledge, the simultaneous doping of two different metal cations (specifically Sr2+ and Ni2+) to both Y3+ and Fe3+ positions in YFeO3 crystal lattice has not yet been reported.
Thus, the aim of this work is to study of how the doping of strontium and nickel, individually and simultaneously, affected the structure, the optical and magnetic properties of YFeO3 orthorhombic nanomaterials. Based on previous works [10,11,12, 23, 24], the concentration of dopants was 20% molar ratio, corresponding to the empirical formulae of YFeO3, Y0.8Sr0.2FeO3, YFe0.8Ni0.2O3, and Y0.8Sr0.2Fe0.8Ni0.2O3.
2 Experimental
2.1 Materials and methods
Y(NO3)3·6H2O (99.9% purity, Sigma-Aldrich), Ni(NO3)2·6H2O (99.8% purity, Acros Organic), Fe(NO3)3·9H2O (99.6% purity, Sigma-Aldrich), Sr(NO3)2 (99.9% purity, Sigma-Aldrich), Na2CO3·10H2O (99.7% purity, Merck), and double distilled water were employed as the starting materials.
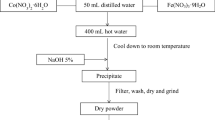
Y1-xSrxFe1-yNiyO3 nanoparticles were synthesized by a simple co-precipitation method based on the preparation of YFe1-xNixO3 [23]. Instead of KOH 5%, Na2CO3 was used as the precipitating agent because Sr2+ cations cannot be precipitated by OH− anions [25].
50 mL of the aqueous solution containing the mixture of Y(NO3)3, Sr(NO3)2, Fe(NO3)3 and Ni(NO3)2 with the molar ratio of Y3+:Sr2+:Fe3+:Ni2+ = (1 − x):x:(1 − y):y (for 4 series of materials: (1) YFeO3 (x = y = 0), (2) Y0.8Sr0.2FeO3 (x = 0.2, y = 0), (3) YFe0.8Ni0.2O3 (x = 0, y = 0.2), and (4) Y0.8Sr0.2Fe0.8Ni0.2O3 (x = y = 0.2) was added dropwise to 400 mL of boiling double distilled water on a heating stirrer (T° > 95 °C). The mixture was then boiled for another 10 min before being cooled down to room temperature (~ 30 °C). Next, 50 mL of Na2CO3 5% solution was added dropwise to the system while continuously stirring. The amount of Na2CO3 5% solution was enough to precipitate all the cations according to the chemical Eqs. (2, 3, 4, 5) [25, 26].
After the addition of the Na2CO3 5% solution, the system was the stirred for 1 h to homogeneously disperse the precipitates. The precipitates were subsided for 30 min then filtered and washed by double distilled water until pH 7 (the filtrate was checked by pH paper). The obtained solid was left to dry at room temperature for 3–5 days then ground, resulting in brownish yellow powder (precursor for Y1-xSrxFe1-yNiyO3 nanoparticles).
2.2 Characterization
Thermogravimetry and differential scanning calorimetry (TG–DSC) curves of the precursor for the synthesis of YFeO3 nanoparticles were recorded under dry air at the heating rate of 10 K·min−1, maximum temperature of 1000 °C, platinum crucibles, using a Labsys Evo (France).
Powder X-ray diffraction analysis (PXRD) of the obtained Y1-xSrxFe1-yNiyO3 samples was carried out using a D8-ADVANCE X-ray diffractometer (Germany) (CuKα radiation, λ = 1.5406 Å, angle range of 2θ = 10°–80°, scanning step of 0.19° s−1). The crystallite sizes of Y1-xSrxFe1-yNiyO3 (DXRD, nm) were determined based on Scherrer’s equation [4, 27].
where FWHM is the full-width at half maximum, and θ is the diffraction angle of the maximum reflection.
Lattice constants (a; b; c, Å) and unit cell volume (V, Å3) were calculated using the formulas presented in Ref [27]:
The morphology and particle size were investigated by transmissions electron microscopy (TEM; Jeol-1400, Japan).
The quantitative and qualitative composition of the samples was determined by energy dispersive X-ray spectroscopy (EDX-analysis) using an FE-SEM S-4800 scanning electron microscope (Japan). The quantitative elemental composition was determined as the average of the values obtained at five different points of each sample.
The UV–Vis absorption spectra of the Y1-xSrxFe1-yNiyO3 nanopowders were studied on a UV–visible spectrophotometer (UV–Vis; JASCO V–550, Japan). The optical energy gap (Eg, eV) was determined by fitting the absorption data to the direct transition as Eq. (9):
where A is the optical absorption coefficient, hν is the photon energy, Eg is the direct band gap and α is a constant [28]. The extrapolation of the linear portions of the curves toward absorption equal to zero (y = 0) gives Eg for direct transitions.
The hysteresis loop and magnetic properties including coercive force (Hc, Oe), remanent magnetization (Mr, emu g−1) and saturation magnetization (Ms, emu·g−1) were recorded at 300 K on a vibrating sample magnetometer (VSM, MICROSENE EV11, Japan) under a magnetic field in the range of − 16 000 Oe to + 16 000 Oe.
3 Result and discussion
As the radii of Fe3+ (0.065 nm) and Ni3+ (0.061 nm) are approximate while those of Y3+ (0.094 nm) and Sr2+ (0.112 nm) have a big difference (Δr = 0.018 nm) [25], the two precursors for YFeO3 and Y0.8Sr0.2FeO3 nanoparticles were chosen for TG–DSC analysis. The results are shown in Fig. 1.
The TG curves of two samples (YFeO3 and Y0.8Sr0.2FeO3) from room temperature to 1000 °C are quite similar. The mass loss percentage are 32.81% for YFeO3 and 31.25% for Y0.8Sr0.2FeO3, and become negligible at around 750 °C for both samples (according to TG curves). Those results can be derived from their resemblance to the average atomic masses of yttrium and strontium, and the formation of stable carbonate precipitates of both Y3+ and Sr2+ cations (Y = 88.91, Sr = 87.62) [25]. The mass losses of the samples when heated were from the dehydration of moisture, dehydration by pyrolysis of iron precipitates, and carbon dioxide loss by base–carbonate pyrolysis of yttrium and strontium carbonate according to the chemical Eqs. (10, 11, 12, 13, 14, 15, 16) [30,31,32,33].
The thermal behaviors of YFeO3 and Y0.8Sr0.2FeO3 samples also have similarity in their endothermic peaks at 128.86 °C/133.33 °C and exothermic peaks at 302.13 °C/321.90 °C on the DSC curves, respectively. The endothermic effects at 70–200 °C come from the removal of moisture, dehydration by pyrolysis of iron (III) and of yttrium and strontium precipitates [25, 30, 31, 33]. The exothermic peaks at 200–400 °C correspond to the γ-Fe2O3 → α-Fe2O3 phase transition according to Eq. (12) [30, 32]. The only difference in the thermal behaviors of YFeO3 and Y0.8Sr0.2FeO3 is the absence of the endothermic peak at 450.17 °C for the YFeO3 sample. This difference originates from the carbonate hydrolysis according to Eq. (16), resulting in a stronger endothermic effect. Finally, exothermic peaks at 700–800 °C were observable for both samples, which indicates the perovskite phase formation as shown in Eqs. (17) and (18).
From the above TG–DSC analysis and previous synthesis of YFe1-xMnxO3 [11], Y1-xSmxFeO3 [12], and YFe1-xNixO3 [23] nanoparticles, the precipitates YFeO3 and Y1-xSrxFe1-yNiyO3 were annealed at 800 °C for 1 h, followed by a structural analysis with PXRD.
The PXRD pattern of the YFeO3 sample (Fig. 2A) has the specific peaks of the YFeO3 orthorhombic phase, Pnma (62) space group (JCPDS: 00-039-1489). The good crystallinity of the YFeO3 sample is proven by its high peak intensity and flat, fine baseline, and no minor impurity peaks were present.
The PXRD patterns of the doped and co-doped nanopowders indicate the same characteristic shape as that of the pristine YFeO3 pattern, with a slight shift in the positions of the peaks (Fig. 2B and Table 1). This is a normal situation for the YFeO3 perovskite, involving the difference in the radii of the cations, according to Goldschmidt [24]. Table 1 shows that, compared to pure YFeO3, 2θ values underwent a left-shift by doping Sr2+ with a bigger radius than that of Y3+, while the doping of Ni3+ with smaller radius than that of Fe3+ resulted in a right-shift of 2θ positions. For Sr and Ni co-doped YFeO3 samples, 2θ positions also had a right-shift. Similar results were reported for Gd and Co co-doped YFeO3 nanopowders [28]. Thus, it is evident that Sr2+ and Ni3+ replaced the Y3+ and Fe3+ ions in YFeO3, respectively. The shift of 2θ values is also consistent with the changes in the crystallite size (DXRD, nm) and lattice cell volume (V, Å3) (Table 1).
Table 1 shows the structural characteristics of Y1-xSrxFe1-yNiyO3 nanocrystals. The crystallite size (DXRD, nm) and unit cell volume (V, Å3) of YFeO3±δ increased with an increase in Sr content and decreased with the addition of Ni because \(r_{{Sr^{2 + } }} > r_{{Y^{3 + } }}\), while \(r_{{Ni^{3 + } }} < r_{{Fe^{3 + } }}\). This is another indication of the successful substitution of Y3+ and Fe3+ ions in the YFeO3 lattice by Sr2+ and Ni2+ ions. The smallest values of DXRD and V correspond to Sr and Ni co-doped YFeO3 samples, because the simultaneous substitution of Y3+ and Fe3+ by Sr2+ and Ni3+ led to an increase in the amount of oxidized Fe3+ to form Fe4+ compared to Y0.8Sr0.2FeO3±δ and YFe0.8Ni0.2O3 (\(r_{{Fe^{4 + } }} < r_{{Fe^{3 + } }}\)), resulting in the decrease in DXRD and V according to Vegard’s law [14, 20, 34, 35]. This result is also in good agreement with the TEM images of Y1-xSrxFe1-yNiyO3 samples in Fig. 3.
The TEM images in Fig. 3 show that the obtained Y1-xSrxFe1-yNiyO3 samples are composed of spherical or slightly angular-shaped particles. The average particle sizes of the test samples of YFeO3, Y0.8Sr0.2O3, YFe0.8Ni0.2O3, and Y0.8Sr0.2Fe0.8Ni0.2O3 were approximately 35.17, 39.86, 34.85, and 32.43 nm, respectively. However, aggregations and clusters are still observable, maybe due to the strong magnetic interaction between the particles.
Elemental composition analysis from EDX shows no impurity elements apart from Y, Sr, Fe, Ni, and O which were detected in the Y1-xSrxFe1-yNiyO3 nanomaterials (Fig. 4). The percentage of atoms of each element is also in accordance with their ratio in the nominal compositions (Table 2). Table 2 also shows that the oxygen content in Y1-xSrxFe1-yNiyO3 materials is much greater than that in the nominal composition, proving the successful substitution of Y3+ and Fe3+ by Sr2+ and Ni3+ in the YFeO3 lattice. When Y3+ was replaced by Sr2+ and Fe3+ was replaced by Ni2+ (Ni2+ in Ni(NO3)2·6H2O), some Fe3+ ions would be oxidized to Fe4+ and Ni2+ to Ni3+ to balance the local charge, resulting in the increase of oxygen content in Y1-xSrxFe1-yNiyO3 [14, 20, 34, 35]. For Sr and Ni co-doped YFeO3 sample, not all Ni2+ ions were oxidized to Ni3+, this sample may have the highest amount of Fe3+ oxidized to Fe4+, and thus it has the highest oxygen content (Table 2). Besides, oxygen adsorption on the surface of Y1-xSrxFe1-yNiyO3 orthoferrite also led to an increased oxygen percentage in the Y1-xSrxFe1-yNiyO3 structure [34, 36], hence the empirical formula of this material series should be Y1-xSrxFe1-yNiyO3+δ.
The substitution of Sr2+ and/or Ni3+ in the YFeO3+δ crystal lattice affected not only the structure but also the optical and magnetic properties of the materials (Figs. 5, 6 and Table 3). The UV–Vis absorption spectra of the Sr-doped, Ni-doped, and Sr, Ni co-doped YFeO3 nanoparticles showed strong absorption in the ultraviolet (~ 250–400 nm) and visible light regions (~ 400–800 nm) (Fig. 5A). While the absorption spectra of pristine and Sr2+ and/or Ni3+ doped YFeO3+δ show no significant difference in the UV region, doped YFeO3+δ samples exhibited much higher absorption in the visible range compared to pure YFeO3+δ, with the highest absorption in the case of Ni-doped YFeO3+δ and Sr, Ni co-doped YFeO3+δ samples. This strong transition corresponds to the electronic transition from the 2p valence orbital of oxygen to the 3d conduction band of iron or nickel [12, 28]. There is a bathochromic shift (toward the longer wavelength) in the absorption edge of Sr-doped, Ni-doped, and Sr, Ni co-doped YFeO3+δ nanoparticles, indicating a decrease in their band gaps. The optical energy gaps (Eg, eV) of the Y1-xSrxFe1-xNiyO3+δ nanoparticles were calculated using the Tauc-plot and are shown in Table 3 and Fig. 5B. The optical band gap for the YFeO3+δ sample was calculated to be 1.87 eV, which is lower than previously reported values [12, 28]. For the Sr-doped YFeO3+δ sample, and especially the Ni-doped YFeO3+δ and Sr and Ni co-doped YFeO3+δ, there is a sudden drop in the band gap values (Fig. 5B and Table 3). The band gap of obtained Y1-xSrxFe1-xNiyO3+δ nanoparticles (1.87–0.60 eV) is much narrower than those of Y1-xSmxFeO3 and Y1-xGdxFe0.95Co0.05O3 nanomaterials (see Table 3) [12, 28]. Besides the intrinsic nature of the materials, according to references [37, 38], reduced particle size leads to a narrow energy gap. Indeed, the size of the obtained YFeO3+δ nanoparticles is remarkably smaller than other YFeO3+δ synthesized by solid-state reaction [28] and sol–gel methods [12]. Furthermore, the size of Y0.8Sr0.2FeO3+δ > Y0.8Sr0.2Fe0.8Ni0.2O3+δ ~ YFe0.8Ni0.2O3+δ, hence the band gap values also decrease accordingly (see Tables 1, 3 and Fig. 3). With a narrow band gap, the Y1-xSrxFe1-xNiyO3+δ nanoparticles synthesized in this work can be used as a photocatalyst in the visible region for organic decomposition processes.
Figure 6 shows the magnetic properties of the Y1-xSrxFe1-yNiyO3+δ nanopowders at room temperature. The doping of Sr and/or Ni into the YFeO3 crystal lattice clearly changed the magnetic nature of the base YFeO3 material. The pure YFeO3 sample, with a narrow hysteresis loop and a very low Mr (0.021 emu g−1) and Hc (50.25 Oe) (Table 3), is a typical weak ferromagnetic material [22]. For Sr and/or Ni-doped YFeO3, a large open region was seen at the center of the hysteresis loops, suggesting ferromagnetic behavior, especially in cases of Sr and Ni co-doped YFeO3 samples (Fig. 6). The Hc (Oe) and Mr (emu g−1) values of the Y0.8Sr0.2O3+δ and YFe0.8Ni0.2O3+δ samples are 40 and 12–21 times greater than those of the pure YFeO3+δ sample, respectively. For Sr and Ni co-doped YFeO3, the values of Hc and Mr are even much higher (80 times higher for Hc, and 104 times for Mr) (Table 3). The Hc value of Y0.8Sr0.2Fe0.8Ni0.2O3+δ at 15,000 Oe was approximately that of Y0.8Gd0.2Fe0.95Co0.05O3 at 60 000 Oe [28] (Table 3). Moreover, the Sr and Ni co-doped YFeO3 sample did not reach saturation at 15,000 Oe, while pure YFeO3, Sr-doped YFeO3, and Ni-doped YFeO3 samples were already saturated at 15,000 Oe. The reasons for the observed improvement in the magnetization can be explained as follows. (1) When the particle size is small, uncompensated surface spins of Fe3+ ions are created, leading to a strong magnetic enhancement [39, 40]. In fact, particle and crystallite sizes are in the order of Y0.8Sr0.2Fe0.8Ni0.2O3+δ < Y0.8Fe0.8Ni0.2O3+δ < Y0.8Sr0.2Fe0.8O3+δ (See Table 1 and Fig. 3). (2) The distorted structure is affected by the doping and co-doping effects. The doping of Sr and/or Ni into the YFeO3 structure increased the crystalline magnetic anisotropy, resulting in the large increase in Hc and Mr [39, 40]. (3) Finally, the substitution of Y3+ and/or Fe3+ by Sr2+ and/or Ni2+/Ni3+ in YFeO3 enhanced the oxidation process, Fe3+ → Fe4+ + e, which led to the increase of super-exchange interactions Fe3+–O2−–Fe4+, Y3+–O2−–Fe3+/Fe4+, Sr2+–O2−–Fe3+/Fe4+, Ni2+/Ni3+–O2−–Fe3+/Fe4+, Ni2+–O2−–Ni3+, Y3+–O2−–Ni2+/Ni3+, Sr2+–O2−–Ni2+/Ni3+ compared to the super-exchange interactions Fe3+–O2−–Fe3+, Y3+–O2−–Y3+ va Y2+–O2−–Fe3+ in pure YFeO3 [41].
In this manner, YFeO3 nanopowder, with its weak ferromagnetic behavior, can be used as a material operating under external fields, such as the cores of transformers, electromagnets, or magnetic conductors. Owing to their high Hc and Mr, Y0.8Sr0.2Fe0.8O3+δ, Y0.8Fe0.8Ni0.2O3+δ, and Y0.8Sr0.2Fe0.8Ni0.2O3+δ can be applied to permanent magnets, magnetic recording materials in hard drives, or magnetic tapes, etc. [22].
4 Conclusions
The single-phase nanostructured YFeO3+δ, Y0.8Sr0.2Fe0.8O3+δ, Y0.8Fe0.8Ni0.2O3+δ, and Y0.8Sr0.2Fe0.8Ni0.2O3+δ perovskites have been synthesized by the simple co-precipitation method. The hydrolysis of Y3+, Sr2+, Fe3+, and Ni2+ cations were carried out in boiling water (T° ≥ 95 °C) with Na2CO3 5% as a precipitant. Sr and/or Ni doping distorted the crystal structure of the base material YFeO3, thus correspondingly modified its optical and magnetic properties. The Sr-doped YFeO3+δ, Ni-doped YFeO3+δ, and Sr and Ni co-doped YFeO3+δ nanopowders have narrow band gaps (of 1.31 eV, 0.60 eV, and 0.76 eV, respectively) and can be used as photocatalysts for the decomposition of organic compounds in the visible light region, which can be recovered easily after their use by rare-earth magnets owing to their high Mr (of 0.261, 0.459, and 2.182 emu g−1, respectively) and Hc (of 2029.37, 2117.32, and 3957.06 Oe, respectively).
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Change history
08 June 2022
A Correction to this paper has been published: https://doi.org/10.1007/s10854-022-08495-0
References
V.I. Popkov, O.V. Almjasheva, A.S. Semenova, D.G. Kellerman, Magnetic properties of YFeO3 nanocrystals obtained by different soft-chemical methods. J. Mater. Sci. 28, 7163–7170 (2017)
M. Wang, T. Wang, Sh. Song, M. Tan, Structure-controllable synthesis of multiferroic YFeO3 nanopowders and their optical and magnetic properties. Materials 10(6), 626 (2017)
R. Maiti, S. Basu, D. Chakravorty, Synthesis of nanocrystalline YFeO3 and its magnetic properties. J. Magn. Magn. Mater. 321, 3274–3277 (2009)
X. Lu, J. Xie, H. Shu, J. Liu, Ch. Yin, J. Lin, Microwave-assisted synthesis of nanocrystalline YFeO3 and study of its photoactivity. Mater. Sci. Eng. B. 138, 289–292 (2007)
M.I. Diez-Garcia, V. Celorrio, L. Calvillo, D. Tiwari, R. Gomez, D. Fermin, YFeO3 photocathodes for hydrogen evolution. Electrochim. Acta 246, 365–371 (2017)
O. Rosales-Gonzalez, F.S.D. Jesus, F. Pedro-Garcia, C.A. Cortes-Escobedo, M. Ramirez-Cardona, A.M. Bolarin-Miro, Enhanced multiferroic properties of YFeO3 by doping with Bi3+. Materials 12, 2054 (2019)
A.T. Nguyen, V.N.T. Pham, TTr.L. Nguyen, V.O. Mittova, Q.M. Vo, M.V. Berezhnaya, IYa. Mittova, T.H. Do, H.D. Chau, Crystal structure and magnetic properties of perovskite YFe1-xMnxO3 nanopowders synthesized by co-precipitation method. Solid State Sci. 96, 105922 (2019)
A.T. Nguyen, H.D. Chau, Tr.L.T. Nguyen, V.O. Mittova, T.H. Do, IYa. Mittova, Structural and magnetic properties of YFe1-xCoxO3 (0.1 ≤ x ≤ 0.5) perovskite nanomaterials synthesized by co-precipitation method. Nanosyst. Phys. Chem. Math. 9(3), 424–429 (2018)
N.O. Khalifa, H.M. Widatallah, A.M. Gismelseed, F.N. Al-Mabsali, R.G.S. Sofin, M. Pekala, Magnetic and Mössbauer studies of pure and Ti-doped YFeO3 nanocrystalline particles prepared by mechanical milling and subsequent sintering. Hyperfine Inter. 237, 46 (2016)
M.V. Berezhnaya, O.V. Al’myasheva, V.O. Mittova, A.T. Nguyen, IYa. Mittova, Sol-gel synthesis and properties of Y1-xBaxFeO3 nanocrystals. Rus. J. Gen. Chem. 88(4), 626–631 (2018)
H. Shen, J. Xu, M. Jin, G. Jiang, Influence of manganese on the structure and magnetic properties of YFeO3 nanocrystal. Ceram. Inter. 38, 1473–1477 (2012)
P.S.J. Bharadwaj, S. Kundu, V.S. Kollipara, Structural, optical and magnetic properties of Sm3+ doped yttrium orthoferrite (YFeO3) obtained by sol-gel synthesis route. J. Phys. Condens. Matt. 32, 035810 (2020)
C. Sasikala, N. Durairaj, I. Baskaran, B. Sathyaseelan, M. Henini, Transition metal titanium (Ti) doped LaFeO3 nanoparticles for enhanced optical structure and magnetic properties. J. All. Compd. 712, 870–877 (2017)
T.A. Nguyen, V.N.T. Pham, H.T. Le, D.H. Chau, V.O. Mittova, L.TTr. Nguyen, D.A. Dinh, T.V.N. Hao, IYa. Mittova, Crystal structure and magnetic properties of LaFe1-xNixO3 nanomaterials prepared via a simple co-precipitation method. Ceram. Inter. 45, 21768–21772 (2019)
C. Feng, S. Ruan, J. Li, B. Zou, J. Luo, W. Chen, W. Dong, F. Wu, Ethanol sensing properties of LaCoxFe1-xO3 nanoparticles: effects of calcination temperature, Co-doping, and carbon nanotube-treatment. Sens. Act. B 155(1), 232–238 (2011)
N.A. Tien, I.Y. Mittova, D.O. Solodukhin, O.V. Al’myasheva, O.V. Mittova, SYu. Demidova, Sol–gel formation and properties of nanocrystals of solid solution Y1-xCaxFeO3. Rus. J. Inor. Chem. 59(2), 40–45 (2014)
A. Somvanshi, Sh. Husain, W. Khan, Investigation of structure and physical properties of cobalt doped nano-crystalline neodymium orthoferrite. J. Alloys Compd. 778, 439 (2019)
M.V. Knurova, I.Y. Mittova, N.S. Perov, O.V. Al’myasheva, N.A. Tien, V.O. Mittova, V.V. Bessalova, E.L. Viryutina, Effect of the degree of doping on the size and magnetic properties of nanocrystals La1-xZnxFeO3 synthesized by the sol–gel method. Rus. J. Inor. Chem. 62(3), 281–287 (2017)
W. Haron, Th. Thaweechai, W. Wattanathana, A. Laobuthee, H. Manaspiya, Ch. Veranitisagul, N. Koonsaeng, Structure characteristics and dielectric properties of La1-xCoxFeO3 and LaFe1-xCoxO3 synthesized via metal organic complexes. Energy Proc. 34, 791–800 (2013)
Z. Habib, K. Majid, M. Ikram, Kh. Sultan, Influence of Ni substitution at B-site for Fe3+ ions on morphological, optical, and magnetic properties of HoFeO3 ceramics. Appl. Phys. A 122, 550 (2016)
A. Bashir, M. Ikram, R. Kumar, P.N. Lisboa-Filho, Structural, electronic structure and magnetic studies of GdFe1-xNixO3 (x ≤ 0.5). J. Alloys Compd. 521, 183–188 (2012)
B.D. Cullity, C.D. Graham, Introduction to magnetic materials, 2nd edn. (Wiley, Canada, 2009)
N.A. Tien, V. Pham, D.H. Chau, V.O. Mittova, I.Y. Mittova, E.I. Kopeychenko, L.TTr. Nguyen, V.X. Bui, A.T.P. Nguyen, Effect of Ni substitution on phase transition crystal structure and magnetic properties of nanostructured YFeO3 perovskite. J. Mol. Struct. 1215, 128293 (2020)
M. Johnsson, P. Lemmens, Crystallography and chemistry of perovskites, 1st edn. (Wiley, Hoboken, 2007)
C.E. Housecroft, A.G. Sharpe, Inorganic Chemistry, 2nd edn. (Prentice Hall, Pearson, 2005)
N. Imanaka, Physical and chemical properties of rare earth oxides, binary rare earth oxides (Kluwer Academic Publishers, Dordrecht, 2004), pp. 111–113
T.A. Nguyen, L.TTr. Nguyen, V.X. Bui, Influence of the synthitic conditions on the crystal structure, magnetic and optical properties of holmium orthoferrite nanoparticles. J. Mater. Sci. 32, 19010–19019 (2021)
M. Wang, T. Wang, Structural, magnetic and optical properties of Gd and Co co-doped YFeO3 nanopowders. Materials 12, 2423 (2019)
N. Ghobadi, Band gap determination using absorption spectrum fitting procedure. Inter. Nano Lett. (2013). https://doi.org/10.1186/2228-5326-3-2
A.G. Belous, E.V. Pashkova, V.A. Elshanskii, V.P. Ivanitskii, Effect of precipitation conditions on the phase composition, particle morphology, and properties of iron (III, II) hydroxide precipitates. Inor. Mater. 36, 343–351 (2000)
P. Caro, M. Lemaitre, M. Blassé, C.R. Reances, Hydroxycarbonates deterres rates Ln2(CO3)x(OH)2(3–x).nH2O. Acad. Sci. Ser. C 269, 687–690 (1969)
T.A. Nguyen, V. Pham, T.L. Pham, L.TTr. Nguyen, I.Y. Mittova, V.O. Mittova, L.N. Vo, B.T.T. Nguyen, V.X. Bui, E.L. Viryutina, Simple synthesis of NdFeO3 by the so-precipitation method based on a study of thermal behaviors of Fe (III) and Nd (III) hydroxides. Curr. Comput.-Aided Drug Des. 10, 219 (2020)
W. Shijin, Zh. Shengliang, W. Zubiao, W. Yuling, W. Shangping, Ch. Jianjun, X. Rong, L. Longfei, Synthesis and characterization of yttrium hydroxide and oxide microtubes. Rare Met. 28(5), 445–448 (2009)
V.V. Kharton, A.V. Kovalevsky, M.V. Patrakeev, E.V. Tsipis, A.P. Viskup, V.A. Kolotygin, A.A. Yaremchenko, A.L. Shaula, E.A. Kiselev, J.C. Waerenborg, Oxygen nonstoichiometry, mixed conductivity, and Mössbauer spectra of Ln0.5A0.5FeO3-δ (Ln = La – Sm, A = Sr, Ba): effect of cation size. Chem. Mater. 20, 6457–6467 (2008)
H.W. Brinks, H. Fjellvag, A. Kjekshus, B.C. Hauback, Structure and magnetism of Pr1-xSrxFeO3-δ. J. Solid State Chem. 150(2), 233–249 (2000)
J.W. Fergus, Perovskite oxides for semiconductor-based gas sensors. Sensors Actuators B 123, 1169–1179 (2007)
C.V. Ramana, R.J. Smith, O.M. Hussain, Grain size effects on the optical characteristics of pulsed-laser deposited vanadium oxide thin films. Phys. Status Solidi A (2003). https://doi.org/10.1002/pssa.200309009
A.J. Deotale, R.V. Nandedkar, Correlation between particle size, strain and band gap of iron oxide nanoparticles. Mater. Today 3(6), 2069–2076 (2016)
T. Moriya, New mechanism of anisotropic superexchange interaction. Phys. Rev. Lett. 4, 228–230 (1960)
I. Dzyaloshinsky, A thermodynamic theory of weak ferromagnetism of antiferromagnetics. J. Phys. Chem. Solids 4(4), 241–255 (1958)
A. Jaiswal, R. Das, S. Adyanthaya, P. Poddar, Surface effects on morin transition, exchange bias, and enchanced spin reorientation in chemically synthesized DyFeO3 nanoparticles. J. Phys. Chem. C 115(7), 2954–2960 (2011)
Acknowledgements
The authors would like to thank Ho Chi Minh City University of Education for the facility support.
Author information
Authors and Affiliations
Contributions
Conceptualization, methodology, and formal analysis: DHTP, LTTN and TAN, validation: TAN and DHC, writing—original draft preparation: TAN, DHC, VXB, writing—review and editing, VOM and IYM. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors maintain that they have no conflict of interest to be described in this communication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised due to affiliation error.
Rights and permissions
About this article
Cite this article
Pham, D.H.T., Nguyen, L.T.T., Mittova, V.O. et al. Structural, optical and magnetic properties of Sr and Ni co-doped YFeO3 nanoparticles prepared by simple co-precipitation method. J Mater Sci: Mater Electron 33, 14356–14367 (2022). https://doi.org/10.1007/s10854-022-08360-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-022-08360-0