Abstract
Lithium-rich manganese-based cathode materials have become one of the most concerned cathode materials for high-energy lithium-ion batteries. In order to improve its electrochemical performance, Li1.2Mn0.54Ni0.13Co0.13O2 with different content LiNbO3 coatings was synthesized by mechanical ball milling. The morphology, microstructure, and electrochemical properties of the samples were investigated by X-ray diffraction, scanning electron microscope, transmission electron microscope, galvanostatic charge/discharge, electrochemical impedance spectroscopy, and cyclic voltammetry. The results show that LiNbO3 coating not only protects the cathode material from the corrosion of electrolyte and HF but also improves the migration rate of Li+ in the interface region. Notably, the 5 wt% LiNbO3-coated Li1.2Mn0.54Ni0.13Co0.13O2 exhibits capacity retention of 89.9% under 0.1 C after 100 cycles. Besides, it has a higher discharge capacity than Li1.2Mn0.54Ni0.13Co0.13O2 at different rates. LiNbO3 coating is an effective way to improve its cycle stability and rate performance of Li1.2Mn0.54Ni0.13Co0.13O2.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Lithium-ion batteries have been widely used in portable devices, electric vehicles, and hybrid vehicles owing to high voltage, high capacity, good safety performance, and environmental friendliness [1–3]. The capacity and stability of cathode materials have become important factors limiting the further development of lithium-ion batteries [4, 5]. Lithium-rich manganese-based cathode material has attracted the interest of many researchers because of its high discharge capacity (> 250 mAh g−1), low cost, and environmental friendliness [6]. More important, it combines the stability of LiCoO2, high capacity of LiNiO2, and low cost of LiMnO2 [4, 7] and its good safety performance has attracted the attention of researchers.
However, there are still many problems with lithium-rich manganese-based cathode, such as low initial efficiency, rapid discharge voltage drop during cycling, poor thermal stability, and cycle performance [8]. The foremost reason for these shortcomings are due to its poor stability of layered structure during charge/discharge which changes from layered to spinel phase [9]. In order to solve these issues, the surface modification has been certified to be an availably way to protect the cathode materials surface from electrolyte erosion, which can significantly enhance the structural stability during cycling [10]. Zhou et al. synthesized Li2SiO3-coated Li1.2Mn0.54Ni0.13Co0.13O2 which greatly enhanced initial coulombic efficiency, cycle stability, and rate capability [11]. He et al. improved initial coulombic efficiency and cycle stability of Li1.2Mn0.54Ni0.13Co0.13O2 cathodes by SmPO4 as a coating material [12]. As a fast lithium-ion conductor, the conductivity of LiNbO3 is approximately 10−6 S cm−1, which can increase the diffusion rate of lithium ions during charge/discharge process. At the same time, LiNbO3 has better thermal and chemical stability.
In this study, Li1.2Mn0.54Ni0.13Co0.13O2 is coated with a layer of LiNbO3 particles. The LiNbO3 layer can serve two functions: (1) preventing the direct contact between Li1.2Mn0.54Ni0.13Co0.13O2 and the electrolyte, thereby reducing the corrosion of the cathode material and (2) reducing lithium-ion transfer barrier at the electrode–electrolyte interface. As a consequence, cycle stability and rate performance of Li1.2Mn0.54Ni0.13Co0.13O2 are obviously improved via LiNbO3 surface modification.
2 Experiment
2.1 Synthesis of LiNbO3
NbCl5 was dissolved in 35% H2O2 solution until completely dissolved, C2H3LiO2·2H2O and citric acid were slowly added and violently stirred, and then the solvent completely evaporated at 65 °C. The resulting colloidal substance was dried and ground. Finally, LiNbO3 (LNO) was obtained by calcination at 600 °C.
2.2 Synthesis of Li1.2Mn0.54Ni0.13Co0.13O2 and LiNbO3-coated Li1.2Mn0.54Ni0.13Co0.13O2
Stoichiometric amounts of MnSO4·H2O, NiSO4·6H2O, and CoSO4·7H2O (the molar ratio was 4:1:1) were dissolved in deionized water to form a mixed solution with a total metal concentration of 2 mol L−1. The mixed solution was added to the reactor through a peristaltic pump in nitrogen as a protective gas. At the same time, Na2CO3 solution was added to the reactor as a precipitant, and NH3·H2O solutions were used to adjust the pH around 7.5. After 12 h, the sediment was filtered, washed with deionized water, and dried. The as-obtained precursor Mn4/6Ni1/6Co1/6CO3 was mixed with Li2CO3 (5 mol% excess) and pre-sintered at 500 °C for 5 h and then calcined at 900 °C for 12 h to prepare Li1.2Mn0.54Ni0.13Co0.13O2 (LMNCO) material. The mixtures of LiNbO3 and Li1.2Mn0.54Ni0.13Co0.13O2 were milled via planet type ball mill and then sintered at 500 °C for 5 h. Different weight contents of LNO-coated LMNCO (1 wt% LNO-LMNCO, 3 wt% LNO-LMNCO, and 5 wt% LNO-LMNCO) were obtained.
2.3 Material characterization
X-ray diffraction (XRD, Cu-Ka, λ = 0.154056 nm) was applied to analyze phase composition and lattice structure of all samples at a voltage of 40 kV, a current of 30 mA, and the scanning 2θ range angle from 10° to 80°. Scanning electron microscope (SEM, Quanta FEI) and transmission electron microscope (TEM, FEI TecnaiG2 F20) were used to observe the morphology and microstructure of all samples. The content of dissolved Ni, Co, and Mn in the electrolyte was detected by Inductively coupled plasma mass spectrometry (ICP-MS).
2.4 Electrochemical measurements
The positive electrode material, acetylene black, and polyvinylidene fluoride were mixed in a mass ratio of 80:10:10 to prepare a positive electrode and coated on the aluminum foil. The anode and electrolyte were lithium metal piece and 1 M LiPF6 solution in the mixture of ethylene carbonate, ethylene methyl carbonate, and dimethyl carbonate (1/1/1 by volume ratio). The galvanostatic charge and discharge tests were implemented on LAND battery test system between 2.0 and 4.8 V (1 C = 250 mAh g−1). Autolab PGSTAT 128 N electrochemical workstation was used to measure cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) tests.
3 Results and discussion
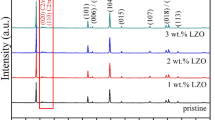
The XRD patterns of the samples are shown in Fig. 1. As shown in the figure, the patterns of as-synthesized LNO are consistent with standard LNO diffraction pattern (JCPDF#20-0631). Furthermore, the XRD patterns of all samples are indexed to a hexagonal α-NaFeO2 structure with R-3 m group space, implying that all samples have a typical layered structure [13, 14]. The minor diffraction peaks between 20° and 25° indicate the Li2MnO3-like unit cell with monoclinic (C2/m) symmetry, which is a typical feature of lithium-rich materials [15]. There are obvious split peaks between (006)/(012) and (018)/(110) peaks, which indicate that all samples have good crystallinity [16,17]. The Rietveld results for lattice parameters are listed in Table 1. In addition, the c/a ratio represented the order of cations in the layered structure [18]. The ratios of all samples were greater than 4.9, indicating a good ordered, layered structure. The 3 wt% LNO-LMNCO and 5 wt% LNO-LMNCO except for 1 wt% LNO-LMNCO clearly show standard patterns of LNO, which is due to low LNO content in 1 wt% LNO-LMNCO.
Figure 2 presents SEM images of LMNCO and 5 wt% LNO-LMNCO. Obviously, two samples appear as the micrometer sphere of the secondary particles. After LNO surface coating, the grain size of LMNCO and 5 wt% LNO-LMNCO has no obvious change. The biggest distinction is that the surface of LMNCO grains appears smooth, while the surface of LNO-LMNCO is relatively rougher than LMNCO. The results show that the rough material on the surface of LMNCO may be LNO particles.
The TEM images of the LMNCO and 5 wt% LNO-LMNCO are shown in Fig. 3, and the LMNCO particles have a smooth edge with clear interference fringes extending to margin. Furthermore, the measured interplanar spacing is 0.47nm, which is consistent with the (003) fringes of the layered LMNCO structure. Moreover, these stripes are uninterrupted and fairly straight, revealing a perfect layered structure. For 5 wt% LNO-LMNCO particles, the measured interplanar spacing is 0.21nm, which corresponds to the (202) fringes of the LiNbO3 structure. The results show that the surface of LMNCO has been successfully coated with a layer of LNO particles.
The galvanostatic charge/discharge curves are shown in Fig. 4a. In the first charge, all the samples exhibit a long potential plateau about 4.5 V. Due to the oxidation of Ni ions (Ni2+ to Ni4+) and Co ions (Co3+ to Co4+), there is a voltage plateau between 3.8 and 4.2 V in the first charging curve [19], which accompanies by Li+ deintercalation from the layered LiMO2 component. Due to the irreversible extraction of lithium ions and the release of oxygen in the Li2MnO3 region, and the subsequent formation of electrochemically active MnO2, there is a long voltage plateau near 4.5 V [9, 20]. As seen in Table 2, the discharge capacity and coulombic efficiency of LMNCO, 1 wt% LNO-LMNCO, 3 wt% LNO-LMNCO, and 5 wt% LNO-LMNCO are 260.0 mAh g−1 and 74.2%, 264.8 mAh g−1 and 75.0%, 278.1 mAh g−1 and 77.5%, and 284.9 mAh g−1 and 79.1%, respectively. This implies that the LNO-coating layer with high lithium-ion diffusion rate can improve coulombic efficiency of LMNCO.
Figure 4b shows the cycle performance of all samples under 1.0 C. The initial discharge capacity of LMNCO was 212.2 mAh g−1, while the capacity retention is only 70.9% after 100 cycles. While the discharge capacity and the capacity retentions of the LNO-modified samples are higher than that of LMNCO, the discharge capacity of 1wt%LNO-LMNCO, 3 wt% LNO-LMNCO, and 5 wt% LNO-LMNCO reaches 217.1, 218.3, and 218.9 mAh g−1, respectively, corresponding to 82.4%, 86.9%, and 89.9% of the capacity retention rate. These results indicate that the LNO layer can prevent the direct contact of LMNCO with the electrolyte and stabilize the cathode crystal structure during cycling.
The 3rd, 10th, 25th, 50th, 75th, and 100th discharge curves of all samples are shown in Fig. 5. It can be observed that the discharge curves of all samples shift to a lower voltage platform. LMNCO exhibits severe voltage attenuation, which means greater polarization. However, LNO coating can reduce voltage attenuation of LMNCO, indicating a higher volume retention rate and a smaller polarization effect. The results show that LNO coating can effectively improve cycle stability of LMNCO.
In order to demonstrate that LNO-coating LMNCO can prevent HF corrosion, the same masses of LMNCO and 5 wt% LNO-LMNCO are added to the same volume of electrolyte. We test the content of dissolved Ni, Co, and Mn in the electrolyte by ICP-MS, and the results are shown in Table 3. Obviously, the 5 wt% LNO-LMNCO dissolves less Ni, Co, and Mn than LMNCO, which demonstrates that LNO coating can protect the cathode material from the corrosion of HF.
The rate performance of all samples at various current densities is shown in Fig. 6. It can be seen that the LNO-coated LMNCO presents better rate capacity than LMNCO during the whole testing operating. Table 4 shows the average discharge capacity of all samples at various rates. The average discharge capacity of LMNCO at 0.1, 0.2, 0.5, 1, 2, and 0.1 C is 259.8, 235.7, 220.8, 210.7, 135.1, and 239.8 mAh g−1, respectively. Notably, the corresponding values of 5 wt% LNO-LMNCO increase to 285.1, 250.2, 231.5, 218.1, 156.8, and 272.4 mAh g−1. The superior rate performance for the LNO layer can increase the diffusion rate of lithium ions on the surface.
The initial three cyclic voltammetry (CV) curves of LMNCO and 5 wt% LNO-LMNCO at a scan rate of 0.1 mV s−1 are shown in Fig. 7. In initial cycle, the oxidation peaks of LMNCO and 5 wt% LNO-LMNCO are related to the oxidation of Ni (Ni2+ to Ni4+) and Co (Co3+ to Co4+) at ~ 4.0 V (vs. Li+/Li). The peak at ~ 4.6 V is mainly related to the irreversible reaction that strips Li2O from the Li2MnO3 component to form MnO2. Due to the reduction of Mn4+, a reduction peak appears at ~ 3.2 V to balance the oxygen vacancy charge caused by the loss of oxygen in initial charge [21]. In the second scan, the other peaks near 2.88 and 2.61 V may be due to the oxidation and reduction process of Mn ions in the spinel composition between +3 and +4 states [22,23]. In subsequent scans, the reversible redox reaction of Mn component can still be observed under 3.5 V. The overlap of the curves after coating is better than that of LMNCO, which proves to have better reversibility.
The EIS test is implemented in order to deeply study the influence of lithium-ion diffusion coefficient on rate performance. The Nyquist plots of the LMNCO and 5wt%LNO-LMNCO are made up of two parts: a straight line in low-frequency region and a semicircle in high-frequency region. The high-frequency semicircle is connected with the ohmic resistance of the electrode (Rs) and the charge transfer resistance (Rct) is connected with the intermediate frequency semicircle [24–26]. The illustration in Fig. 8a shows the equivalent circuit model. Table 5 shows the fitted impedance data. The linear relationship between Z′ and σ is shown in Fig. 8b. Eq. (1) is used to estimate the DLi+:
The ideal gas constant and Faraday constant are R (8.314J K−1) and F (96485 C mol−1), respectively. The surface area of the cathode and absolute temperature are defined as A (cm2) and T (298 K). The number of transferred electrons and the concentration of lithium ions are represented by n and C. There is a linear relationship between Z′ and ω−1/2, and its slope is related to the Warburg factor (σ), which was related to the real impedance (Z′) in Eq. (2) [27]. The 5 wt% LNO-LMNCO has a smaller Rct value than pure LMNCO. Lithium-ion diffusion coefficients of 9.40 × 10−14 and 1.15 × 10−13 cm2 S−1 were obtained for LMNCO and 5 wt% LNO-LMNCO, respectively. The results apply that the introduction of LNO into the LMNCO samples can decrease the resistance of interfaces and improve lithium ions diffusion rate, thus, achieving better rate performance.
The electrochemical performances of LMNCO are coated by various substances previously reported in the literature are shown in Table 6. Obviously, overall electrochemical performance of LNO-LMNCO is better than that of lithium-rich cathode materials coated by various materials. Especially, LNO-LMNO has higher cycle stability and capacity retention.
4 Conclusions
In summary, the electrochemical properties of LMNCO are greatly improved by LNO-coating modification. The LNO-coating layer improves the diffusion rate of lithium ions, and availability inhibits the side reaction between electrode and electrolyte, which contributes to improving rate capacity and cycle stability of LMNCO. It is found through testing that the optimum LNO content is 5%. The 5 wt% LNO-LMNCO has a highest discharge capacity (284.9 mAh g−1) and coulombic efficiency (79.09%) under 0.1 C. Besides, it reveals a higher capacity retention of 89.9% (vs. 70.9% of LMNCO) with a high discharge capacity of 180.2 mA h g−1 (vs. 149.3 mA h g−1 of LMNCO) after 100 cycles under 1.0 C. Therefore, the surface modification with LNO is an effective way to improve the electrochemical properties of LMNCO.
References
M.H. Sun, S.Z. Huang, L.H. Chen, Y. Li, X.Y. Yang, Z.Y. Yuan, B.L. Su, Applications of hierarchically structured porous materials from energy storage and conversion, catalysis, photocatalysis, adsorption, separation, and sensing to biomedicine. Chem. Soc. Rev. 45, 3479–3563 (2016)
W.D. Li, B.H. Song, A. Manthiram, High-voltage positive electrode materials for lithium-ion batteries. Chem. Soc. Rev. 46, 3006–3059 (2017)
L. Kang, M. Zhang, J. Zhang, S. Liu, N. Zhang, W. Yao, Y. Ye, C. Luo, Z. Gong, C. Wang, X. Zhou, X. Wu, S.C. Jun, Dual-defect surface engineering of bimetallic sulfide nanotubes towards flexible asymmetric solid-state supercapacitors. J. Mater. Chem. A 8, 24053–24064 (2020)
L. Kang, C. Huang, J. Zhang, M. Zhang, N. Zhang, S. Liu, Y. Ye, C. Luo, Z. Gong, C. Wang, X. Zhou, X. Wu, S.C. Jun, Effect of fluorine doping and sulfur vacancies of CuCo2S4 on its electrochemical performance in supercapacitors. Chem. Eng. J. 390(2020)
S. Liu, L. Kang, J. Hu, E. Jung, J. Zhang, S.C. Jun, Y. Yamauchi, Unlocking the potential of oxygen-deficient copper-doped Co3O4 nanocrystals confined in carbon as an advanced electrode for flexible solid-state supercapacitors. ACS Energy Lett. 6, 3011–3019 (2021)
M.M. Thackeray, S.H. Kang, C.S. Johnson, J.T. Vaughey, R. Benedek, S.A. Hackney, Li2MnO3-stabilized LiMO2 (M = Mn, Ni, Co) electrodes for lithium-ion batteries. J. Mater. Chem. 17, 3112–3125 (2007)
H. Arai, S. Okada, Y. Sakurai, J. Yamaki, Reversibility of LiNiO2 cathode. Solid State Ion. 95, 275–282 (1997)
M. Gu, I. Belharouak, J.M. Zheng, H.M. Wu, J. Xiao, A. Genc, K. Amine, S. Thevuthasan, D.R. Baer, J.G. Zhang, N.D. Browning, J. Liu, C.M. Wang, Formation of the spinel phase in the layered composite cathode used in Li-ion batteries. ACS Nano 7, 760–767 (2013)
D. Mohanty, S. Kalnaus, R.A. Meisner, K.J. Rhodes, J.L. Li, E.A. Payzant, D.L. Wood, C. Daniel, Structural transformation of a lithium-rich Li1.2Co0.1Mn0.55Ni0.15O2 cathode during high voltage cycling resolved by in situ X-ray diffraction. J. Power Sources 229, 239–248 (2013)
M.K. Shobana, Metal oxide coated cathode materials for Li ion batteries—a review. J. Alloys Compd. 802, 477–487 (2019)
L. Zhou, Y.N. Wu, J. Huang, X. Fang, T. Wang, W.M. Liu, Y. Wang, Y. Jin, X.C. Tang, Enhanced electrochemical performance of Li1.2Mn0.54Ni0.13Co0.13O2 cathode material coated with Li+-conductive Li2SiO3 for lithium ion batteries. J. Alloys Compd. 724, 991–999 (2017)
L. He, J.M. Xu, T. Han, H. Han, Y.J. Wang, J. Yang, J.R. Wang, W.K. Zhu, C.J. Zhang, Y.H. Zhang, SmPO4-coated Li1.2Mn0.54Ni0.13Co0.13O2 as a cathode material with enhanced cycling stability for lithium ion batteries. Ceram. Int. 43, 5267–5273 (2017)
Y. Li, C. Wu, Y. Bai, L. Liu, H. Wang, F. Wu, N. Zhang, Y.F. Zou, Hierarchical mesoporous lithium-rich LiLi0.2Ni0.2Mn0.6O2 cathode material synthesized via ice templating for lithium-ion battery. ACS Appl. Mater. Interfaces 8, 18832–18840 (2016)
K. Redel, A. Kulka, A. Plewa, J. Molenda, High-performance Li-rich layered transition metal oxide cathode materials for Li-ion batteries. J. Electrochem. Soc. 166, A5333–A5342 (2019)
R.Z. Yu, Z.J. Zhang, S. Jamil, J.C. Chen, X.H. Zhang, X.Y. Wang, Z.H. Yang, H.B. Shu, X.K. Yang, Effects of nanofiber architecture and antimony doping on the performance of lithium-rich layered oxides: enhancing lithium diffusivity and lattice oxygen stability. ACS Appl. Mater. Interfaces 10, 16561–16571 (2018)
Y.B. Cao, X.Y. Qi, K.H. Hu, Y. Wang, Z.G. Gan, Y. Li, G.R. Hu, Z.D. Peng, K. Du, Conductive polymers encapsulation to enhance electrochemical performance of Ni-rich cathode materials for Li-ion batteries. ACS Appl. Mater. Interfaces 10, 18270–18280 (2018)
G.R. Hu, X.Y. Qi, K.H. Hu, X.W. Lai, X. Zhang, K. Du, Z.D. Peng, Y.B. Cao, A facile cathode design with a LiNi0.6Co0.2Mn0.2O2 core and an AlF3-activated Li1.2Ni0.2Mn0.6O2 shell for Li-ion batteries. Electrochim. Acta 265, 391–399 (2018)
S.L. Pang, M. Zhu, K.J. Xu, X.Q. Shen, H.R. Wen, Y.J. Su, G.M. Yang, X. Wu, S.W. Li, W.Z. Wang, X.M. Xi, H.B. Wang, Enhanced electrochemical performance of Li1.2Mn0.54Ni0.13Co0.13O2 via L-ascorbic acid-based treatment as cathode material for Li-ion batteries. J. Electrochem. Soc. 165, A1897–A1902 (2018)
L. Li, B.H. Song, Y.L. Chang, H. Xia, J.R. Yang, K.S. Lee, L. Lu, Retarded phase transition by fluorine doping in Li-rich layered Li1.2Mn0.54Ni0.13Co0.13O2 cathode material. J. Power Sources 283, 162–170 (2015)
X.D. Xiang, W.S. Li, Self-directed chemical synthesis of lithium-rich layered oxide Li Li0.2Ni0.2Mn0.6O2 with tightly interconnected particles as cathode of lithium ion batteries with improved rate capability. Electrochim. Acta 127, 259–265 (2014)
Q.Y. Wang, J. Liu, A.V. Murugan, A. Manthiram, High capacity double-layer surface modified LiLi0.2Mn0.54Ni0.13Co0.13O2 cathode with improved rate capability. J. Mater. Chem. 19, 4965–4972 (2009)
F. Wu, N. Li, Y.F. Su, L.J. Zhan, L.Y. Bao, J. Wang, L. Chen, Y. Zheng, L.Q. Dai, J.Y. Peng, S. Chen, Ultrathin spinel membrane-encapsulated layered lithium-rich cathode material for advanced Li-ion batteries. Nano Lett. 14, 3550–3555 (2014)
J.C. Zhang, R. Gao, L.M. Sun, Z.Y. Li, H. Zhang, Z.B. Hu, X.F. Liu, Understanding the effect of an in situ generated and integrated spinel phase on a layered Li-rich cathode material using a non-stoichiometric strategy. Phys. Chem. Chem. Phys. 18, 25711–25720 (2016)
T.F. Yi, Y.M. Li, S.Y. Yang, Y.R. Zhu, Y. Xie, Improved cycling stability and fast charge-discharge performance of cobalt-free lithium-rich oxides by magnesium-doping. ACS Appl. Mater. Interfaces 8, 32349–32359 (2016)
T.-F. Yi, X. Han, S.-Y. Yang, Y.-R. Zhu, Enhanced electrochemical performance of Li-rich low-Co Li1.2Mn0.56Ni0.16Co0.08–xAlxO2 (0≤x≤0.08) as cathode materials. Sci. China Mater. 59, 618–628 (2016)
T.-F. Yi, B. Chen, Y.-R. Zhu, X.-Y. Li, R.-S. Zhu, Enhanced rate performance of molybdenum-doped spinel LiNi0.5Mn1.5O4 cathode materials for lithium ion battery. J. Power Sources 247, 778–785 (2014)
S.Y. Yang, X.Y. Wang, X.K. Yang, Y.S. Bai, Z.L. Liu, H.B. Shu, Q.L. Wei, Determination of the chemical diffusion coefficient of lithium ions in spherical LiNi0.5Mn0.3Co0.2O2. Electrochim. Acta 66, 88–93 (2012)
C.S. Xu, H.T. Yu, C.F. Guo, Y. Xie, N. Ren, T.F. Yi, G.X. Zhang, Surface modification of Li1.2Mn0.54Ni0.13Co0.13O2 via an ionic conductive LiV3O8 as a cathode material for Li-ion batteries. Ionics 25, 4567–4576 (2019)
S.Q. Yang, P.B. Wang, H.X. Wei, L.B. Tang, X.H. Zhang, Z.J. He, Y.J. Li, H. Tong, J.C. Zheng, Li4V2Mn(PO4)4-stablized LiLi0.2Mn0.54Ni0.13Co0.13O2 cathode materials for lithium ion batteries. Nano Energy 63(2019)
J. Duan, W. Tang, R. Wang, X. Tang, J. Li, M. Tang, P. Li, Inhibited voltage decay and enhanced electrochemical performance of the Li-rich layered Li1.2Mn0.54Ni0.13Co0.13O2 cathode material by CeAlOδ surface coating modification. Appl. Surf. Sci. 521(2020)
L. Zhao, Y. Sun, K. Song, F. Ding, Enhanced electrochemical performance of Li-rich Li[Li0.2Mn0.52Ni0.13Co0.13V0.02]O2 cathode materials for lithium ion batteries by Li1.13Mn0.47Ni0.2Co0.2O2 coating. Ionics 26, 4455–4462 (2020)
C. Chen, T.F. Geng, C.Y. Du, P.J. Zuo, X.Q. Cheng, Y.L. Ma, G.P. Yin, Oxygen vacancies in SnO2 surface coating to enhance the activation of layered Li-Rich Li1.2Mn0.54Ni0.13Co0.13O2 cathode material for Li-ion batteries. J. Power Sources 331, 91–99 (2016)
X.D. Zhang, J.J. Hao, L.C. Wu, Z.M. Guo, Z.H. Ji, J. Luo, C.G. Chen, J.F. Shu, H.M. Long, F. Yang, A.A. Volinsky, Enhanced electrochemical performance of perovskite LaNiO3 coating on Li1.2Mn0.54Ni0.13Co0.13O2 as cathode materials for Li-ion batteries. Electrochim. Acta 283, 1203–1212 (2018)
Y. Liu, Z. Yang, J. Zhong, J. Li, R. Li, Y. Yu, F. Kang, Surface-functionalized coating for lithium-rich cathode material to achieve ultra-high rate and excellent cycle performance. ACS Nano 13, 11891–11900 (2019)
Y.X. Hao, F.N. Yang, D.D. Luo, J.H. Tian, Z.Q. Shan, Improved electrochemical performances of yttrium oxyfluoride-coated LiLi0.2Mn0.54Ni0.13Co0.13O2 for lithium ion batteries. J. Energy Chem. 27, 1239–1246 (2018)
W.X. Zhang, Y.T. Liu, J.L. Wu, H.X. Shao, Y.F. Yang, Surface modification of Li1.2Mn0.54Ni0.13Co0.13O2 cathode material with Al2O3/SiO2 composite for lithium-ion batteries. J. Electrochem. Soc. 166, A863–A872 (2019)
X. Nie, Z. Xu, L. Chen, J. Li, J. Cheng, Q. Sun, Y. Qiao, X. Xu, Y. Zhang, D. Li, R. Peng, L. Ci, Enhanced electrochemical performance of Li1.2[Mn0.54Co0.13Ni0.13]O2 enabled by synergistic effect of Li1.5Na0.5SiO3 modification. Adv. Mater. Interfaces 7, 2000378 (2020)
F. Yang, S.Z. Lin, Z.M. Guo, Y.R. Shao, B. Zhang, X.D. Zhang, S.H. Yan, A.A. Volinsky, Suppressed voltage decay and improved electrochemical performance by coating LiAl5O8 on the surface of Li1.2Mn0.54Ni0.13Co0.13O2. J. Alloys Compd. 805, 1034–1043 (2019)
Y.X. Zuo, B. Huang, C.M. Jiao, R.G. Lv, G.C. Liang, Enhanced electrochemical properties of LiLi0.2Mn0.54Ni0.13Co0.13O2 with ZrF4 surface modification as cathode for Li-ion batteries. J. Mater. Sci.-Mater. Electron. 29, 524–534 (2018)
C. Song, W. Feng, Z. Shi, Z. Huang, Coating TiO2 on lithium-rich Li1.2Mn0.54Ni0.13Co0.13O2 material to improve its electrochemical performance. Ionics 27, 457–468 (2020)
C.D. Li, Z.L. Yao, J. Xu, P. Tang, X. Xiong, Surface-modified LiLi0.2Mn0.54Ni0.13Co0.13O2 nanoparticles with LaF3 as cathode for Li-ion battery. Ionics 23, 549–558 (2017)
Funding
This work received support from the Chongqing Technology Innovation and Application Development project of Chongqing Science and Technology Commission (No.cstc2019jscx-msxmX0358), the Key Project of Science and Technology Research of Chongqing Education Commission of China (No.KJZDK201801103), the Scientific and Technological Research Foundation of Chongqing Municipal Education Commission (No.KJQN201901110), the Venture & Innovation Support Program for Chongqing Overseas Returnees (No.cx2019128), and the General program of Chongqing Natural Science Foundation (No.cstc2019jcyj-msxmX0165).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, J., Wu, K., Xu, C. et al. LiNbO3-coated Li1.2Mn0.54Ni0.13Co0.13O2 as a cathode material with enhanced electrochemical performances for lithium-ion batteries. J Mater Sci: Mater Electron 32, 28223–28233 (2021). https://doi.org/10.1007/s10854-021-07199-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-021-07199-1