Abstract
In this paper, we have investigated the effect of annealing temperature on the structural, optical, and Mössbauer properties of nanocrystalline (NC) nickel ferrites (NFOs) synthesized by the sol–gel auto-combustion method. The NFOs were characterized by X-Ray diffraction (XRD), Raman spectroscopy, Diffusion reflectance spectroscopy (DRS), and Mössbauer spectroscopy techniques. The XRD results show that the average crystallite size increases from 27.5 to 54.3 nm when increasing the annealing temperature from 200 to 1000 °C. The Ultraviolet–Visible Diffuse Reflectance Spectroscopy (UV-DRS) measurement is used to find the optical band gap observed between 1.92 and 1.75 eV for NFOs annealed at 200 and 1000 °C, respectively. The Mössbauer study confirmed that the structure transforms from mixed spinel to inverse spinel structure when moving to higher annealing temperature.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
During the last few decades, nanocrystalline (NC) spinel ferrites having structural formula AFe2O4 (where A = Mn, Fe, Co, Ni, Zn, Mg, etc.) are the most studied material due to their wide technological and medical applications like the microwave devices, magnetic memory devices, drug delivery, recording media, gas sensors, biosensors, photo-catalysis, magnetic resonance, electrodes, converters, ferroelectric devices, magnetic disks, phase shifters, etc. [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19]. Among all the spinel ferrites, nickel ferrites (NFOs) have been investigated widely due to their peculiar properties like high chemical stability, catalytic behavior, ferromagnetic behavior, high resistivity, and low coercivity [2,3,4,5,6,7, 13,14,15,16,17,18]. Nickel ferrites have an inverse spinel structure with space group Fd3m where Fe3+ ions are equally distributed in between the octahedral and tetrahedral site and Ni2+ ion occupies an octahedral site which may be represented as (Fe3+)T(Ni2+Fe3+)OO2−4 [3, 5, 7, 14]. In recent years, several methods have been employed to synthesize nanocrystalline or nanoparticle NFOs like hydrothermal, sol–gel, solid-state reaction, chemical co-precipitation, plasma deposition, high-energy ball milling, microwave processing, etc. [9,10,11,12,13,14,15,16,17]. Among these methods, the sol–gel auto-combustion method is an excellent technique to synthesize low-cost, high-purity, homogeneous, and ultrafine nanoparticle powder. It is well known that the factors that affect the physical and chemical properties of nanocrystalline ferrites are the synthesis procedure, cation distribution, annealing temperature, surface spin, etc.
The electrochemical performance of Ni-ferrite as a supercapacitor electrode has been evaluated by Patil et al. and their utility for energy storage applications is highlighted [2]. Wang et al. have studied the photocatalytic activities of NiFe2O4 3D nanosphere prepared by Glycerol-assisted microwave solvothermal method [4]. The authors found that the 3D NFO has a high degradation ability which can rapidly degrade organic pollutants. The magnetic and biological properties of NiFe2O4 nanoparticles prepared by the polymeric precursor method have been evaluated by Santos et al. [5]. They explore the possibility of S-NiFe2O4 nanoparticles as drug delivery agents in targeted cancer therapy. Pottker et al. [7] have investigated the effect of structural order–disorder on the optical and magnetic properties of NiFe2O4 nanoparticles synthesized by the co-precipitation method and calcined in the ambient atmosphere at 700, 800, 900, and 1000 °C temperatures. The synthesis of porous NiFe2O4 microspheres by the one-step solvothermal method has been reported by Zhang et al. [16]. The authors show that NiFe2O4 microspheres have promising applications as high-performance acetone gas sensors. Majid et al. have investigated the magnetic, structural, and dielectric properties of NiFe2O4 prepared by hydrothermal and sol–gel routes [20]. The results show that the synthesis method affects NiFe2O4 properties; therefore, a suitable method can be used for the desired properties. Baig et al. have synthesized NiFe2O4/SiO2 nanostructure material for high-rated supercapacitors electrode [21]. Mandal et al. [22, 23] have detailed morphology studies on NiFe2O4 to enhance their microwave applications. Nanocrystalline NiFe2O4 is a soft material with low coercivity and saturation magnetization. Senthilkumar et al. have synthesized sub-microns size NiFe2O4 particles by the molten salt technique at 900 °C with different weight ratios of NaCl:KCl. The values of saturation magnetization (MS) were found to be increased from 31.58 to 44.19 emu/g, whereas the value of coercivity (HC) was decreased from 246.7 to 86.7 Oe with increasing the NaCl:KCl ratio from 4:0 to 0:4 [24]. Gao et al. have studied metal–organic frameworks (MOFs)-derived magnetic porous carbon microspheres constructed by core–shell Ni@C structure and found similar magnetic properties like NiFe2O4 naoparticles [25]. The values of MS and HC were found between 21–25 emu/g and 19.5–26.3 Oe, respectively [25].
In the present study, nanocrystalline nickel ferrites were synthesized by the sol–gel method following different annealing temperatures. The structural, optical, and Mössbauer properties of annealed nanocrystalline nickel ferrites were evaluated and correlated with crystallite size, lattice parameter, etc. The Mössbauer spectroscopy measurements assessed the effect of annealing temperature on the cations distribution.
2 Experimental work
Nanocrystalline (NC) nickel ferrites (NFOs) were prepared by the sol–gel method using SRL high-purity nickel nitrate (purity ≥ 99%) and ferric nitrate (purity ≥ 98%), and citric acid (purity ≥ 99.7%) as starting material and fuel, respectively. The raw material for NFOs was prepared by dissolving stoichiometric amounts of the metal nitrates and citric acid in the same molar ratio (1:1). The raw materials solution was stirred at 50 °C for half an hour using a magnetic agitator with a hot plate. After that, the temperature was raised to 100 °C to get the gel form of a solution which produces powder of NFOs by achieving the auto-combustion. This powder was ground in an agate mortar and then annealed at different temperatures i.e., 200 (hereafter referred to as NFO-01), 400 (referred as NFO-02), 600 (referred as NFO-03), 800 (referred as NFO-04), and 1000 °C (referred as NFO-05) for 6 h.
The crystal structure of annealed NFOs was confirmed by using Cu-Kα lines (wavelength λ = 1.540 Å) of Rigaku Ultima IV X-Ray diffractometer (XRD) with a step size of 0.02° in the 2θ range between 20 and 70°. The Confocal Raman spectrometer (STR-500) with 5 mW optimized laser power of 532 nm DPSS laser was carried out to study the electronic properties of annealed NFOs. The optical properties of annealed NFOs were carried out using UV–Vis DRS spectroscopy measurement in the wavelength range 200–800 nm. The room temperature Mössbauer spectra of NFOs were recorded in transmission geometry using a constant acceleration mode Mössbauer spectrometer with 57Co source in Rh matrix of 5 mCi strength. High-purity 57Fe metal foil was used for velocity calibration. The Win-Normos site fitting program was performed to fit all the Mössbauer spectra.
3 Results and discussion
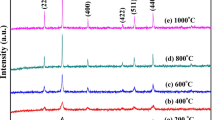
The XRD patterns for nanocrystalline NFOs synthesized via the sol–gel method and annealed at a different temperature from 200 to 1000 °C are shown in Fig. 1a. The intensity peaks of the XRD pattern confirm the formation of the single phase cubical spinel structure of nickel ferrite with space group Fd3m, which is in good agreement with the JCPDS card 74-2081. The crystallite size (D) was calculated using the Debye–Scherrer formula as in the following equation [26, 27]:
a X-ray diffraction pattern of nanocrystalline Ni-ferrite annealed at different temperature i.e., 200 (NFO-01), 400 (NFO-02), 600 (NFO-03), 800 (NFO-04), and 1000 °C (NFO-05). b Variation in crystallite size (D) and surface area (S) with increasing the annealing temperature. c Variation in strain (ξ) with band gap energy (Eg) with increasing the annealing temperature
where λ the wavelength of X-Ray and β is broadening of the peak (311) at a diffraction angle (θ), while the theoretical X-Ray density (dx) and dislocation density (δ) were calculated using the following equations [27]:
where M is the compound’s molecular weight and NA and a are Avogadro’s number and the lattice parameter, respectively. The crystallite size calculated using the most intense peak and the lattice constant, density, and strain are tabulated in Table 1. The XRD result confirms the formation of nanocrystalline material. The XRD results show that the crystallinity increases with the annealing temperature as the intensity of the most intense diffraction peak increases and peak width decrease with increasing annealing temperature which shows an enhancement in the phase crystallinity of annealed NFOs [28,29,30,31,32,33,34,35,36]. The crystallite size increases from ~ 28 to 54 nm with an increase in annealing temperature from 200 to 1000 °C, respectively, while strain decreases with an increase in annealing temperature (Fig. 1b, c). Almessiere et al. have studied [Ni0.4Cu0.2Zn0.4](Fe2-xTbx)O4(x ≤ 0.1) nanospinel ferrites and found the crystallite size to be increased from 12 to 19 nm, whereas there is no such trend in the average particle size and it fluctuated between 19 and 70 nm [37]. The small particles combined to form larger grain boundaries under the re-crystallization process (reduce interparticle distance) as increasing annealing temperature decreases the lattice strain and defects (indicates an improvement in the crystallinity). The slight variation in lattice parameters with increasing annealing temperature may be due to ordering in unit cells. The present pattern/trend of XRD results are in good agreement with the earlier reported data [28, 29].
In mostly cubic spinel ferrites XRD data, the peaks (220) and (422) belong to tetrahedral sites (8a), while the peak at (222) belongs to octahedral sites (16d) and we can get the cationic distribution ratio by using these peaks intensity or area. Thus, the ratios I220/I222 and I220/I222 are an indication of the A- and B-sites occupation. The experimental relative intensities I220/I222 and I422/I222 of NiFe2O4 as a function of annealing temperature are given in Table 1. The observed values (I220/I222) lie between 1.67 and 3.93, and show an increase with the sintering temperature. The observed values of I220/I222 are compatible with the reported values (0.8–3.6) [38]. The increase in the I220/I222 ratio with annealing temperature indicates that the cation concentration in the A-site increases when compared to that of the B-site, this suggests the formation of a inverse spinel structure from a mixed spinel structure.
The room temperature Raman spectroscopy measurement has been carried out to interpret vibrational and structural properties of nanocrystalline NFOs [28,29,30,31, 39]. The Lorentzian line shape least square-fitted Raman spectra of NFOs in the range of 160–800 cm−1 are shown in Fig. 2. The five or more active Raman modes in the Factor group analysis confirm the spinel structure formation of NFOs [28, 29]. The measured Raman modes of NFOs are tabulated in Table 2. Earlier reports on Raman spectroscopy analysis of spinel ferrites show that Raman modes in the region 660–720 cm−1 and 460–640 cm−1 explored the nature of the tetrahedral and octahedral sites [28,29,30,31]. The symmetric stretching vibrations of the Metal (M)—Oxygen (O) bond at the tetrahedral site creates A1g mode, while Eg mode is produced by the symmetric bending of the M–O bond. The T2g(2) and T2g(3) modes are due to the asymmetric stretching of M–O ions and bending of oxygen ion, respectively, at the octahedral site, while the translation movement of the metal ion together with four oxygen atoms at the tetrahedral site attribute to T2g(1) mode. A double-peak-like structure of A1g mode (splitting of A1g mode into doublet like peak) in all NFOs may be due to the distribution of Fe/Ni–O bond length (as ionic radii of Fe and Ni ions are different), where one peak corresponds to the Fe–O bond and the other one is due to the mixing of Fe and Ni ions band with oxygen. Thus, Raman spectroscopy analysis confirms the formation of mixed spinel structure of NFOs. The present result shows that Raman mode shifts toward high wavenumber as increasing the annealing temperature, indicating that mixed spinel structure moves toward the inverse spinel structure with increasing the annealing temperature.
To understand the influence of annealing temperature on the optical properties of the nanocrystalline NFOs, the Ultraviolet–Visible Diffuse Reflectance Spectroscopy (UV–Vis DRS) measurement has been performed. The UV–Vis DRS results are shown in Fig. 3, which shows a significant change in absorbance spectra with the annealing temperature. The spectroscopy data were analyzed using the Kubelka Munk function using the reflectance and absorption data such as follows[6, 39,40,41,42,43,44]:
where F(R) is the Kubelka–Munk function, α is the absorption coefficient, and R is the reflection coefficient. The energy bandgap of all the NC NFOs is calculated using the Tauc equations such as the following[6, 43, 44]:
where hυ, n, Eg, and A are photon energy, index of the optical transition, bandgap energy, and constant depending on the material, respectively. The index (n) value could be 2 or 1/2 depending on the direct or indirect optical transition, respectively. Figure 4 shows the energy bandgap for all annealed NC NFOs. Our results show that the bandgap of NFO-05 (1.75 eV) is narrower than that of NFO-01 (1.92 eV). The estimated band values of NFO-01, NFO-02, NFO-03, NFO-04, and NFO-05 were 1.92, 1.85, 1.81, 1.75, and 1.75 eV, respectively. This shows that the energy band of nanocrystalline NFOs has shifted toward the lower energy side (redshift) with increasing the annealing temperature. It is found that the energy bandgap value can be affected by various factors like annealing temperature, preparation method, doping, crystallite size, etc. [39,40,41,42,43,44,45]. Researchers have reported that the band gap energy decreases with an increase in annealing temperature [41, 46]. The decrease in bandgap values with increasing annealing temperature may be due to the grain growth (re-crystallization process) and the presence of the defects. Our result also shows similar behavior for annealed nanocrystalline NFOs.
Mössbauer spectra for annealed nanocrystalline NFOs were recorded at room temperature using a Mössbauer spectrometer operated in constant acceleration mode (triangular wave) in transmission geometry shown in Fig. 5 and the analysis results are given in the Table S1. The Mössbauer spectra of NFO-01 and NFO-02 are fitted with three sextets, whereas Mössbauer spectra of NFO-03, NFO-04, and NFO-05 are fitted with two sextets. The two sextets are originated due to the tetrahedral and octahedral environment of Fe ions, while the third sextet (with less hyperfine field area) in NFO-01 and NFO-02 may be due to the canted Fe3+ spin ions due to comparatively small particles [18, 34, 36, 47, 48]. The various hyperfine interaction parameters like isomer shift (δ), quadrupole splitting (∆) magnetic hyperfine field (Hf), relative area (RA), outer line width of sextets (Г), and average outer line width of sextets (< Г >) obtained from Mössbauer spectra and the variation in these parameters with annealing temperature are shown in Fig. 6a–e, respectively. Earlier studies show that the room temperature Mössbauer spectra of NFOs nanocrystalline are typically a superposition of quadrupole doublet and magnetic sextets [18, 48]. In contrast, in the present study, we have observed only sextets. Our results show that the intensity of sextet’s increases and the average values of outer line width (< Γ >) absorption spectra become sharper with increasing the annealing temperature (Fig. 6e). This sharpness is due to the increasing crystallite size and distribution of Ni2+ and Fe3+ ions at B-site creating a hyperfine field at A-site. The isomer shift (δ) for octahedral Fe3+ ions (sextet B) is more than that for the tetrahedral Fe3+ ions which shows that Fe3+ ions are more covalent at the tetrahedral site [49,50,51,52].
The value of isomer shift of Fe3+ ions at A- and B-sites remains nearly constant as increasing annealing temperature which shows a small migration of Ni ions from A-site to B-site (Fig. 6a). The values of isomer shift for the tetrahedral site are found between δ = 0.23–0.26 mm/s and for the octahedral site they are found between δ = 0.34–0.68 mm/s with respect to α-Fe (δ = 0.0 mm/s) foil. The range of isomer shift values indicates Fe ions are in Fe3+ valence state with high spin state configuration [53,54,55,56].
The magnetic hyperfine field of the A-site is less than that of the B-site may be due to the covalent nature of the tetrahedral site. The relative area ratio under the resonance curve of Mössbauer spectra is used to determine the Fe sites occupancy and cations distribution in the prepared samples [48, 57]. Cation distribution derived from Mössbauer study of nanocrystalline NFOs heated at different temperatures is shown in Table 3. Our result indicates that the Ni concentration at B-site increases as increasing annealing temperature. This demonstrates that nanocrystalline NFOs structure transforms from mixed spinel to inverse spinel structure when moving to higher annealing temperature. The sample annealed at 1000 °C is showing a pure inverse spinel structure. Thus Mössbauer study supports Raman spectra results.
4 Conclusions
The influence of annealing temperature on the structural, optical, and Mössbauer properties of nanocrystalline NFOs synthesized via the sol–gel method has been investigated in this study. The XRD results revealed that the value of crystallite size increases from 28 to 54 nm with increase in annealing temperature. The Raman spectra of nanocrystalline NFOs confirm the formation of spinel structure with five Raman active modes. The Raman mode shifts toward high wavenumber as increasing the annealing temperature indicates that mixed spinel structure moves toward the inverse spinel structure with increasing the annealing temperature. Using UV-DRS spectra, the optical bandgap energy of nanocrystalline NFOs was calculated between ranges 1.75 to 1.92 eV. Results show that the energy band shifted toward the lower energy side (redshift) to increase the annealing temperature. In Mössbauer spectra, the value of isomer shift of Fe3+ ions at sites A and B remains nearly constant with increasing annealing temperature, which shows a minimal migration of Ni ions from A-site to B-site which indicates structure transformation from mixed spinel to inverse spinel as increasing annealing temperature.
References
T.V. Sagar, T.S. Rao, K.C.B. Naidu, Ceram. Int. 46, 11515 (2020)
P.D. Patil, S.R. Shingte, V.C. Karade, J.H. Kim, T.D. Dongale, S.H. Mujawar, A.M. Patil, P.B. Patil, J. Energy Storage 40, 102821 (2021)
D.K. Dinkar, B. Das, R. Gopalan, B.S. Dehiya, Mater. Chem. Phys. 218, 70 (2018)
Y. Wang, H. Wang, Y. Yang, B. Xin, Ceram. Int. 47, 14594 (2021)
J.G. Santos, H. Lopes, H. Moreno, M.A. Ramirez, F.G. Garcia, A.Z. Simoes, Ceram. Int. 47, 16152 (2021)
K. Kombaiah, J.J. Vijaya, L.J. Kennedy, K. Kaviyarasu, Mater. Chem. Phys. 221, 11 (2019)
W.E. Pottker, R. Ono, M.A. Cobos, A. Hernando, J.F.D.F. Araujo, A.C.O. Bruno, S.A. Lourenco, E. Longo, F.A.L. Porta, Ceram. Int. 44, 17290 (2018)
Y. Pan, Y. Zhang, X. Wei, C. Yuan, J. Yin, D. Cao, G. Wang, Electrochim. Acta 109, 89 (2013)
K.C.B. Naidu, W. Madhuri, Mater. Chem. Phys. 187, 164 (2017)
Z.V. Mocanu, M. Airimioaei, C.E. Ciomaga, L. Curecheriu, F. Tudorache, S. Tascu, A.R. Iordan, N.N.M. Palamaru, L. Mitoseriu, J. Mater. Sci. 49, 3276 (2014)
K.C.B. Naidu, S.R. Kiran, W. Madhuri, Mater. Res. Bull. 89, 125 (2017)
T.A. Taha, A.A. Azab, M.A. Sebak, J. Mol. Struct. 1181, 14 (2019)
M.A. Almessiere, Y. Slimani, S. Güner, A. Baykal, I. Ercan, J. Rare Earths 37, 871 (2019)
S.V. Bhosale, P.S. Ekambe, S.V. Bhoraskar, V.L. Mathe, Appl. Surf. Sci. 441, 724 (2018)
X.F. Wang, W. Ma, F. Jiang, E.S. Cao, K.M. Sun, L. Cheng, X.Z. Song, Chem. Eng. J. 338, 504 (2018)
S. Zhang, W. Jiang, Y. Li, X. Yang, P. Sun, F. Liu, X. Yan, Y. Gao, X. Liang, J. Ma, G. Lu, Sensors Actuators B Chem. 291, 266 (2019)
J. Jia, L. Qu, Z. Luo, Z. Wu, S. Jiang, X. Wu, B. Zhang, J. Xie, J. Li, J. Yang, J. Environ. Chem. Eng. 4, 2865 (2016)
K. El-Sayed, M.B. Mohamed, S. Hamdy, S.S. Ata-Allah, J. Magn. Magn. Mater. 423, 291 (2017)
M.Y. Rafique, M. Ellahi, M.Z. Iqbal, Q. Javed, L. Pan, Mater. Lett. 162, 269 (2016)
F. Majid, J. Rauf, S. Ata, I. Bibi, A. Malik, S.M. Ibrahim, A. Ali, M. Iqbal, Mater. Chem. Phys. 258, 123888 (2021)
M.M. Baig, E. Pervaiz, M. Azad, Z. Jahan, M.B.K. Niazi, S.M. Baig, Ceram. Int. 47, 12557 (2021)
D. Mandal, A. Gorai, K. Mandal, J. Magn. Magn. Mater. 485, 43 (2019)
D. Mandal, M. Alam, K. Mandal, Phys. B Condens. Matter 554, 51 (2019)
B. Senthilkumar, R.K. Selvan, P. Vinothbabu, I. Perelshtein, A. Gedanken, Mater. Chem. Phys. 130, 285 (2011)
S. Gao, G. Zhang, Y. Wang, X. Han, Y. Huang, P. Liu, J. Mater. Sci. Tech. 88, 56 (2021)
R. Verma, A. Chauhan, K.M. Neha, R. Batoo, M. Kumar, M. Hadhi, E.H. Raslan, Ceram. Int. 47, 3680 (2021)
M. Irfan, N. Dogan, A. Bingolbali, F. Aliew, J. Magn. Magn. Mater. 537, 168150 (2021)
S. Dhaka, S. Kumar, K. Poonia, V. Singh, K. Dhaka, H.S. Mund, J. Mater. Sci. Mater. Electron. 32, 16392 (2021)
P. Prajapat, S. Dhaka, H.S. Mund, J. Elec. Mater. 50, 4671 (2021)
R.S. Yadav, J. Havlica, J. Masilko, L. Kalina, J. Wasserbauer, M. Hajdúchová, V. Enev, I. Kuřitka, Z. Kožáková, J. Magn. Magn. Mater. 394, 439 (2015)
Z. Cvejic, E. Ðurdic, G.I. Ivandekic, B. Bajac, P. Postolache, L. Mitoseriu, V.V. Srdic, S. Rakic, J. Alloys Compd. 649, 1231 (2015)
A.R. Nitika, Mater. Today Proceed. 45, 5444 (2021)
S.V. Bhandare, R. Kumar, A.V. Anupama, H.K. Choudhary, V.M. Jali, B. Sahoo, Ceram. Int. 46, 17400 (2020)
N.A.S. Nogueira, V.H.S. Utuni, Y.C. Silva, P.K. Kiyohara, I.F. Vasconcelos, M.A.R. Miranda, J.M. Sasaki, Mater. Chem. Phys. 163, 402 (2015)
T. Dippong, E.A. Levei, C. Leostean, O. Cadar, J. Alloys Compd. 868, 159203 (2021)
G. Chandra, R.C. Srivastava, V.R. Reddy, H.M. Agrawal, J. Magn. Magn. Mater. 427, 225 (2017)
M.A. Almessiere, Y. Slimani, B. Unal, T.I. Zubar, A. Sadaqat, A.V. Trukhanov, A. Baykal, J. Mater. Res. Technol 9, 10608 (2020)
J.H. Liu, L. Wang, F.S. Li, J. Mater. Sci. 40, 2573 (2005)
S.K. Paswan, S. Kumari, M. Kar, A. Singh, H. Pathak, J.P. Borah, L. Kumar, J. Phys. Chem. Solids 151, 109928 (2021)
A. Manikandan, J.J. Vijaya, L.J. Kennedy, M. Bououdina, J. Mol. Struct. 1035, 332 (2013)
A. Loganathan, K. Kumar, Appl. Nanosci. 6, 629 (2016)
M.A.S. Amulya, H.P. Nagaswarupa, M.R.A. Kumar, C.R. Ravikumar, S.C. Prashantha, K.B. Kusuma, Appl. Surf. Sci. Adv. 1, 100023 (2020)
T. Tangcharoen, J.T. Thienprasert, C. Kongmark, J. Adv. Ceram. 8, 352 (2019)
A.K.H. Bashir, N. Matinise, J. Sackey, K. Kaviyarasu, I.G. Madiba, L. Kodseti, F. Ezema, M. Maaza, Physica E 119, 114002 (2020)
S. Joshi, M. Kumar, S. Chhoker, G. Srivastava, M. Jewariya, V.N. Singh, J. Mol. Struct. 1076, 55 (2014)
S. Swathi, R. Yuvakkumar, P.S. Kumar, G. Ravi, D. Velauthapillai, Chemosphere 281, 130903 (2021)
M.V. Ushakov, B. Senthilkumar, R. Kalai Selvan, I. Felner, M.I. Oshtrakh, Mater. Chem. Phys. 202, 159 (2017)
A. Ahlawat, V.G. Sathe, V.R. Reddy, A. Gupta, J. Magn. Magn. Mater. 323, 2049 (2011)
S.S. Shinde, S.S. Meena, S.M. Yusuf, K.Y. Rajpure, J. Phys. Chem. C 115, 3731 (2011)
M. Hashim, S.S. Meena, R.K. Kotnala, S.E. Shirsath, A.S. Roy, A. Parveen, P. Bhatt, S. Kumar, R.B. Jotania, R. Kumar, J. Alloys Compd. 602, 150 (2014)
A. Mitra, J. Mohapatra, S.S. Meena, C.V. Tomy, M. Aslam, J. Phys. Chem. C. 118, 19356 (2014)
H.N. Chaudhari, P.N. Dhruv, C. Singh, S.S. Meena, S. Kavita, R.B. Jotania, J. Mater. Sci. Mater. Electron. 31, 18445 (2020)
K. Sharma, S. Singh, C.L. Prajapat, S. Bhattacharya, M.R. Jagannath, M.R. Singh, S.M. Yusuf, G.P. Kothiyal, J. Magn. Magn. Mater. 321, 3821 (2009)
K. Sharma, Dixit, S. Singh, S. Jagannath, C.L. Bhattacharya, P.K. Prajapat, S.M. Sharma, A.K. Yusuf, A.K. Tyagi, G.P. Kothiyal, J. Mater. Sci. and Eng. C 29, 2229 (2009)
K. Sharma, C.L. Prajapat, S.S. Meena, M.R. Singh, S.M. Yusuf, L. Montagne, G.P. Kothiyal, J. Magn. Magn. Mater. 345, 24 (2013)
K. Sharma, S.S. Meena, S. Saxena, S.M. Yusuf, A. Srinivasan, G.P. Kothiyal, Mater. Chem. Phys. 133, 144 (2012)
M. Hashim, S.S. Meena, R.K. Kotnala, S.E. Shirsath, P. Bhatt, S. Kumar, E. Şentürk, R. Kumar, N. Gupta, Alimuddin, J. Magn. Magn. Mater. 360, 21 (2014)
Acknowledgements
The authors are thankful to the Head, Department of Physics M.L. Sukhadia University, Udaipur for providing XRD facility and MRC, MNIT Jaipur for Raman spectroscopy measurements and also grateful to Prof. L. K. Kennedy Department of Physics, VIT Chennai for providing DRS facility. One of us (HSM) is grateful to Science and Engineering Research Board (SERB), New Delhi for proving grant under Core Research Grant Scheme (CRG/2020/002826). The authors are thankful to Dr. Vidyadhar Singh, Dept. of Physics, J P University, Chapra for his support during the revision.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mund, H.S., Prajapat, P., Dhaka, S. et al. Impact of annealing temperature on structural, optical, and Mössbauer properties of nanocrystalline NiFe2O4. J Mater Sci: Mater Electron 32, 27232–27242 (2021). https://doi.org/10.1007/s10854-021-07089-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-021-07089-6