Abstract
The present study focuses on pure and antimony (Sb)-doped tin oxide thin film and its influence on their structural, optical, and electrical properties. Both undoped and Sb-doped SnO2 thin films have been grown by using simple, inexpensive pyrolysis spray technique. The deposition temperature was optimized to 450 °C. X-ray diffractions pattern have revealed that the films are polycrystalline and have tetragonal rutile-type crystal structure. Undoped SnO2 films grow along (110) preferred orientation, while the Sb-doped SnO2 films grow along (200) direction. The size of Sb-doped tin oxide crystals changes from 26.3 to 58.0 nm when dopant concentration is changed from 5 to 25 wt%. The transmission spectra revealed that all the samples are transparent in the visible region, and the optical bandgap varies between 3.92 and 3.98 eV. SEM analysis shows that the surface morphology and grain size are affected by the doping rate. All the films exhibit a high transmittance in the visible region and show a sharp fundamental absorption edge at about 0.38–0.40 nm. The maximum electrical conductivity of 362.5 S/cm was obtained for the film doped with 5 wt% Sb. However, the carrier concentration is increased from 0.708 × 1018 to 4.058 × 1020 cm3. The electrical study reveals that the films have n-type electrical conductivity and depend on Sb concentration. We observed a decrease in sheet resistance and resistivity with the increase in Sb dopant concentration. For the dopant concentration of 5 wt% of Sb in SnO2, the Rs and ρ were found minimum with the values of 88.55 (Ω cm−2) and 2.75 (Ω cm), respectively. We observed an increase in carrier concentration and a decrease in mobility with the addition of Sb up to 25 wt%. The highest figure of merit values 2.5 × 10–3 Ω−1 is obtained for the 5wt% Sb, which may be considered potential materials for solar cells' transparent windows.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Transparent conducting oxide (TCO) films have gained a lot of interest owing to their various optoelectronic devices viz., gas sensors, solar cells, varistors, and flat-panel displays [1,2,3]. The TCO films have gained much interest specific to applications in solar cells. Among the TCO materials, Tin oxide makes the best choice because of the chemical inertness, mechanical strength, and heat-resistant nature of this material. The optical properties of any conducting material are governed by the energy bandgap between conduction and valence band, and this bandgap can be tweaked or modified by using various intrinsic or extrinsic dopants. These impurities may improve the free charge carriers, which is found to be important in adjusting optical transparency as well as electrical conductivity in the oxides. TCO material's electrical conductivity can be enhanced by decreasing the bandgap energy which is possible in two ways: either by (i) enhancing the number of free charge carriers via intrinsic imperfections, like oxygen vacancies, or (ii) extrinsic dopants, usually higher-valence metal cations [7]. Antimony is the material of choice for extrinsic doping of SnO2 owing to the non-toxic characteristic properties, it is also gaining versatility due to its cost-effectiveness in comparison to some commonly used dopants, such as indium (In) and fluorine (F) [4,5,6]. Sb:SnO2 thin film can be deposited using various approaches like spray pyrolysis, sol–gel coating, sputtering, and chemical vapor deposition [8,9,10,11,12,13].This study/work focuses on the experimental findings of structural, morphological, optical, and electrical parameters change in pristine SnO2 as well as Sb-doped SnO2 thin films. Simple and economical spray pyrolysis methods were employed to create thin films. In this research, antimony-doped tin dioxide (ATO) films were deposited on glass substrates at T = 550 °C through spray pyrolysis. Different weight percentages from 5% to 25% of Sb were doped in the SnO2.The effects of antimony doping on the structural, optical, and electrical properties of the thin films were investigated. Tin chloride (SnCl4_5H2O) and antimony chloride (SbCl3) were used as a host and a dopant precursor, respectively. X-ray diffraction (XRD) analysis indicated that the undoped SnO2 thin film exhibited a preferred (110) orientation. As the Sb doping concentration increased, a different preferred (200) orientation was observed. As the doping concentration increased, the average grain size initially increased and then decreased; electrical resistance initially decreased and subsequently increased; the carrier concentration and Hall mobility initially increased and then decreased. The optical bandgap of the thin films ranged from 3.92 to 3.98 eV. Hall effect and thermoelectric studies revealed that the films exhibited an n-type conductivity. The innovative part of this work is to bring change in optical and electrical properties of SnO2 by increasing the dopant concentration. We can make new materials with very good optical and electrical properties if we take into consideration materials like SnO2 with dopant concentration.
2 Experimental details
A chemical spray pyrolysis process was used to create Tin oxide (SnO2 and Sb:SnO2) and Sb-doped SnO2 thin films. Stannous chloride (0.2 M SnCl2⋅2H2O) was applied as a precursor which was dissolving in Methanol. Sb (antimony chloride, SbCl3) was dissolved in methanol separately and applied to SnCl2⋅2H2O precursor solution. The concentration of antimony was varied as 0.0, 5.0, 7.0, 15.0, and 25.0 wt% in the starting solution. The solution was kept on a magnetic stirrer to obtain the homogenous solution. Further, the glass substrates were rinsed with organic solvents like acetone, ethanol as well as deionized water in the ultrasonic bath before use. Fine droplets were formed from the spray liquid by passing it through a pyrolysis mechanism. Precursor’s droplets were poured out over the glass substrates. The substrate was kept at a distance of 25 cm from the spray nozzle. A substrate holder heated to a temperature of 450 °C was placed below the glass substrate. FTIR analysis was carried out using a PerkinElmer 2000 infrared spectrometer in the range of 500–2000 cm−1. Powder X-ray diffraction study was carried out using Bruker AXS-8D diffractometer with CuKα X-ray source to determine the crystal structure of SnO2 thin film. To examine the optical properties of deposited thin films, the optical transmission spectrometer of Horiba Jobin Yvon Spectrofluorometer (Fluoromax-4) was used. The morphology of the prepared samples were measured using SEM (JEOL JSM 6390LV). The sheet resistance of the films and the Hall effect data were determined using the van der Pauw configuration.
3 Results and discussions
3.1 Structural properties
3.1.1 XRD analysis
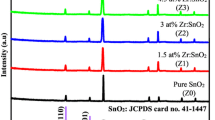
Figure 1 demonstrates the XRD analysis recorded for pristine SnO2 thin films and Sb-doped (Sb: 5, 7, 15, 25 wt%) deposited on glass substrates. It is usual for amorphous glass to have a diffractogram background of 10°–30° [14]. The undoped SnO2 thin film has a preferred to be (110) orientation. The (110) peak's intensity dropped in comparison to the (200) peaks as the quantity of Sb doping raised. Therefore, it can be concluded that the preferred orientation changes with Sb doping. Apart from (110) and (200) orientations, the existence of other peaks like (101), (211). (220), (310), and (301) were also noted with significant intensities for doped and undoped SnO2 films. The patterns of XRD do not show the Sb2O3 and Sb2O5 phases [10,11,12,13,14,15]. It has been found that the concentration of the preferred orientation increases with the rise in doping levels. Increases in the main (200) plane's intensity reveal that crystallinity rises as Sb doping levels rise [16], similar behavior/result was also reported by Elangovan et al. [16]. The tetragonal structure's lattice constants a and c were found using relation (1) [17]:
Using Scherrer's formula (2) [18], the films crystallite size D was determined from the XRD data. Here, β indicates the whole width at half-maximum of the high-intensity peak, λ signifies the X-rays wavelength used, k is the dimensionless shape factor whose value is close to unity, and θ indicates the Braggs’ angle in radians.
Table 1 presents the primary crystalline calculated parameters of the Sb:SnO2 thin films. Figure 2 clearly indicates that the sample undoped presented a much higher crystallite size value of 63 nm in each principal plane (110), (101), and (200) evaluated to the samples Sb-doped SnO2 thin films. The computed D value for undoped film decreases from 58.04 to 26.3 nm with 5 wt% Sb doping and then increases to 49.63 nm with 15 wt% Sb doping. The observed decrease in average crystallite size may be attributed to enhancing (200) orientation [19]. The grain size decreases again to 39.16 nm in doping level 25 wt%. Using Eq. (3) [20], the “texture coefficient” TC(hkl) for the planes is calculated, which provides information on the random or preferential development of polycrystalline thin films.
Here, I0(hkl) & I(hkl) represent the measured X-ray reflection intensity and associated standard intensity using JCPDS data card Number 41-1445, respectively. The number of reflections seen in the pattern of the XRD is denoted by M. The computed values of TC (hkl) are represented in Table 1. The films' polycrystalline composition is confirmed by the peaks that are less than unity. Nonetheless, the undoped film represented a possible oriented growth along the (110) plane direction. With an increase in the doping concentration, there occurs further growth-oriented along the (200) plane direction, as shown in (Fig. 3).
The microstrain (ε) produced in the SnO2 thin films was computed through Eq. (4): [17] Here, β indicates the whole width at half-maximum of the (101) peak and D signifies the crystallite size. The values of ε are shown in Table 1.
3.1.2 FTIR spectroscopic analysis
The “Fourier Transform Infrared” (FTIR) spectrum was recorded to monitor/detect any change in the functional groups from pristine SnO2 to Sb-doped SnO2 thin films. Figure 4 shows the FTIR spectrum of the SnO2 thin films as a dopant function (Sb) content (0.0, 5.0, 7.0, 15.0, and 25.0 wt%) in transmission mode. The physisorbed water molecules’ hydroxyl groups are linked to (OH-) groups on the SnO2 surface by weak hydrogen, as shown by the absorption peak at 3430 cm−1 [21, 22]. The bands found between 1080 and 970 cm−1 are caused by vibration of surface cation–oxygen bonds Sn=O as well as Sn–O, [23]. The weak adsorption at 1635 cm−1 is correlated with the deformation vibration for physisorbed water's H–O–H bonds. The usual M–O–M (in this example, Sn–O–Sn) vibrations are seen in the 530–770 cm−1 range [24].
3.2 Microstructure properties
3.2.1 Scanning electron microscopy (SEM) analysis
SEM descriptions of Sb:SnO2 thin film formed on a glass substrate with different Sb doping concentrations were taken (Fig. 5). Sb doping concentration has a significant impact on the film's surface morphology. As can be seen, the films are crack-free, continuous, and uniform with continuous grain distribution. It indicates that the sample has grains of various sizes [25, 26]. The presence of large grains suggests that the crystallites are designed using coalescence. The grains are randomly distributed, resulting in a dispersion effect that lowers transmittance. The grain size is reduced with rising the doping ratio which is in keeping with the XRD study that indicates the reduction of the crystallite size in the doped film. Figure 6 depicts the cross-sectional view of the SnO2 thin film having 7% by weight Sb concentration.
3.3 Optical properties
3.3.1 UV–vis analysis
Figure 7 indicates the change of transmittance concerning the wavelength of SnO2 thin films formed at different substrate temperatures. The visible region's average transmission ranges from 60 to 86%, based on concentration. A rise in the transmission is detected with the rise of Sb content. In the visible portion of the spectra, transmission is often fairly high (high enough to examine interference fringes). Low reflectivity and no (or little) absorption owing to optical interference effects allow for maximum thin-film transmission in a certain wavelength range [21]. A considerably greater transmittance of approximately 87% at 700 nm for both films 5 and 7 wt% Sb:SnO2 has been observed, which is vital for the applications of the Sb:SnO2 films as transparent conducting oxide (TCO). Figure 8 shows the (αhν)1/2 Tauc plot vs. Photon energy (hν) plot for pure SnO2 films and Urbach tail width. For indirect bandgap semiconductors, the Tauc formula (3) is used to compute the film bandgaps [27];
Here, α indicates the absorption coefficient, h represents Planck’s constant, B signifies constant, Eg denotes the energy bandgap, and ν indicates “incident photon frequency.” As shown in Table 2, the film's bandgap is increased from 3.87 to 3.92 eV, with the antimony doping ratio increasing from 5 to 7 wt% and then decreasing from 25 wt% to 3.92 eV value. The undoped SnO2 bandgap value is a good accord with the presented data in the literature; it is recognized that the optical bandgap of SnO2 films ranges from 3.4 to 4.6 eV [28,29,30,31]. The Burstein–Moss effect [32, 33] is responsible for the widening of the bandgap, as the concentration of free carriers increases. The low-energy photon absorption is due to the band tail states induced through film network disorder. The absorption coefficient in this range is determined by the following equation [34]:
here α0 indicates the pre-exponential factor, E00 denotes the disorder energy or band tail width called Urbach tail and hν signifies the photon energy [35]. E00 may be determined from the “inverse slope” of the linear plot of ln(α) vs. (hν).The fluctuations of the computed bandgap and the band tail width E00 with antimony doping levels are shown in Fig. 9 and Table 2. As could be observed, the fluctuations of bandgap and Urbach energies have opposite trends. That proposed that the disorder also controls the fluctuation of the optical gap in the film network.
3.3.2 Photoluminescence (PL) analysis
The PL spectra of SnO2 and Sb-doped SnO2 films are demonstrated in Fig. 10. The spectrum shows two emission peaks in the Ultraviolet. We observed that with the rise in Sb concentration, the PL peaks to higher wavelengths and reduces their intensity. The existence of the ultraviolet emission peak is owing to the electron's transition from the conduction band (CB) to holes in the valence band (VB) [36,37,38]. As shown in Fig. 10, the PL intensity falls as the Sb level rises and achieves a minimal value of 25 wt% for the Sb doping concentration. This decrease in intensity may be described by reducing the electron–hole combination rate and Sb’s new trapping energy level suppressing the electron–hole pairs, which enhances the photocatalytic efficiency [36]. However, at doping contents higher than 25 wt%, the PL intensity increases where the Sb doping turns into an electron–hole pairs recombination center and reduces the photocatalytic activity of the films [39, 40].
4 Electrical properties
4.1 Hall Effect
Different electrical properties like resistivity, mobility, as well as free carrier concentration of spray, deposited films of undoped as well as antimony-doped tin oxide, were assessed using Hall Effect tests, which are summarized in Table 3. The n-type film's conductivity was verified by the negative sign of the “Hall coefficient.” Figure 11 depicts the impact of Sb doping on the SnO2 films' mobility (μ) and carrier concentration (n). With a rise in Sb doping concentration, the film's carrier concentration rises, and its mobility decreases, as seen in the figure. While doped films have the lowest electrical resistivity, the undoped film's resistivity is still within acceptable limits when evaluated to the previously noted values. The low resistivity might be explained by the existence of a chlorine impurity as a dopant. In SnO2 lattices, Sb atoms are substituted for the Sn4+ atoms when Antimony is applied. Sb may exist in two distinct oxidation states in antimony-doped SnO2 films: Sb3+ and Sb5+. The Sb5+ replaced on Sn4+ sites operate as donor and generate surplus electrons in the first addition of Sb ([Sb]/[Sn]\(\le\) 5 weight percent) in the film [14, 41,42,43,44]. Therefore, increased carrier concentration and decreased resistivity are seen with Sb incorporation up to 5% by weight. A portion of the Sb5+ ions decrease to the Sb3+ beyond 5 wt% of Sb doping, leading to the creation of acceptor sites and the carrier’s loss [45,46,47]. The Sb3+ to Sb5+ reduction was confirmed by Kojima et al. [42] by estimating the lattice parameter of the doped SnO2 films. The ionic radius of Sn4+ is below that of Sb3+ but greater as compared to Sb5+, a rise in the SnO2 stage’s lattice parameter is noted over 5 wt% percent of Sb doping. The film's figure of merit (ϕ) plays a significant part in device application. The Haacke formula [32] is used to compute it:
Here, ‘T’ indicates the transmittance at λ = 550 nm (close to solar maximum) and ‘Rs’ represents the sheet resistance. The sheet resistance is computed with the Van der Pauw method [10]. Table 4 shows the transmittance values at 550 nm, sheet resistance, and computed figures of merits for the investigated films. It is obvious from Eq. (6) that the merit figure is dependent on the sheet resistance. As a consequence, the smaller the value of the figure of merit, the greater the sheet resistance, and vice versa. Furthermore, it is essential to mention that the figure of merit is influenced by the thickness of the film. Thus, the sheet resistance of a thicker film would be reduced, resulting in a greater figure of merit. Thus, the figure of merit is a useful tool for comparing optical and electrical characteristics [34]. It was discovered (Table 4) that the maximum merit is found for the film, which is noted in the case of 5 wt% Sb content (ϕ = 2.5 × 10–3(Ω sq−1)−1. This is possible owing to the creation of good film quality in terms of conductivity and transmittance. The noted (ϕ) values are in good accord with the related fluorine values (F:SnO2) [11], Antimony (Sb:SnO2) [48], and Niobium (Nb:SnO2)-doped SnO2 thin films [49]. The (Sb:SnO2) thin films with high transparency and conducting (namely figure of merit) show promise in a range of optoelectronic usages, particularly as flat-panel displays and solar cells window layers.
5 Conclusions
The pure and Sb-doped tin oxide films were prepared using spray pyrolysis technique. Investigations of doping of Sb on structural, morphological, optical, and electrical properties of SnO2 were studied successfully. X-ray diffraction confirmed tetragonal rutile crystalline phase of Sb-doped and undoped tin oxide. It was observed that the preferential orientation changes with the addition of Sb doping from (110) to (200). The electrical study reveals that the films have n-type electrical conductivity and depend upon Sb concentration. We observed a decrease in sheet resistance and resistivity with the increase in Sb dopant concentration.
Data availability
The data sets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
I. Saadeddin, B. Pecquenard, J.P. Manaud, R. Decourt, C. Labrugère, T. Buffeteau, G. Campet, Synthesis and characterization of single- and co-doped SnO2 thin films for optoelectronic applications. Appl. Surf. Sci. 253(12), 5240–5249 (2007)
R.S. Niranjan, Y.K. Hwang, D.K. Kim, S.H. Jhung, J.S. Chang, I.S. Mulla, Nanostructured tin oxide: synthesis and gas-sensing properties. Mater. Chem. Phys. 92(2–3), 384–388 (2005)
A.Z. Simões, A. Ries, E.C. Souza, L. Perazolli, M. Cilense, E. Longo, J.A. Varela, Investigation of electrical properties of tantalum doped SnO2 varistor system. Ceram. Int. 31(3), 399–404 (2005)
W. Yang, S. Yu, Y. Zhang, W. Zhang, Properties of Sb-doped SnO2 transparent conductive thin films deposited by radio-frequency magnetron sputtering. Thin Solid Films 542, 285–288 (2013)
A.A. Yadav, S.C. Pawar, D.H. Patil, M.D. Ghogare, Properties of (200) oriented, highly conductive SnO2 thin films by chemical spray pyrolysis from non-aqueousmedium: effect of antimony doping. J. Alloy. Compd. 652, 145–152 (2015)
A.R. Babar, S.S. Shinde, A.V. Moholkar, C.H. Bhosale, J.H. Kim, K.Y. Rajpure, Physical properties of sprayed antimony doped tin oxide thin films: the role of thickness. J. Semiconductors 32(5), 053001 (2011)
H. Bendjedidi, A. Attaf, H. Saidi, M.S. Aida, S. Semmari, A. Bouhdjar, Y. Benkhetta, Properties of n-type SnO2 semiconductorprepared by spray ultrasonic technique for photovoltaic applications. J. Semiconductors 36(12), 123002 (2015)
E. Kuantama, D.W. Han, Y.M. Sung, J.E. Song, C.H. Han, Structure and thermal properties of transparent conductive nanoporous F: SnO2 films. Thin Solid Films 517(14), 4211–4214 (2009)
B. Thangaraju, Structural and electricalstudies on highlyconducting spray deposited fluorine and Antimony doped SnO2 thin films from SnCl2 precursor. Thinsolid films 402(1–2), 71–78 (2002)
A. Rahal, A. Benhaoua, C. Bouzidi, B. Benhaoua, B. Gasmi, Effect of antimony doping on the structural, optical and electricalproperties of SnO2 thin films prepared by spray ultrasonic. Superlattices Microstruct. 76, 105–114 (2014)
B. Benhaoua, S. Abbas, A. Rahal, A. Benhaoua, M.S. Aida, Effect of film thickness on the structural, optical and electrical properties of SnO2: F thin films prepared by spray ultrasonic for solarcells applications. Superlattices Microstruct. 83, 78–88 (2015)
G. Gasparro, J. Pütz, D. Ganz, M.A. Aegerter, Parameters affecting the electrical conductivity of SnO2: Sb sol–gel coatings. Sol. Energy Mater. Sol. Cells 54(1–4), 287–296 (1998)
K.Y. Rajpure, M.N. Kusumade, M.N. Neumann-Spallart, C.H. Bhosale, Effect of Sb doping on properties of conductive spray deposited SnO2 thin films. Mater. Chem. Phys. 64(3), 184–188 (2000)
S. Gupta, B.C. Yadav, P.K. Dwivedi, B. Das, Microstructural, optical and electrical investigations of Sb-SnO2 thin films deposited by spray pyrolysis. Mater. Res. Bull. 48(9), 3315–3322 (2013)
J. Kim, B.J. Murdoch, J.G. Partridge, K. Xing, D.C. Qi, J. Lipton-Duffin et al., Ultrasonic spray pyrolysis of antimony-doped tin oxide transparent conductive coatings. Adv. Mater. Interfaces 7(18), 2000655 (2020)
E. Elangovan, K. Ramesh, K. Ramamurthi, Studies on the structural and electrical properties of spray deposited SnO2: Sb thin films as a function of substrate temperature. Solid State Commun. 130(8), 523–527 (2004)
A. Abdelkrim, S. Rahmane, O. Abdelouahab, N. Abdelmalek, G. Brahim, Effect of solution concentration on the structural, optical and electrical properties of SnO2 thin films prepared by spray pyrolysis. Optik 127(5), 2653–2658 (2016)
K. Derrar, M. Zaabat, I. Zerrouk, A. Hafdallah, N. Rouabah, B. Gasmi, Optical and structural properties of SnO2 thin films deposited by spray pyrolysis technique: effect of solution concentration. in Defect and Diffusion Forum (vol. 397, pp. 179–186, 2019)
C. Nassiri, A. Hadri, F.Z. Chafi, A. El Hat, N. Hassanain, M. Rouchdi et al., Structural, optical and electrical properties of Fe doped SnO2 prepared by spray pyrolysis. J. Mater. Environ. Sci 8(2), 420–425 (2017)
A. Benhaoua, A. Rahal, B. Benhaoua, M. Jlassi, Effect of fluorine doping on the structural, optical and electrical properties of SnO2 thin films prepared by spray ultrasonic. Superlattices Microstruct. 70, 61–69 (2014)
B. Benrabah, A. Bouaza, A. Kadari, M.A. Maaref, Impedance studies of Sb doped SnO2 thin film prepared by sol gel process. Superlattices Microstruct. 50(6), 591–600 (2011)
T. Bezrodna, G. Puchkovska, V. Shymanovska, J. Baran, H. Ratajczak, IR-analysis of H-bonded H2O on the pure TiO2 surface. J. Mol. Struct. 700(1–3), 175–181 (2004)
S. Lenaerts, J. Roggen, G. Maes, FT-IR characterization of tin dioxide gas sensor materials under working conditions. Spectrochim. Acta Part A 51(5), 883–894 (1995)
L.M. Fang, X.T. Zu, Z.J. Li, S. Zhu, C.M. Liu, W.L. Zhou, L.M. Wang, Synthesis and characteristics of Fe3+-doped SnO2 nanoparticles via sol–gel-calcination or sol–gel-hydrothermal route. J. Alloy Compd. 454(1–2), 261–267 (2008)
S.H. Saeedabad, G.S. Selopal, S.M. Rozati, Y. Tavakoli, G. Sberveglieri, From transparent conducting material to gas-sensing application of SnO2: Sb thin films. J. Electron. Mater. 47(9), 5165–5173 (2018)
S.Y. Lee, B.O. Park, Structural, electrical and optical characteristics of SnO2: Sb thin films by ultrasonic spray pyrolysis. Thins Solid Films 510(1–2), 154–158 (2006)
A. Hafdallah, K. Derrar, M.S. Aida, N. Attaf, Effet de la solution précurseur sur les propriétés structurales et optiques des couches minces de ZnO préparées par spray pyrolyse. Afr. Sci. 12(3), 26–33 (2016)
A. Adjimi, M.S. Aida, N. Attaf, Y.S. Ocak, Gadolinium doping effect on SnO2 thin films optical and electrical properties. Mater. Res. Express 6(9), 096405 (2019)
X. Feng, J. Ma, F. Yang, F. Ji, C. Luan, H. Ma, Structural and optical properties of SnO2 films grown on α-Al2O3 (0 0 0 1) by MOCVD. J. Cryst. Growth 310(2), 295–298 (2008)
A. Czapla, E. Kusior, M. Bucko, Optical properties of non-stoichiometric tin oxide films obtained by reactive sputtering. Thin Solid Films 182(1–2), 15–22 (1989)
A.E. Rakhshani, Y. Makdisi, H.A. Ramazaniyan, Electronic and optical properties of fluorine-doped tin oxide films. J. Appl. Phys. 83(2), 1049–1057 (1998)
E. Burstein, Anomalous optical absorption limit in InSb. Phys. Rev. 93(3), 632 (1954)
T.S. Moss, The interpretation of the properties of indium antimonide. Proc. Phys. Soc. Sect. B 67(10), 775 (1954)
F. Urbach, The long-wavelength edge of photographic sensitivity and of the electronic absorption of solids. Phys. Rev. 92(5), 1324 (1953)
I. Singh, R.K. Bedi, Studies and correlation among the structural, electrical and gas response properties of aerosol spray deposited self assembled nanocrystalline CuO. Appl. Surf. Sci. 257(17), 7592–7599 (2011)
H.J. Lin, T.S. Yang, M.C. Wang, C.S. Hsi, Structural and photodegradation behaviors of Fe3+-doping TiO2 thin films prepared by a sol–gel spin coating. J. Alloy Compd. 610, 478–485 (2014)
A.M. Selman, Z. Hassan, M. Husham, Structural and photoluminescence studies of rutile TiO2 nanorods prepared by chemical bath deposition method on Si substrates at different Ph values. Measurement 56, 155–162 (2014)
L. Kernazhitsky, V. Shymanovska, T. Gavrilko, V. Naumov, L. Fedorenko, V. Kshnyakin, J. Baran, Room temperature photoluminescence of anatase and rutile TiO2 powders. J. Lumin. 146, 199–204 (2014)
A.J. Freeman, K.R. Poeppelmeier, T.O. Mason, R.P.H. Chang, T.J. Marks, Chemical and thin-film strategies for new transparent conducting oxides. MRS Bull. 25(8), 45–51 (2000)
P.V. Bhuvaneswari, P. Velusamy, R.R. Babu, S.M. Babu, K. Ramamurthi, M. Arivanandhan, Effect of fluorine doping on the structural, optical and electrical properties of spray deposited cadmium stannate thin films. Mater. Sci. Semicond. Process. 16(6), 1964–1970 (2013)
C.A. Vincent, The nature of semiconductivity in polycrystalline tin oxide. J. Electrochem. Soc. 119(4), 515 (1972)
M. Kojima, H. Kato, M. Gatto, Blackening of tin oxide thin films heavily doped with Antimony. Philos. Mag. B 68(2), 215–222 (1993)
E. Elangovan, K. Ramamurthi, Studies on optical properties of polycrystalline SnO2: Sb thin films prepared using SnCl2 precursor. Cryst. Res. Technol. 38(9), 779–784 (2003)
E. Elangovan, K. Ramamurthi, Effect of substrate temperature on electrical and optical properties of spray deposited SnO2: Sb thin films. J. Optoelectron. Adv. Mater. 5(2), 415–420 (2003)
A.F. Carroll, L.H. Slack, Effects of additions to SnO2 thin films. J. Electrochem. Soc. 123(12), 1889 (1976)
I.S. Mulla, H.S. Soni, V.J. Rao, A.P.B. Sinha, Deposition of improved optically selective conductive tin oxide films by spray pyrolysis. J. Mater. Sci. 21(4), 1280–1288 (1986)
T.M. Hammad, N.K. Hejazy, Structural, electrical and optical properties of ATO thin films fabricated by dip coating method (2011)
A.R. Babar, K.Y. Rajpure, Effect of intermittent time on structural, optoelectronic, luminescence properties of sprayed antimony doped tin oxide thin films. J. Anal. Appl. Pyrolysis 112, 214–220 (2015)
G. Turgut, E.F. Keskenler, S. Aydın, E. Sönmez, S. Doğan, B. Düzgün, M. Ertuğrul, Effect of Nb doping on structural, electrical and optical properties of spray deposited SnO2 thin films. Superlattices Microstruct. 56, 107–116 (2013)
Acknowledgements
This research was funded by Princess Nourah bint Abdulrahman University Researchers Supporting Project Number (PNURSP2022R19), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia. Also, the author Dr Abualnaja appreciated Taif University Researchers Supporting Project Number (TURSP-2020/267), Taif University, Taif, Saudi Arabia.
Funding
This research was funded by Princess Nourah bint Abdulrahman University Researchers Supporting Project Number (PNURSP2022R19), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia. Also, the author Dr Abualnaja appreciated Taif University Researchers Supporting Project Number (TURSP-2020/267), Taif University, Taif, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
K D performed all the experimental work, collected and analyzed the data, and wrote the first draft of the manuscript. RN Approved the final version of the manuscript. MZ and RN revised the manuscript and approved the final version of the manuscript. NR, FH, AH, SAK, NSA, KMK, and KMA conceptualized and designed the work.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Derrar, K., Zaabat, M., Rouabah, N. et al. Preparation of Sb:SnO2 thin films and its effect on opto-electrical properties. J Mater Sci: Mater Electron 33, 10142–10153 (2022). https://doi.org/10.1007/s10854-022-08004-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-022-08004-3