Abstract
A novel visible-light-driven InVO4/Bi2S3/g-C3N4 (VBSCN) nanocomposite photocatalyst was successfully synthesized by a wet-impregnation method. The phase, morphology, chemical composition, microstructure, and optical properties of the prepared pure g-C3N4 and ternary InVO4/Bi2S3/g-C3N4 heterojunctions were measured in detail by various characterization techniques, including powder XRD, FT-IR, TEM, SEM, BET, UV–Vis DRS, PL, the photocurrent response, and EIS analyses. The fabricated nanocomposites Bi2S3/g-C3N4 with the InVO4 doping mass ratio of 5% exhibited superior photocatalytic degradation of Reactive blue 19 dye under visible light irradiation. Furthermore, O2− was identified as the main active species by free radical trapping. This efficient catalysis was benefited from the doping of InVO4, which reduced the wide intrinsic band gap and improved the absorption and utilization ability of visible light. Meanwhile, a dual Z-scheme heterostructure interface was constructed to realize the rapid separation of photogenerated electron–hole pairs. This investigation may provide referential significance for the exploration and fabrication of new and efficient g-C3N4-based heterostructure.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Nowadays, rapid industrialization growth discharges a large number of non-biodegradable pollutants, causing great harm to the ecological environment. Especially in the manufacturing process of textile industry, 10–50% of printing and dyeing wastewater containing complex aromatic structure will be produced [1]. Therefore, how to effectively degrade printing and dyeing wastewater is one of the most urgent problems to be solved in wastewater treatment. At present, technologies have been reported to remove organic pollutants from wastewater, such as photocatalytic technology, membrane filtration, reverse osmosis, and adsorption [2, 3]. Among different technologies, semiconductor photocatalysis is considered as an efficient, environmental-friendly and low-cost treatment method to degrade contaminants, and its photocatalytic function is attributed to the light-induced generation of charge carrier pairs which can transfer to the surface of the material and exhibit unique redox properties [4, 5]. However, traditional photocatalysts, such as pure TiO2, cannot be fully applied to photocatalysis field due to its broad band gap (about 3.2 eV) only responding in the ultraviolet region and high photoelectron-hole compound rate [6, 7]. Therefore, in order to enhance the utilization rate of sunlight, it has great significance to develop new photocatalysts with superior visible light response.
The emerging graphite-like phase carbon nitride (g-C3N4) material is a polymeric layered semiconductor with defect-rich N-bridged tri-s-triazine or s-triazine as the basic structural unit. Generally, g-C3N4 has been widely used as a material for photocatalytic treatment of pollutants due to its simple preparation method, stable physical and chemical properties, and suitable optical band gap (about 2.7 eV) [8,9,10]. Nevertheless, the certain deficiencies of g-C3N4, including inadequate sunlight absorption ability, low photo response current, and high photoinduced electron–hole pairs composite rate, result in poor photocatalytic efficiency [11, 12]. To overcome the above defects, Zhou et al. have summarized a series of strategies including heteroatom doping, structure modification, shape-control synthesis, semiconductor coupling, and dye-sensitization to improve the photocatalytic activity of g-C3N4 [13]. In recent years, coupling it with the different types of semiconductor materials, such as metal oxides, metal sulfides, oxometallates, and bismuth oxyhalides [14,15,16,17,18], to fabricate II type or Z-scheme heterojunction, is an effective way to enhance the photocatalytic efficiency by broadening the light response range and prolonging the lifetime of the photoinduced charge carriers [19, 20]. Especially, Z-scheme heterojunction is helpful to maintain the superior redox ability of electrons and holes after charge transfer [21]. Moreover, many researchers are devoted to constructing ternary heterojunctions to further improve the photocatalytic performance of catalytic materials [22, 23].
Nanocomposite photocatalysts have been attracted much research attention because of their comprehensive advantages and synergistic effects which contributes to the separation of photogenerated electrons and holes. [24, 25]. As a typical layered semiconductor, Bi2S3 is endowed with a direct band gap of about 1.2–1.7 eV [26]. The narrow band gap is the direct advantage of choosing Bi2S3 as photocatalyst. Bi2S3 was successfully loaded onto g-C3N4 by ultrasonic method [27] and one-pot method [28], which can enhance the absorption of visible light and provide energy for the rapid transfer of photogenerated carriers, thus showing excellent photocatalytic performance. In addition, narrow band gap semiconductor InVO4 (Eg = 2.3 eV) is also an important visible-driven catalytic material due to its high visible light utilization rate, adjustable morphology and size, and simple synthesis method [29]. Studies have shown that the energy levels of g-C3N4 and InVO4 are well matched overlapping band structures [30]. Therefore, it is predicted that the introduction of InVO4 in Bi2S3/g-C3N4 Z-scheme system could construct dual Z-scheme heterojunction, which further expands the reduction or oxidation surfaces to improve the photocatalytic performance [31].
In order to further analyze the optical response and light catalytic properties of g-C3N4-based composites, on the basis of Bi2S3/g-C3N4, ternary dual Z-scheme InVO4/Bi2S3/g-C3N4 heterojunctions were firstly synthesized in this work. The crystal structure, micromorphology, chemical composition, and optical properties of the ternary photocatalyst were analyzed by means of various characterization methods. The photocatalytic activity was measured by degradation of anion dye Reactive Blue 19 (RB19), and the photocatalytic degradation mechanism was explored by free radical capture experiment. In comparison with pure g-C3N4, InVO4 and Bi2S3/g-C3N4, the as-prepared InVO4/Bi2S3/g-C3N4 composites displayed higher visible light absorption efficiency, stronger catalytic activity, and better stability. With the optimal content of 5 wt% InVO4 in the composite, 5wt% InVO4/Bi2S3/g-C3N4 performed the excellent visible light catalytic activity for the degradation of RB19, and the degradation rate of RB19 (15 mg/L) could reach 97% after 100 min of visible light irradiation. To prove the advantages of InVO4/Bi2S3/g-C3N4, Table 1 presents a retrospective analysis using multiple photocatalysts for earlier studies on the degradation of dye under visible light.
2 Experimental section
2.1 Chemicals and materials
Urea (H2NCONH2, 99%) and melamine (C3H6N6, 99%) were bought from Tianjin Damao Chemical Reagent Factory (China). Bi(NO3)3·5H2O (99%) was obtained from Tianjin Kaitong Chemical Reagent Co., Ltd. Na2S2O3·5H2O (99%) was provided from Tianjin Guangfu Technology Development Co., Ltd. InCl3·4H2O (99%) and NH4VO3 (99%) were from Tianjin Jinke Fine Chemical Research Institute. RB19 was purchased from Shanghai Maclean Biochemical Technology Co., Ltd. P-benzoquinone (BQ, 99%) was from Tianjin Qinghua Jinying Technology Co., Ltd. Tert-butanol (TBA, 99%) came from Tianjin Beichen Founder Reagent Factory. Ammonium oxalate (AO, 99%) was from Tianjin Zhiyuan Chemical Reagent Co., Ltd. Nitrotetrazolium Blue chloride (NBT) was from Hefei BSF Biology Science and Technology Co., Ltd. In this research, all of the materials were analytical grade without further purification. In addition, distilled water was used during the whole experiment process.
2.2 Catalyst preparation
2.2.1 Preparation of Bi2S3/g-C3N4
Pure g-C3N4 was prepared by calcining urea and melamine at high temperature [36]. Typically, 10.5 g urea and 4.5 g melamine were added to a covered alumina crucible and placed in a muffle furnace for 4 h at 550 °C, in which heating rate is 5 °C/min. After cooling to room temperature, the obtained light yellow g-C3N4 was ground into powder for further use.
Bi2S3 was prepared by a wet-impregnation method [37]. Firstly, 1.9403 g Bi(NO3)3·5H2O and 0.7445 g Na2S2O3·5H2O were uniformly dissolved in 50 mL methanol by ultrasonication for 10 min, and then stirred on a magnetic agitator for 60 min. Then, the suspension was transferred into a 100-mL polytetrafluoroethylene-lined stainless autoclave, heated at 180 °C for 12 h. Finally, the products were cooled to room temperature naturally, collected by centrifugation, and washed with anhydrous ethanol, distilled water for 3 times, and fully dried in an oven at 60 °C to obtain the grassy black Bi2S3 sample.
Bi2S3/g-C3N4 composite was synthesized by a simple wet-impregnation and calcination. In detail, 1 g g-C3N4 was dispersed in 80 mL methanol and treated with ultrasonic for 30 min to obtain solution A. Then, Bi2S3 with a mass doping ratio of 5% was dissolved in g-C3N4 solution, and the solution B was obtained after ultrasonic treatment for 60 min. Then, A and B were mixed through magnetic stirring for 24 h. After the reaction, the product was dried at 60 °C and finally calcined at 250 °C for 3 h. After grinding, the light yellow powdery photocatalyst of Bi2S3/g-C3N4 was collected and denoted as 5BS/CN.
2.2.2 Preparation of InVO4/Bi2S3/g-C3N4
InCl3·4H2O and NH4VO3 were used to fabricate bare InVO4. Usually, 0.0638 g InCl3·4H2O and 0.0255 g NH4VO3 were dissolved in 50 mL distilled water. Subsequently, the NaOH solution was added dropwise to the above mixture and the pH was adjusted to 6–8. The InVO4 was obtained by ultrasonic for 30 min.
A series of InVO4/Bi2S3/g-C3N4 hybrid with different doping ratios of InVO4 (denoted as xV/5BSCN, x = 3, 5, 10, 15, and 20 wt% with x representing the weight percentage of InVO4) were synthesized by the following method. 1 g 5BS/CN uniformly dispersed in 80 mL distilled water by ultrasonication for 10 min. Then, a certain amount of InVO4 was added into the above suspension. Next, the mixture was continued to be sonicated for 30 min and further magnetic stirred for 24 h at room temperature. Finally, ternary InVO4/Bi2S3/g-C3N4 photocatalyst was fully dried at 60 °C and collected by ground.
2.3 Catalyst characterization
The phase purity and crystal structure information were determined by X-ray diffraction measurement (XRD) (LABXRD-6000) using Cu-Kα source (λ = 0.154 nm) with a step size of 0.02° in the range of 10°–80° at the scan rate of 2°/min at 30 mA and 40 kV. The functional groups and chemical bonds were qualitative analyzed by Fourier transform infrared (FT-IR) spectra on thermo Scientific Nicolet iS10 spectrometer taking KBr as the reference in the range of 500–4000 cm−1. The morphologies of the photocatalysts were observed. The morphologies and microstructures of the samples were investigated by the transmission electron microscopy (TEM) (JEM-2500SE) at 200 kV and the field emission scanning electron microscopy (FE-SEM) (JSM-7100F) at 15 kV. Energy dispersive X-ray spectroscopy (EDS) attached to SEM was used to determine the chemical composition of the composite. N2 adsorption and desorption isotherms at − 196 °C were obtained using a Micromeritics ASAP 2020.The BET (Brunauer–Emmett–Teller) method and BJH (Barrett–Joyner–Halenda) method were used to calculate the specific surface area and pore size distribution, respectively. The absorption edge of the catalysts was measured by UV–Vis diffuse reflectance spectra (UV–Vis DRS) (Hitachi U-3900, Japan) using Ba2SO4 as the background in the range from 200 to 800 nm. The catalysts photoluminescence (PL) spectra were recorded on a Shimadzu RF-6000 fluorescence spectrophotometer with slit width of 3 nm in the range from 400 to 700 nm at the scan rate of 6000 nm/min. The photocurrent response and electrochemical impedance spectroscopy (EIS) were measured in Na2SO4 (0.1 M) electrolyte solution by Chi660E electrochemical work station with a three-electrode cell, and the platinum electrode, ITO glass (25 × 15 × 1.1 mm) which was coated by the samples, Ag+/AgCl electrode were used as counter electrode, working electrode, and reference electrode, respectively.
2.4 Catalytic experiments
The photocatalytic activities were evaluated by the degradation of RB19 under visible light irradiation. The visible light source was a 300 W Xenon lamp (CEL-HXF300) equipped with a 420 nm cutoff filter, 15 A current, and 14 V voltage. Typically, 200 mg of photocatalysts with different mass ratios of InVO4 was added to a 250 mL 20 mg/L of RB19 dyes solution and then placed in a 300 mL glass reactor. Before the irradiation reaction, the above suspension was firstly dispersed by ultrasound for 10 min and then stirred in the dark for 40 min to establish an adsorption–desorption equilibrium. Afterward, the suspension was placed under visible light in the photocatalytic chamber for 100 min. To eliminate the effect of temperature, the reaction vessel was endowed with a large beaker containing an appropriate amount of cold water (25 ± 1 °C). Meanwhile, 10 mL of photoreacted solution was sampled at regular irradiation time intervals (20 min) and centrifuged twice for 5 min each (10,000 rpm). Lastly, the concentration of RB19 was tested on a UV-1800PC spectrophotometer (MAPAD, Shanghai, China) (λmax = 594 nm). The photocatalytic efficiency (η) was calculated according to the following formula:
where C0 is the initial concentration of RB19 dyes solution and C is the real-time concentration of RB19 dyes solution during the reaction. Furthermore, the first-order rate constant kobs of the photocatalytic process is measured by the following formula [38]:
where kobs (min−1) is the first-order rate constant, C0 is the RB19 dyes concentration after 40 min dark adsorption (mg/L), and C is the instantaneous concentration of the RB19 solution at illuminated time t (min).
In addition to the experiment of optimal xV/BSCN doping ratio, the influence of catalyst dosage, initial concentration of RB19, initial pH, and the stability of the photocatalyst were also studied. The photocatalytic properties of xV/BSCN were explored from different perspectives.
2.5 Reactive species experiments
2.5.1 Active species trapping experiments
In order to explore the reaction mechanism and determine the generation of main active species in the process of the photocatalytic degradation of RB19, 2 mL TBA, 0.0621 g AO, and 0.0054 g BQ quenchers were used as scavengers of hydroxyl radicals (·OH), photo-holes (h+), and superoxide radicals (O2−), respectively, were added to the reaction system after the adsorption–desorption equilibrium, and the remaining steps were the same as for other degradation experiments.
2.5.2 NBT transformation experiments
NBT was often used to quantitatively analyze the·O2− in photocatalytic reaction [39]. Briefly, 50 mg as-prepared catalysts were dispersed in 250 mL distilled water by ultrasonication for 10 min. Next, 10.2205 mg NBT was added in the above dispersion to react. After a given time interval, the concentration of NBT was measured with recording variations of the absorption band maximum (259 nm) in the UV–Vis spectra to react with the amount of ·O2−.
3 Results and discussion
3.1 Optimal doping ratio selection
Figure 1a and b shows the photodegradation efficiency of 25 mg/L RB19 and the first-order rate constants of each reaction over the g-C3N4, InVO4 (V), 5BS/CN, and the ternary catalysts with different InVO4 doping ratios. It could be clearly seen from Fig. 1a that the adsorption capacity of g-C3N4 to RB19 solution was larger, reaching 41.2%, and the photocatalytic degradation rate of RB19 solution under visible light was lower and degraded to 85.3% after 100 min of light exposure. Pure InVO4 had no adsorption and degradation ability at all. The binary catalyst 5BS/CN improved the degradation capacity of pure g-C3N4 to 91.2% after 100 min of visible light irradiation. The photocatalytic performance of 5BS/CN was further increased by doping InVO4. Obviously, the catalytic activity of the xV/5BSCN composites was closely related to their doping ratios. The photocatalytic rates of 3 V/5BSCN, 5 V/5BSCN, 10 V/5BSCN, 15 V/5BSCN, and 20 V/5BSCN were 91.2%, 95.5%, 89.1%, 86.6%, and 85.3%. Clearly, as the doping ratio of InVO4 increased from 3 to 5%, the photodegradation efficiency increased gradually; however, when the loading percent of InVO4 further increased from 5 to 20%, the photocatalytic rate for RB19 tended to a significant decrease. It indicated that 5 V/5BSCN had the best photocatalytic activity. This might be due to excessive InVO4 deposition, which was not well dispersed on the surface of the material, affecting its absorption of light, reducing the number of effective heterogeneous junctions in the composite material, and influencing charge transfer between heterogeneous junction interfaces [30]. Similarly, the first-order rate constant of 5 V/5BSCN also was the largest at 40 min. Therefore, the 5 V/5BSCN were selected as the optimal photocatalyst for the following studies.
3.2 Characterization
3.2.1 Structure and morphology
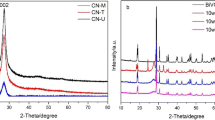
The crystal structure of as-synthesized g-C3N4, Bi2S3, InVO4, 5BS/CN, 5 V/5BSCN were detected by XRD pattern and are shown in Fig. 2. Obviously, two diffraction peaks of pure g-C3N4 were observed at 13.02° and 27.52°corresponding to (100) and (002) planes of pristine g-C3N4 (JCPDS 87-1526), which were derived from the tri-s-thiazine units and the stacking of the conjugated aromatic systems, respectively [40, 41]. The crystal structures of bare Bi2S3 were orthorhombic crystal structure and their diffraction peaks at 23.44°, 29.1°, 31.94°, 45.56°, 52.46°, and 62.40° could be indexed to (130), (211), (221), (002), (351), and (152) crystal planes respectively, which matched well with the standard card (JCPDS 17-0320) [42, 43]. In the XRD pattern of 5BS/CN, the diffraction peaks of g-C3N4 and Bi2S3 appeared simultaneously, which indicated the successful loading of Bi2S3 without impurity. The XRD spectra of pure InVO4 showed that the diffraction peaks at 19.8°, 21.5°, 31.8°, 33.0°, 41.6°, 51.0°, and 60.9° could be ascribed to (110), (020), (200), (112), (202), (042), and (242) crystal planes of orthorhombic phase InVO4 (JCPDS. No 48-0898), [44]. With the addition of InVO4 to 5BSCN, it could be found that the main diffraction peaks of InVO4 and 5BS/CN existed at the same time in the XRD pattern of 5 V/5BSCN hybrid, which illustrated the successful preparation of the ternary photocatalysts. Furthermore, the weak the characteristic peak intensity of InVO4 in the composite might be due to the low content of InVO4.
The functional groups and bonds of g-C3N4, 5BS/CN, and 5 V/5BSCN were tested on the FT-IR spectra, the results of which are displayed in Fig. 3. It could be seen from Fig. 3 that the characteristic peaks of the three materials were mainly concentrated in the range of 500–3500 cm−1. In the FT-IR spectrum of g-C3N4, the sharp absorption band at 807 cm−1 was from the distinctive vibration of tri-s-triazine ring units [45], the absorption peaks in the range of 1200–1700 cm−1 were caused by stretching vibrations of C–N and C=N bonds [46]. A wide absorption band at 3250 cm−1 was the stretching vibrations of O–H and N–H due to the adsorption of water molecules and residue of amino groups on the surface of g-C3N4. In the FT-IR spectra of 5BS/CN, the absorption peaks at 3254 cm−1 and 811 cm−1 which also existed in the FT-IR spectra of pristine Bi2S3, corresponding to the stretching vibrations of the residual N–H and Bi–S groups respectively [47], thus demonstrating the existence of Bi2S3 in 5BS/CN composite. The diffraction peak of 5 V/5BSCN appeared at 809 cm−1 owing to the interaction between tri-s-triazine ring units of g-C3N4 and Bi–S groups of Bi2S3, which also suggested the coexistence of g-C3N4 and Bi2S3. Moreover, the diffraction peak of 5 V/5BSCN at 3253 cm−1 was due to the stretching vibrations of N–H. However, no characteristic peaks of InVO4 could be observed in the pattern of 5 V/5BSCN, which might be attributed to low amount of InVO4 loading on 5BS/CN. In fact, the existence of InVO4 in the nanocomposite could be further verified by TEM based on the recognition of the morphology and microstructure.
Figure 4 displays TEM images of g-C3N4, Bi2S3, 5 V/5BSCN, and HR-TEM images of 5 V/5BSCN. Figure 4a shows that the internal structure of g-C3N4 was layered and overlapped. And Fig. 4b presents that Bi2S3 had a rod-like structure. TEM image of 5 V/5BSCN (Fig. 4c) showed that the bar Bi2S3 and the irregular stacked layered g-C3N4 were uniformly covered with dark-colored flake substances, indicating that the InVO4 was doped into the 5BS/CN composite material to form the ternary photocatalyst, which was consistent with the XRD analysis. In addition, Fig. 4d shows that the lattice spacing of the lattice stripes was 0.346 nm attributed to the (220) crystal plane of InVO4 [48].
The distribution of InVO4 in the 5 V/5BSCN composite was further demonstrated by elemental mapping. As shown in Fig. 5, C, N, O, S, Bi, In, and V elements were uniformly distributed within 5 V/5BSCN composite. Meanwhile, the weight percentages of each element in 5 V/5BSCN were detected in Fig. 5i, further implying the coexistence of InVO4, Bi2S3 and g-C3N4 without other impurities.
The textural properties of pure g-C3N4 and 5 V/5BSCN were inferred from their N2 adsorption–desorption isotherms (Fig. 6). All photocatalysts showed type-IV adsorption isotherms with H3 type hysteresis loops, indicating the existence of slit-like pores created by the stacked nanosheets [49]. BET surface area for g-C3N4 and 5 V/5BSCN was 63.4485 m2g−1 and 59.7659 m2g−1, respectively. Compared to the pure g-C3N4, the composite materials delivered low surface area. The decrease in the surface area of g-C3N4 might be due to the fact that spherical InVO4 dispersed on the surface of CN blocks the pores of CN. These results could be affirmed by the above-mentioned SEM. It was also a good explanation that the adsorption capacity of RB19 of composite material was lower than that of pure CN. Moreover, 5 V/5BSCN showed more micropores (2 nm) that might be generated by stacked nanosheets.
3.3 Optical properties
The light harvesting capacities of g-C3N4, Bi2S3, InVO4, and 5BS/CN, 5 V/5BSCN composites can be evaluated by UV–Vis diffuse reflectance (DRS) spectra. As shown in Fig. 7a, the optical absorption edges of g-C3N4, Bi2S3, and InVO4 were 475, 1130, and 620 nm respectively, while the optical absorption edges of the doped composite materials 5BS/CN and 5 V/5BSCN were 485 and 680 nm, respectively. This indicated that the light absorption edges of the composites had red shift compared with that of pure g-C3N4, and the visible light absorption region was significantly broadened, thus improving the utilization efficiency of visible light.
The band gap energy (Eg) of photocatalysts can be calculated by the following Kubelka–Munk Eq. [50]:
where α is the absorption coefficient, Eg is the band gap energy, A, h, and v are a proportionality constant, plank constant, and light frequency, respectively, the value of n depends on the type of optical transition of semiconductors (n = 1 for direct transition and n = 4 for indirect transition). According to the previous research, g-C3N4, Bi2S3 and InVO4 are all direct semiconductors, so the value of n is 1 for them [41, 44, 51]. According to the plots of (αhv)1/2 versus hv (Fig. 7b), the calculated Eg values of g-C3N4, Bi2S3 and InVO4 were 2.74, 1.90, and 2.34 eV respectively. After the introduction of Bi2S3, the band gap of 5BS/CN composites was 2.25 eV, which was 0.49 eV smaller than that of pure g-C3N4. Moreover, InVO4 was further introduced, the band gap of the 5 V/5BSCN was continuously reduced to 1.96 eV. This indicated that heterogeneous junctions were constructed between g-C3N4, Bi2S3 and InVO4, which effectively reduced the interface contact barrier, strengthened the electron coupling between semiconductors, and thus enhanced the photocatalytic activity [52]. In addition, the valence band (VB) and conduction band (CB) of semiconductors can be calculated by the following formula [50]
where EVB and ECB are the valence band potential and conduction band potential of semiconductors. X is the electronegativity of the semiconductor, which is the geometric average value of the electronegativity of constituent atoms, and the X values of g-C3N4, Bi2S3, and InVO4 are 4.67, 5.56, and 5.03 eV, respectively [53,54,55]. Ee is the energy of free electrons on the hydrogen scale (about 4.5 eV). Using the Eqs. (4, 5), the VB edge potentials of g-C3N4, Bi2S3, and InVO4 were 1.54 eV, 2.01 eV, and 1.70 eV, respectively, and the CB edge potentials of them were − 1.20 eV, 0.11 eV, and − 0.64 eV, respectively.
3.4 Photocatalytic activities
3.4.1 Effect of catalyst dosage
In order to explore the effect of the photocatalyst dosage on degradation of RB19, the dosage gradient of 5 V/5BSCN was set at 0.4, 0.6, 0.8, 1.0, and 1.2 g/L. Accordingly, the degradation rate of 25 mg/L RB19 and the first-order rate constant under 40 min illumination were studied, as shown in Fig. 8. Obviously, with the increase of the catalyst dosage, the adsorption capacity of the dark reaction was augmented, and when the catalyst dosage reached the maximum of 1.2 g/L, the dark adsorption capacity of RB19 achieved the maximum of 38.16% accordingly. This was because physical adsorption occurred during the dark reaction, in which the more the catalyst, the more the pollutant molecules were adsorbed. Figure 8a exhibits that the final degradation rate of RB19 also increased with the increase of dosage, which was 39.05%, 47.66%, 95.46%, 97.34%, and 97.42% respectively. Remarkably when the dosage of 5 V/5BSCN increased from 0.6 to 0.8 mg/L, the degradation rate increased significantly, and then the dosage further increased to 1.2 mg/L, the degradation rate increased slowly. It could be seen from Fig. 8b that the rate constants corresponding to the dosage were 0.351 × 10–2, 0.580 × 10–2, 2.931 × 10–2, 2.905 × 10–2, and 2.884 × 10–2 min−1, which showed a trend similar to that of degradation rate. This illustrated that an appropriate amount of catalyst could significantly improve the photocatalytic degradation efficiency, but increasing the amount of catalyst further had little effect on the degradation of pollutants and could even inhibit the process. This was because a small amount of the catalyst did not produce enough active charge carriers to afford excess amount of RB19 molecules, with the increase of its dosage, the more active sites could be contributed to enhance light utilization efficiency [56]. Nevertheless, when catalyst dosage surpassed a certain amount, the light transmittance began lower down, so less light could be irradiated to the surface of photocatalysts, which reduced the generation of the photogenerated electrons and holes, eventually resulting in the decrease of photocatalytic efficiency [56, 57]. Therefore, 0.8 g/L was chosen as the optimal dosage for 5 V/BSCN for subsequent exploratory experiments.
3.4.2 Effect of initial concentration of RB19 solution
In order to investigate the effect of initial concentration of RB19 solution on degradation of RB19, the concentration gradient of RB19 was set at 10 mg/L, 15 mg/L, 20 mg/L, 25 mg/L, and 30 mg/L, and the dosage of 5 V/5BSCN was 0.8 g/L. As shown in Fig. 9, 5V/5BSCN degrading 15 mg/L and 25 mg/L RB19 performed better catalytic activity considering the dark adsorption capacity and degradation rate. And there was an inflection point when the initial concentration of the pollutant increased from 25 to 30 mg/L, the degradation rate decreased dramatically. The result indicated that RB19 solution of high concentration suppressed photocatalytic effect. This was because with the increase of the concentration of RB19, the increasing adsorbed pollutants on the surface of the catalyst consumed photoelectron-hole pairs gradually which were hindered to recombination, thus showing a high degradation rate. When the concentration of pollutant solution reached to a high level certainly, on the one hand, the massive sedimentary of RB19 molecules reduced the visible light transmittance; on the other hand, the intermediate by-products that cannot be decomposed in time competed with pollutant molecules for active sites, retarding the reaction, thus leading to the reduction of photocatalytic efficiency [57]. Figure 9b shows that when the initial concentration of RB19 was 15 mg/L, the corresponding first-order rate constant presented a maximum value. So, 15 mg/L was the most suitable initial concentration of RB19 solution among them.
3.4.3 Effect of initial pH
In order to study the effect of the initial pH of the reaction solution on degradation of RB19, the photocatalytic degradation efficiency of RB19 was tested by 10ABCN at initial pH 3, 5, 7, 9, and 11. The initial concentration of RB19 was 15 mg/L, and the dosage of 5 V/5BSCN was 0.8 g/L. Figure 10 presents that the dark adsorption capacities of RB19 at the initial pH of 3, 5, 7, 9, and 11 were 92.17%, 48.50%, 25.93%, 25.93%, and 31.46%, respectively. It manifested that the higher the acidity, the greater the adsorption capacity, because pH affected the electronegativity of the catalyst [50, 58]. Under acidic conditions, the surface of the catalyst was positively charged, while RB19 was a negatively charged anionic dye, then the electrostatic attraction occurred between the photocatalysts and pollutants giving rise to a plentiful adsorption of RB19 molecules on the surface of 5 V/5BSCN. On the contrary, in the alkaline environment, the surface of the catalyst was negatively charged, so there was electrostatic repulsion with the negatively charged RB19, which leaded to the weakening of the adsorption capacity of 5 V/5BSCN. As for the degradation effect, the degradation trend performed faster under alkaline conditions, especially when pH 11. This was because O2 adsorbed on the surface of the catalyst reacted with the negative charge on the surface to generate active species·O2− [59], then RB19 was rapidly degraded. However, after 40 min of photoreaction, the degradation rate of RB19 under alkaline conditions gradually slowed down, and the degradation under pH 11 reaction conditions almost stopped, mainly because the sulfur group of RB19 was substituted to form vinyl sulfone in alkaline environment and 2-hydroxyethyl sulfone [60], the stronger the basicity was, the stronger the substitution effect of the sulfur group was, thus slowing down the photocatalytic reaction process.
3.4.4 Stability experiment
The stability of 5 V/5BSCN was tested by photocatalytic degradation of RB19 in four cycles with the dye concentration of 15 mg/L. As shown in Fig. 11a, the degradation rate of RB19 decreased slightly after each cycle, reaching about 76% after 4 cycles, indicating that the 5 V/5BSCN composite material was relatively stable during photocatalytic degradation of RB19 dyes. Moreover, XRD (Fig. 11b) and SEM (Fig. 11c, d) displayed that the crystal structure of 5 V/5BSCN heterojunction was not destroyed in the progress of the photocatalytic reaction. The stability might be due to the tight heterojunction interface conducive to the rapid separation of photogenerated electrons and holes.
3.5 Photocatalytic mechanism
Fluorescence spectroscopy (PL) is an important method to study the photogenerated electron–hole separation ability. The stronger the peak of the fluorescence emission spectrum was, the higher the electron–hole recombination rate was [7, 61]. Figure 12 depicts the fluorescence spectra of pure g-C3N4, 5BS/CN, and 5 V/5BSCN. The PL spectrum of pure g-C3N4 had a strong emission peak caused by the band transition at 460 nm, which revealed that the electron–hole recombination rate was quite fast [62, 63]. Compared with pure g-C3N4, the PL peak of the 5BS/CN composite material was significantly weaker, illustrating that the construction of heterojunction could effectively inhibit the recombination of photogenerated carriers. Moreover, the PL peak of 5 V/5BSCN was further weaker, indicating that the continuous loading of InVO4 on the basis of 5BS/CN composite to construct a ternary heterojunction could further inhibit the recombination of photogenerated carriers and improve the photocatalytic degradation efficiency.
The photocurrent response and EIS further demonstrated the separation and transfer of photoinduced charge carriers. The photocurrent response of CN, 5BS/CN, and 5 V/5BSCN was monitored for several on–off cycles at open-circuit potential. Higher photocurrent density means a stronger carrier separation capacity [64]. As shown in Fig. 13a, the 5 V/5BSCN exhibited the highest photocurrent density, which were 2.3 and 2 times greater than g-C3N4 and 5BS/CN, respectively, indicating that 5 V/5BSCN had a higher carrier separation ability. The result of EIS is shown in Fig. 13b, the smaller the radius in EIS diagram was, the smaller the charge transfer resistance was at the electrode interface, suggesting the faster interfacial electron transport [65]. The EIS measurements revealed that the radius of Nyquist curve of 5 V/5BSCN was smaller than that of CN and 5BS/CN, and therefore, 5 V/5BSCN was proved to possess higher photogenerated charge transferability than pure CN.
Figure 14 shows the results of active species trapping experiments. It could be seen that the addition of TBA had almost no effect on the degradation rate of RB19, which indicated OH slightly participated in the photocatalytic degradation process. After adding BQ acting as a scavenger of O2−, the degradation rate had a sharp decline. Also, AO slightly inhibited the reaction as h+ scavenger. Therefore, O2− played a main part during the photocatalytic reaction, and h+ was secondary. In order to further determine the main active species deciding RB19 degradation, NBT was introduced to the experiment. Figure 15 shows the spectra of the NBT transformation percentage in the presence of 5 V/5BSCN. As the photoreaction proceeded, NBT was gradually transformed, and the absorbance of NBT decreased significantly at 120 min, which implied varieties of ·O2− radicals were generated during the process.
Based on the above analysis and band structure matching, dual Z-scheme path was favorable for the photocatalytic mechanism and charge transfer pathways of 5 V/5BSCN composite, as shown in Fig. 16. Under the irradiation of visible light, the electrons on the VB of Bi2S3, g-C3N4, and InVO4 both were excited to CB, leaving holes on the VB, respectively. Then, the electrons on the CB of Bi2S3 and InVO4 recombined with the holes on the VB of g-C3N4 through the heterojunction interface, and photogenerated electrons that accumulated on the CB of g-C3N4 reduced O2 to·O2−, leading to the decomposition of RB19 molecules. Since the VB potential of Bi2S3 was positive than E0(H2O/OH) potential (1.99 eV vs. NHE),·OH radicals were generated on the VB of Bi2S3. Meanwhile, the photogenerated holes left behind on the VB of InVO4 would directly participate in the reaction of degrading RB19. In addition, because g-C3N4 accounted for the largest proportion of 5 V/5BSCN, O2− radicals generated by g-C3N4 were the main active species for oxidizing RB19 to degradation products, which was consistent with the results of the active species trapping and NBT conversion experiments. Therefore, the 5 V/5BSCN photocatalytic material realized the rapid separation of photogenerated carriers by constructing a dual Z-scheme heterojunction successfully, and improved the yield and lifetime of photogenerated carriers at the same time, which was also confirmed in the above-mentioned PL spectrum and electrochemical analysis results. The specific reaction process was shown in the following formula:
4 Conclusions
In summary, the ternary dual Z-scheme InVO4/Bi2S3/g-C3N4 heterojunction photocatalyst was successfully synthesized by the wet impregnation method. Under visible light, the 5 V/5BSCN composite, i. e., the loading ratio of InVO4 was 5%, performed the excellent photocatalytic degradation activity for RB19. After 100 min visible light irradiation, the degradation rate of RB19 achieved 95.5%. The improvement of the degradation rate of RB19 by InVO4/Bi2S3/g-C3N4 was ascribed to generate tight heterogeneous interfaces to construct the double Z-scheme heterostructure, which effectively inhibited the recombination of photogenerated carriers on the one hand, and enhanced the oxidation on the other hand. The active species trapping experiment showed that the role of free radicals in the degradation experiment followed ·O2− > h+ > OH. According to the results of this experiment, the dual Z-scheme charges transfer mechanism could provide an effective strategy to design and fabricate g-C3N4-based nanocomposites for wastewater treatment.
Data availability
All data generated or analyzed during this study are included in this published article. And the original datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
S. Rajagopal, B. Paramasivam, K. Muniyasamy, Photocatalytic removal of cationic and anionic dyes in the textile wastewater by H2O2 assisted TiO2 and micro-cellulose composites. Sep. Purif. Technol. 252, 117444–117454 (2020)
C.T. Hou, H.Y. Liu, F.B. Mohammad, Preparation of ordered mesoporous F-H2Ti3O7 nanosheets using orthorhombic HTiOF3 as a precursor and their highly efficient degradation of tetracycline hydrochloride under simulated sunlight. J Solid State Chem. 300, 122288–122302 (2021)
G. Gopal, S.A. Alex, N. Chandrasekaran, A. Mukherjee, A review on tetracycline removal from aqueous systems by advanced treatment techniques. RSC Adv. 10, 27081–27095 (2020)
U. Rafiq, K. Majid, Mitigating the charge recombination by the targeted synthesis of Ag2WO4/Bi2Fe4O9 composite: the facile union of orthorhombic semiconductors towards efficient photocatalysis. J. Alloys Compd. 842, 155876–155884 (2020)
C.T. Hou, H.Y. Liu, Y.J. Li, The preparation of three-dimensional flower-like TiO2/TiOF2 photocatalyst and its efficient degradation of tetracycline hydrochloride. RSC Adv. 11, 14957–14969 (2021)
H.J. Yu, Y.F. Zhao, C. Zhou, L. Shang, Y. Peng, Y.H. Cao, L.Z. Wu, C.H. Tung, T.R. Zhang, Carbon quantum dots/TiO2 composites for efficient photocatalytic hydrogen evolution. J. Mater. Chem. A. 2, 3344–3351 (2014)
W. Chen, Y.X. Hua, Y. Wang, T. Huang, T.Y. Liu, X.H. Liu, Two-dimensional mesoporous g-C3N4 nanosheet-supported MgIn2S4 nanoplates as visible-light-active heterostructures for enhanced photocatalytic activity. J. Catal. 349, 8–18 (2017)
X. Zhang, X.Z. Yuan, L.B. Jiang, J. Zhang, H.B. Yu, H. Wang, G.M. Zeng, Powerful combination of 2D g-C3N4 and 2D nanomaterials for photocatalysis: recent advances. Chem. Eng. J. 390, 124475–124503 (2020)
Y. Yang, C. Zhang, D.L. Huang, G.M. Zeng, J.H. Huang, C. Lai, C.Y. Zhou, W.J. Wang, H. Guo, W.J. Xue, R. Deng, M. Cheng, W.P. Xiong, Boron nitride quantum dots decorated ultrathin porous g-C3N4: Intensified exciton dissociation and charge transfer for promoting visible-light-driven molecular oxygen activation. Appl. Catal. B. 245, 87–99 (2019)
Y. Yang, X. Li, C.Y. Zhou, W.P. Xiong, G.M. Zeng, D.L. Huang, C. Zhang, W.J. Wang, B.A. Song, X. Tang, X.P. Li, H. Guo, Recent advances in application of graphitic carbon nitride-based catalysts for degrading organic contaminants in water through advanced oxidation processes beyond photocatalysis: a critical review. Water Res. 184, 116200–116217 (2020)
X.L. Liu, R. Ma, L. Zhuang, B.W. Hu, J.R. Chen, X.Y. Liu, X.K. Wang, Recent developments of doped g-C3N4 photocatalysts for the degradation of organic pollutants. Crit. Rev. Environ. Sci. Technol. 51, 751–790 (2020)
L. Chen, Y.M. Xu, Z. Yang, K. Zhang, B.L. Chen, Cobalt (II)-based open-framework systems constructed on g-C3N4 for extraordinary enhancing photocatalytic hydrogen evolution. Appl. Catal. B. 277, 119207–119215 (2020)
L. Zhou, H.Y. Zhang, H.Q. Sun, S.M. Liu, M.O. Tade, S.B. Wang, W.Q. Jin, Recent advances in non-metal modification of graphitic carbon nitride for photocatalysis: a historic review. Catal. Sci. Technol. 6, 7002–7023 (2016)
X.J. Zhou, C.L. Shao, X.H. Li, X.X. Wang, X.H. Guo, Y.C. Liu, Three dimensional hierarchical heterostructures of g-C3N4 nanosheets/TiO2 nanofibers: controllable growth via gas-solid reaction and enhanced photocatalytic activity under visible light. J. Hazard. Mater. 344, 113–122 (2018)
F.F. Mei, K. Dai, J.F. Zhang, W.Y. Li, C.H. Liang, Construction of Ag SPR-promoted step-scheme porous g-C3N4/Ag3VO4 heterojunction for improving photocatalytic activity. Appl. Surf. Sci. 488, 151–160 (2019)
S.P. Huang, Z.Q. Wei, X.J. Wu, J.W. Shi, Optical properties and theoretical study of Mn doped ZnAl2O4 nanoparticles with spinel structure. J. Alloys Compd. 825, 154004–154012 (2020)
J.H. Ma, Z.Q. Wei, L. Li, L. Ma, C. Li, S.P. Huang, Synthesis and photoelectrochemical properties of visible-light response g-C3N4@CdS heterojunctions photocatalyst. Desalin. Water. Treat. 231, 287–296 (2021)
S.P. Huang, Z.Q. Wei, M.J. Ding, C. Li, Q. Lu, Photo-electrochemical and photocatalytic properties of hierarchical flower-like BiOI/CoFe2O4 nanocomposites synthesized by co-precipitation method. Opt Mater. 111, 110643–110652 (2021)
M. Jourshabani, B.K. Lee, Z. Shariatinia, From traditional strategies to Z-scheme configuration in graphitic carbon nitride photocatalysts: recent progress and future challenges. Appl. Catal. B. 276, 119157–119184 (2020)
Y.A. Wang, Y.Q. Zeng, X.Y. Chen, Q.Y. Wang, S.P. Wan, D.Y. Wang, W. Cai, F.J. Song, S.L. Zhang, Q. Zhong, Tailoring shape and phase formation: Rational synthesis of single-phase BiFeWOx nanooctahedra and phase separated Bi2WO6-Fe2WO6 microflower heterojunctions and visible light photocatalytic performances. Chem. Eng. J. 351, 295–303 (2018)
N.Q. Thang, A. Sabbah, L.C. Chen, K.H. Chen, T. Minh, P.V. Viet, High-efficient photocatalytic degradation of commercial drugs for pharmaceutical wastewater treatment prospects: a case study of Ag/g-C3N4/ZnO nanocomposite materials. Chemosphere 282, 130971–130982 (2021)
W.H. Zhao, Z.Q. Wei, X.D. Zhang, M.J. Ding, S.P. Huang, S.G. Yang, Magnetic recyclable MnFe2O4/CeO2/SnS2 ternary nano-photocatalyst for photo-Fenton degradation. Appl. Catal. A-Gen. 593, 117443–117452 (2020)
Y.F. Jia, H.X. Ma, C.L. Liu, Au nanoparticles enhanced Z-scheme Au-CoFe2O4/MoS2 visible light photocatalyst with magnetic retrievability. Appl. Surf. Sci. 463, 854–862 (2019)
S.Q. Liu, Y.Y. Liu, G.P. Dai, X.Q. Bao, N. Huang, R. Peng, Y. Zhou, Synthesis and characterization of novel Bi2S3/BiOCl/g-C3N4 composite with efficient visible-light photocatalytic activity. Mater. Lett. 241, 190–193 (2019)
W.H. Zhao, Z.Q. Wei, X.D. Zhang, M.J. Ding, S.P. Huang, PH-controlled MnFe2O4@ SnS2 nanocomposites for the visible-light photo-Fenton degradation. Mater. Res. Bull. 124, 110749–110755 (2020)
Y.J. Wang, J.R. Jin, W.G. Chu, D. Cahen, T. He, Synergistic effect of charge generation and separation in epitaxially grown BiOCl/Bi2S3 nano-heterostructure. ACS Appl. Mater. Interfaces 10, 15304–15313 (2018)
Q. Hao, C. Xie, Y.M. Huang, D.M. Chen, Y.W. Liu, W. Wei, B.J. Ni, Accelerated separation of photogenerated charge carriers and enhanced photocatalytic performance of g-C3N4 by Bi2S3 nanoparticles. Chin. J. Catal. 41, 249–258 (2020)
T.P. Hu, K. Dai, J.F. Zhang, G.P. Zhu, C.H. Liang, One-pot synthesis of step-scheme Bi2S3/porous g-C3N4 heterostructure for enhanced photocatalytic performance. Mater. Lett. 257, 126740–126743 (2019)
J.D. Hu, D.Y. Chen, N.J. Li, Q.F. Xu, H. Li, J.H. He, J.M. Lu, Fabrication of graphitic-C3N4 quantum dots/graphene-InVO4 aerogel hybrids with enhanced photocatalytic NO removal under visible-light irradiation. Appl. Catal. B. 236, 45–52 (2018)
W.L. Shi, F. Guo, J.B. Chen, G.B. Che, X. Lin, Hydrothermal synthesis of InVO4/graphitic carbon nitride heterojunctions and excellent visible-light-driven photocatalytic performance for rhodamine B. J. Alloys Compd. 612, 143–148 (2014)
K. Saravanakumar, C.M. Park, Rational design of a novel LaFeO3/g-C3N4/BiFeO3 double Z-scheme structure: photocatalytic performance for antibiotic degradation and mechanistic insight. Chem. Eng. J. 423, 130076–130087 (2021)
R. Rajendran, S. Vignesh, A. Sasireka, P. Priya, S. Suganthi, V. Raj, J.K. Sundar, M. Srinivasan, S. AlFaify, Investigation on novel Cu2O modified g-C3N4/ZnO heterostructures for efficient photocatalytic dye degradation performance under visible-light exposure. Colloids Interface Sci. Commun. 44, 100480–100490 (2021)
E. Jang, D.W. Kim, S.H. Hong, Y.M. Park, T.J. Park, Visible light-driven g-C3N4@ZnO heterojunction photocatalyst synthesized via atomic layer deposition with a specially designed rotary reactor. Appl. Surf. Sci. 487, 206–210 (2019)
Z. Cui, H. Yang, X. Zhao, Enhanced photocatalytic performance of g-C3N4/Bi4Ti3O12 heterojunction nanocomposites. Mater. Sci. Eng. B. 229, 160–172 (2018)
M.T. Zhu, T.A. Kurniawan, F. Song, T. Ouyang, M.H.D. Othman, M. Rezakazemi, S. Shirazian, Applicability of BaTiO3/graphene oxide (GO) composite for enhanced photodegradation of methylene blue (MB) in synthetic wastewater under UV-vis irradiation. Environ. Pollut. 255, 113182–113191 (2019)
X.Q. Geng, S. Chen, X. Lv, W. Jiang, T.H. Wang, Synthesis of g-C3N4/Bi5O7I microspheres with enhanced photocatalytic activity under visible light. Appl. Surf. Sci. 462, 18–28 (2018)
X.Y. Weng, B.Q. Shi, A.N. Liu, J.Y. Sun, Y. Xiong, H.Q. Wan, S.R. Zheng, L. Dong, Y.W. Chen, Highly dispersed Pd/modified-Al2O3 catalyst on complete oxidation of toluene: role of basic sites and mechanism insight. Appl. Surf. Sci. 497, 143747–143757 (2019)
M. Pirhashemi, A. Habibi-Yangjeh, Simple and large scale one-pot method for preparation of AgBr-ZnO nanocomposites as highly efficient visible light photocatalyst. Appl. Surf. Sci. 283, 1080–1088 (2013)
H.N. Che, G.B. Che, H.J. Dong, W. Hu, H. Hu, C.B. Liu, C.M. Li, Fabrication of Z-scheme Bi3O4Cl/g-C3N4 2D/2D heterojunctions with enhanced interfacial charge separation and photocatalytic degradation various organic pollutants activity. Appl. Surf. Sci. 455, 705–716 (2018)
W.L. Shi, M.Y. Li, X.L. Huang, H.J. Ren, C. Yan, F. Guo, Facile synthesis of 2D/2D Co3(PO4)2/g-C3N4 heterojunction for highly photocatalytic overall water splitting under visible light. Chem. Eng. J. 382, 122960–122968 (2020)
K.D. Zhong, J.W. Feng, H.B. Gao, Y.M. Zhang, K.R. Lai, Fabrication of BiVO4@g-C3N4(100) heterojunction with enhanced photocatalytic visible-light-driven activity. J. Solid State Chem. 274, 142–151 (2019)
S. Li, Z.R. Wang, X.Y. Xie, G.W. Liang, X.W. Cai, X.L. Zhang, Z.W. Wang, Fabrication of vessel-like biochar-based heterojunction photocatalyst Bi2S3/BiOBr/BC for diclofenac removal under visible LED light irradiation: mechanistic investigation and intermediates analysis. J. Hazard. Mater. 391, 121407–121419 (2020)
N. Askari, M. Beheshti, D. Mowla, M. Farhadian, Synthesis of CuWO4/Bi2S3 Z-scheme heterojunction with enhanced cephalexin photodegradation. J. Photochem. Photobiol. A-Chem. 394, 112463–112476 (2020)
X. Zhang, J. Zhang, J.Q. Yu, Y. Zhang, F.K. Yu, L. Jia, Y.L. Tan, Y.M. Zhu, B.R. Hou, Enhancement in the photocatalytic antifouling efficiency over cherimoya-like InVO4/BiVO4 with a new vanadium source. J. Colloid Interface Sci. 533, 358–368 (2019)
X.S. Zhao, Y.Y. You, S.B. Huang, Y.X. Wu, Y.Y. Ma, G. Zhang, Z.H. Zhang, Z-scheme photocatalytic production of hydrogen peroxide over Bi4O5Br2/g-C3N4 heterostructure under visible light. Appl. Catal. B-Environ. 278, 119251–119261 (2020)
Y.J. Wang, X.J. Bai, C.S. Pan, J. He, Y.F. Zhu, Enhancement of photocatalytic activity of Bi2WO6 hybridized with graphite-like C3N4. J. Mater. Chem. 22, 11568–11573 (2012)
S.V.P. Vattikuti, P.C. Nagajyothi, J. Shim, Fabrication of CdS quantum dot/Bi2S3 nanocomposite photocatalysts for enhanced H2 production under simulated solar light. J. Mater. Sci. Mater. Electron. 30, 5681–5690 (2019)
X. Lin, X.Y. Guo, W.L. Shi, F. Guo, G.B. Che, H.J. Zhai, Y.S. Yan, Q.W. Wang, Ag3PO4 quantum dots loaded on the surface of leaf-like InVO4/BiVO4 heterojunction with enhanced photocatalytic activity. Catal. Commun. 71, 21–27 (2015)
C. Yang, Q.Y. Tan, Q. Li, J. Zhou, J.J. Fan, B. Li, J. Sun, K.L. Lv, 2D/2D Ti3C2 MXene/g-C3N4 nanosheets heterojunction for high efficient CO2 reduction photocatalyst: Dual effects of urea. Appl. Catal. B 268, 118738–118748 (2020)
M. Chen, C.S. Guo, S. Hou, J.P. Lv, Y. Zhang, H. Zhang, J. Xu, A novel Z-scheme AgBr/P-g-C3N4 heterojunction photocatalyst: excellent photocatalytic performance and photocatalytic mechanism for ephedrine degradation. Appl. Catal. B 266, 118614–118626 (2020)
C.Z. Zhu, T.T. Gong, Q.M. Xian, J.M. Xie, Graphite-like carbon nitride coupled with tiny Bi2S3 nanoparticles as 2D/0D heterojunction with enhanced photocatalytic activity. Appl. Surf. Sci. 444, 75–86 (2018)
Y.F. Li, R.X. Jin, X. Fang, Y. Yang, M. Yang, X.C. Liu, Y. Xing, S.Y. Song, In situ loading of Ag2WO4 on ultrathin g-C3N4 nanosheets with highly enhanced photocatalytic performance. J. Hazard. Mater. 313, 219–228 (2016)
N. Tian, H.W. Huang, Y. He, Y.X. Guo, T.R. Zhang, Y.H. Zhang, Mediator-free direct z-scheme photocatalytic system: BiVO4/g-C3N4 organic-inorganic hybrid photocatalyst with highly efficient visible-light-induced photocatalytic activity. Dalton Trans. 44, 4297–4307 (2015)
J.J. Xi, H. Wang, B.H. Zhang, F.Q. Zhao, B.Z. Zeng, Novel molecularly imprinted photoelectrochemical sensor for rutin based on Bi2S3/ZnIn2S4 heterojunction. Sens. Actuators B-Chem. 320, 128409–128416 (2020)
U. Lamdab, K. Wetchakun, S. Phanichphant, W. Kangwansupamonkon, N. Wetchakun, InVO4-BiVO4 composite films with enhanced visible light performance for photodegradation of methylene blue. Catal. Today 278, 291–302 (2016)
Y. Chen, K.R. Liu, Preparation and characterization of nitrogen-doped TiO2/diatomite integrated photocatalytic pellet for the adsorption-degradation of tetracycline hydrochloride using visible light. Chem. Eng. J. 302, 682–696 (2016)
Y.C. Deng, L. Tang, C.Y. Feng, G.M. Zeng, J.J. Wang, Y.Y. Zhou, Y.N. Liu, B. Peng, H.P. Feng, Construction of plasmonic Ag modified phosphorous-doped ultrathin g-C3N4 nanosheets/BiVO4 photocatalyst with enhanced visible-near-infrared response ability for ciprofloxacin degradation. J. Hazard. Mater. 344, 758–769 (2018)
M. Yasmina, K. Mourad, S.H. Mohammed, C. Khaoula, Treatment heterogeneous photocatalysis; factors influencing the photocatalytic degradation by TiO2. Energy Procedia 50, 559–566 (2014)
A. Nezamzadeh-Ejhieh, M. Karimi-Shamsabadi, Decolorization of a binary azo dyes mixture using CuO incorporated nanozeolite-X as a heterogeneous catalyst and solar irradiation. Chem. Eng. J. 228, 631–641 (2013)
R.P.F. Melo, E.L.B. Neto, M.C.P.A. Moura, T.N.C. Dantas, A.A.D. Neto, H.N.M. Oliveira, Removal of Reactive Blue 19 using nonionic surfactant in cloud point extraction. Sep. Purif. Technol. 138, 71–76 (2014)
C.Y. Jin, M. Wang, Z.L. Li, J. Kang, Y. Zhao, J. Han, Z.M. Wu, Two dimensional Co3O4/g-C3N4 Z-scheme heterojunction: mechanism insight into enhanced peroxymonosulfate-mediated visible light photocatalytic performance. Chem. Eng. J. 398, 125569–125582 (2020)
M. Humayun, Z.W. Hu, A. Khan, W. Cheng, Y. Yuan, Z.P. Zheng, Q.Y. Fu, W. Luo, Highly efficient degradation of 2,4-dichlorophenol over CeO2/g-C3N4 composites under visible-light irradiation: detailed reaction pathway and mechanism. J. Hazard. Mater. 364, 635–644 (2019)
F. Raziq, Y. Qu, X.L. Zhang, M. Humayun, J. Wu, A. Zada, H.T. Yu, X.J. Sun, L.Q. Jingo, Enhanced cocatalyst-free visible-light activities for photocatalytic fuel production of g-C3N4 by trapping holes and transferring electrons. J. Phys. Chem. C 120, 98–107 (2015)
C.J. Zhang, M.Y. Jia, Z.Y. Xu, W.P. Xiong, Z.H. Yang, J. Cao, H.H. Peng, H.Y. Xu, Y.P. Xiang, Y. Jing, Constructing 2D/2D N-ZnO/g-C3N4 S-scheme heterojunction: efficient photocatalytic performance for norfloxacin degradation. Chem. Eng. J. 430, 132652–132661 (2022)
M.L. Tang, Y.H. Ao, C. Wang, P.F. Wang, Facile synthesis of dual Z-scheme g-C3N4/Ag3PO4/AgI composite photocatalysts with enhanced performance for the degradation of a typical neonicotinoid pesticide. Appl. Catal. B 266, 118614–118626 (2020)
Acknowledgements
This work was supported by the Natural Science Foundation of Shanxi (201901D111068) and Key Research and Development (R&D) Projects of Shanxi Province (201803D31152).
Funding
The funded was provided by Natural Science Foundation of Tianjin Municipal Science and Technology Commission (Grant No: 201901D111068), Key Research and Development (R&D) Projects of Shanxi Province (Grant No: 201803D31152).
Author information
Authors and Affiliations
Contributions
YL: responsible for putting forward research ideas, designing research schemes, guiding, writing, editing, revising, and delivering paper. ST: responsible for doing some experiments, collecting and analyzing data, and writing paper. YM: responsible for the experiment and data analysis. YX: revising paper. LG: revising paper. WC: revising paper.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
The authors declare that the whole paper is original and related experiments have been carried out. The paper has not been submitted or published in other places, and it will not be submitted to other places until the program of periodical editorial department is completed. All the authors listed in the paper have agreed to sign their names and submit their papers for publication, and the order of authors has reached a consensus with all authors before submitting their papers.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, Y., Tan, S., Meng, Y. et al. Fabrication ternary dual Z-scheme InVO4/Bi2S3/g-C3N4 heterojunction photocatalyst for the highly efficient visible-light-driven degradation of Reactive Blue 19. J Mater Sci: Mater Electron 33, 13887–13904 (2022). https://doi.org/10.1007/s10854-022-08320-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-022-08320-8