Abstract
The effect of light Ni-substituted Manganese on the physical properties of La0.2Sr0.8MnO3 perovskite prepared by the standard solid-state reaction method was investigated. Field Emission Scanning Electron microscopy confirms the particle size composition and grain boundaries in these samples. Moreover, the presence of all the chemical elements with atomic ratios coordinating with the general formula La0.2Sr0.8Mn1−xNixO3 (x = 0.0 and 0.1) was confirmed by Energy-dispersive X-ray spectroscopy. X-ray diffractions analyses indicate that both compounds have a rhombohedral structure with an R3C space group, for which the cell parameter increases with nickel addition. A competition between ferromagnetic and antiferromagnetic moments is evidenced at room temperature for both compounds by measuring the magnetization versus magnetic field. Saturation magnetization and the corresponding residual magnetization decrease with the Ni substitution; however, the coercive field increases. These changes are correlated with those evidenced by structural properties. Conductance measurements as a function of frequency from 102 Hz to 5 MHz are analyzed in the temperature range 300 K–420 K in order to evidence the conduction mechanism.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Due to their variety of applications, manganite perovskites materials are recently devoted with continuous structural, magnetic, and electronic investigations. These materials exhibit various electric and magnetic phenomena depending on the composition of each element, such as ferromagnetic, antiferromagnetic, charge, and orbital ordering [1]. The properties of these materials are principally derived from the double-exchange interaction Mn3+–O–Mn4+ [2]. Consequently, they can drive excellent magneto-transport properties, based on both ferromagnetic and antiferromagnetic complexes allowing their use as active layer in spintronics devices [3] and sensors [4]. Indeed, the discovery of colossal magneto-resistance (CMR) [5] in these manganites represents a starting point for benefitting technological applications such as sensors for magnetometry applications [6], storage media [7], and supercapacitors [8]. These exotic applications are based on the competition between spin and freedom lattice degree [9].
Among these manganites, SrMnO3 perovskite has been used as active layers for humidity monitoring applications due to its low cost, low contamination impact, and good stability of the resistivity and environmental factors [10]. The SrMnO3 has been also extended for super-capacitor prototype with higher cycling stability [8]. Moreover, a combination of optoelectronic, magnetic, and thermoelectric study with DFT calculations supports its applications for UV visible optical devices [11].
Scientific communities try continuously to understand the mechanism in manganite materials formation in order to improve their properties which can lead to their use in specific applications. Consequently, much attention has been devoted to explain the fundamental mechanisms combining their magnetic and transport properties. To improve physical functionalities, we often proceed by a substitution ABO3 in A or B sites with a series of alkaline earth or rare earth metal ions. Particularly, the substitution induces change in the Curie temperature and saturation magnetization [12], due to the fact that the rare earth substitution leads to a change of valence states of manganese which plays a core role in the transport properties. The quaternary La1−xSrxMnO3 has been classified among the materials with excellent CMR effects. The increase of the Sr concentration more than 0.5 leads to some change due to the straightness of the bonds Mn–O–Mn [9]. The ferromagnetism of these materials is essentially derived from structural properties such as oxygen vacancies [13], double-exchange interaction Mn3+/Mn4+, and the strain. Moreover, the simple substitution of Ni in Mn site of La0.8Sr0.2MnO3 is an efficient way to tune its bifunctional catalytic activity [14]. Added to the higher relative cooling power (RCP), it has been demonstrated that the transition temperature and MCE are tuned to nearly room temperature by Ni substitution for Ni concentration of 0.1 [15, 16].
Recent progress are nowadays focused on the rare earth element substitution on perovskite manganite at the nano-scale, exhibiting new experimental behaviors which can be used for new nano-electronic devices [10]. In this work, we are interested in the nickel-substituted manganese on the perovskite oxides La0.2Sr0.8MnO3 at lower Ni concentration prepared with the standard solid-state reaction method. Changes of structural properties have been studied by X-ray diffraction (XRD) analyses and field emission scanning electron microscope (FESEM) coupled with energy-dispersive X-ray (EDX) analysis. Then, in order to evaluate the effect of manganese substitution on the magnetic properties, magnetization has been measured using vibrating sample magnetometer. Moreover, conductance measurements are analyzed in large frequential domain (102 Hz–5 MHz) in order to evidence the effect of Ni-substituted Mn on the conduction mechanism.
2 Experimental details
Perovskite oxides La0.2Sr0.8MnO3 (LSMO) and La0.2Sr0.8Mn0.9Ni0.1O3 (LSMNO) powders were prepared by the standard solid-state reaction method [16]. The precursors LaO3, SrO3, MnO3, and NiO with purity better than 99.99% were weighed with the stoichiometric ratios and mixed using an agate mortar and then calcined at 1000 °C for 8 h. The resulting powders were ground, pelletized, and sintered at 1500 °C for 48 h with one intermediate grinding. The powders of both samples, thus, obtained were well ground again and were compressed into pellets of 8 mm (1 mm) diameter (thickness). On the other hand, in order to decrease their porosities, both materials were annealed at 1500 °C for 24 h under oxygen atmosphere.
The structural properties are studied using a Philips X pert, X-ray diffractometer with CuKα radiation (λ = 1.54 Å). FESEM is employed to examine the morphological characteristics of the samples and also EDX analysis was used to check the presence of chemical elements introduced during the sample preparation using a FESEM, TeScan—Mira III, Czech Republic. A vibrating sample magnetometer (8600 Series VSM Model lake shore) was employed to study the magnetic properties of both samples at room temperature. Conductance measurement data are deduced from impedance spectroscopy measurements using an N4L-NumetriQ type PSM1735.
3 Results and discussion
3.1 X-ray diffraction results
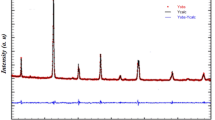
Figure 1 shows XRD patterns of both samples LSMO and LSMNO. In both cases, the interplanar lines spacing was calculated in order to investigate the phase formation and probe the structure of the synthesized La0.2Sr0.8Mn1−xNixO3. As shown, XRD pattern of both samples reveals that a pure and single-phase perovskite structure has been successfully formed. No trace of the secondary phase was detected within the sensitivity limit of the experiment. All the patterns are in good accordance with the JCPDS card no. 01-075-0440 as shown in Table 1. It is concluded that the lines in the pattern’s major planes (012), (020), (110), (202), (024), (116), (214), (208), (006), and (128) indicate rhombohedral (hexagonal setting) perovskite structure with space group R3C. As shown in Table 1, no additional lines are detected after nickel addition, reflecting that both materials exhibit the same phase.
The average of lattice parameters was calculated for all lines in each sample according to the following formula [17, 18]:
where dhkl is the interplanar spacing (recorded automatically) and h, k, and l are the Miller indices of each plane. The lattice parameters for LSMO sample were a = b = 5.4929 Å and c = 13.3235 Å, while for the LSMNO sample they were a = b = 5.5072 and c = 13.3875 Å which exactly match the value given in this card (a = b = 5.4694 and c = 13.4183 Å). Unit cell volume and lattice parameters increase after Nickel addition as summarized in Table 2 and follow the same trend found by Wang et al. [19] and Kuharuangrong et al. [20]. The nickel ion can be present as Ni2+ as the most stable state with radius 0.70 Å. Other states of nickel can also be present such low Ni3+ high spin, Ni3+ lower spin, and Ni4+ with radii, respectively, of 0.56 Å, 0.60 Å, and 0.48 Å. However, as previously reported, Mn ions are likely to be in the Mn3+ low spin or Mn4+ states with radii of 0.58 Å and 0.53 Å, respectively [21]. However, it has been largely reported in the literature that when substituting Mn by Ni via X-ray Photoelectron spectroscopy (XPS) and/or Electron paramagnetic resonance (EPR) studies Nickel exist as Ni2+ ions [14, 19, 22], especially at lower Ni concentration.
Furthermore, as shown in Fig. 2, we show a shift of plane (110) position toward smaller angle, accompanied with a peak broadening and a decrease of the XRD intensity. Similar behavior has been previously reported for La0.7Sr0.3Mn1−xCrxO3 [23]. These effects are attributed to the crystallite size growth and/or to the decrease in the degree of crystallinity. The value of integral line breadth (area under the peak/maximum peak intensity) was used to obtain the pure broadening of the characteristic peak for both LSMO and LSMNO perovskites [24]. The integral breadth of observation (B) in the sample is corrected by subtracting the value of the instrumental broadening (b) to give only that of pure sample breadth (β). Figure 3 shows the integral breath of both LSMO and LSMNO perovskites. As is evident from the figure, the broadening of the XRD peaks increases with the addition of nickel which may be the consequence of smaller grain sizes [21]. It is noted that the phase of LSMO after adding nickel is not changed, due to the fact that both Ni2+-ion and Mn3+-ion have similar ionic radii (70 pm and 72 pm respectively), giving rise to easier substitution process. The manganite LSMNO exhibits a more large bandwidth reflecting a weak electron and phonon interaction. This weak electron–phonon interaction indicates that lattice effects are minor by comparison to those induced by electronic and magnetic structure modifications. Therefore, Ni2+-ion can only be used as a control parameter to change the magnetic and transport properties of these manganite’s [25,26,27,28,29].
The physical, mechanical, and morphological properties of perovskites depend on the degree of crystallinity and on the growth of the preferred orientation in the crystal part. Usually, both quantities depend on the different parameters used on the preparation method, such as the sintering temperature, the heating time, and the cooling rate [30, 31]. The heat treatment of the samples reduces residual stored stress, internal stress, average crystallite sizes, and beak breadths. Thus, perovskite is usually a mixture of the crystalline and amorphous phases [32]. The XRD data were used to calculate the degree of crystallinity of the semi-crystalline perovskite [33,34,35,36]. The procedure consists on calculating each of the peaks area corresponding to (012), (020), (110), (202), (024), (116), (214), (208), (006), and (128) planes and by assuming their sum as the crystalline part. The amorphous contribution is, however, estimated by the wide diffraction band of the spectrum, approximated by the background curve. A typical fitting procedure is used in this work to separate the crystalline peaks from the amorphous pattern of LSMO and LSMNO. The crystalline part was separated from the amorphous part by EVA software using the Hermans–Weidinger method [37]. Then, the degree of crystallinity, XC, was obtained for each sample as a ratio between the area under the crystal peaks, AC, and the total area under the diffraction curve, AT = AA + AC.
The degree of crystallinity and the degree of Amorphousity are estimated and recorded in Table 2. As it is shown, the amorphousity is increased after Ni addition.
An XRD profile analysis is one of the most accurate techniques for estimating the crystallite size [38,39,40,41] and the irregular displacement strain of the atoms with respect to the positions of the reference lattice [42].
The three most important sources of line broadening are the instrumental factor, crystallite size, and micro-strain. The calculation is based on the shape of the peak where there are two ways. Full width at half maximum (FWHM) and integral breadth are applied to the shape of the peaks and leads to more accurate results as in the following relationships [43]:
The crystallite size and micro-strain are calculated according to the Debye–Scherrer’s relationships [17, 43, 44]:
In Eq. (6), K is the shape factor taken in our case which is equal to 0.94, since there is not another analysis to estimate the average of crystallites shapes to evaluate its proper value. λ is the incident X-ray wavelength (λ = 0.1542 nm), D is the crystallite size, and 〈ε〉 is the average micro-strain. The crystallite size and internal micro-strain were calculated according to the FWHM and integral breadth for both LSMO and LSMNO. All results are summarized in Table 2. It should be noted that the obtained nano-crystallite sizes remains an estimation due to the instrumental limits and the crystallite shape distribution [45]. The estimated average value of nano-crystallite is considered to be decreased as the case of many Ni-substituted Mn in large variety manganites [15, 19, 22, 46, 47]. That is, as the strength of the chemical bonds between the elements decrease, and consequently, the crystallization decreases. On the other hand, the internal micro-strain exhibited a contrast behavior, i.e., a gradual increase when adding nickel ions is evidenced. This difference may be due to lower degree of preferred direction and lower structural defects including grain boundaries. Because of the high degree of preferred orientation, a single meditation arrangement can be measured with sufficient accuracy.
3.2 Surface morphology and compositional analysis
In order to study the surface morphology of the perovskite samples using a FESEM and estimate the proportions of the elements using EDX, the powder samples were affixed to a glass (SiO2) holder. So we notice the presence of the characteristic line of the silicon element in the spectrum of the two samples. The microstructure of LSMO and LSMNO obtained by FESEM shows the effect of nickel-substituted Mn on the surface morphology as shown in Fig. 4. Where many microscopic images were photographed at different position and magnifications starting from 10 to 135kx for each sample, and the large magnification was chosen to show the most accurate details of the surface morphology. Figures 4a and b illustrate the FESEM micrographs obtained for LSMO and LSMNO at the same magnification (5kx) to make easier comparison showing a decreasing trend in particle sizes after Ni substitution. The structure shows strong agglomeration of nanoparticles in all samples constituted by grains and grain boundaries. This is evidence of the good growth of the crystallization during the thermal preparation process. It has been confirmed that Ni substitution leads to a decreased particle size of perovskite oxides, as recently reported in the case of La0.7Sr0.3Mn1−xNixO3 [48]. The reduction in particle size leads to an increase in the surface area of the granules specified for perovskite oxide, as LSMNO exhibits a greater surface area of the grains, nearly twice the surface area of the non-activated LSMO. The average particle size in the case of LSMO (180 nm to 270 nm) was strongly reduced for LSMNO (80 to 190 nm) and calculated according to the analysis of several FESEM obtained images [27, 49]. In Fig. 4, images (c) and (d), having the same magnification of 70kx, we notice cracking and demolition in crystal agglomeration and a decrease in particle size after adding nickel. Same effects are also seen for images (e) and (f), which are taken at the same magnification of 135kx. Disintegration, destruction of crystal agglomeration, and reduction of particle size are the most revealed effects with the addition of the Nickel element.
High-resolution scanning electron microscopy (HRSEM) micrographs of the LSMO (a, c, e) and LSMNO (b, d, f) samples prepared at 1500 °C for 24 h at different magnifications; as the zoom for the two images (a) and (b) was 50kx, and for the two images (c) and (d) was 70kx, while the zoom for the two images (e) and (f) was 135kx
In addition, EDX spectra shown in Fig. 5 reveal the presence of all chemical elements (La, Sr, Ni, Mn and O) that were introduced along the development of LSMO and LSMNO samples, confirming that there was no loss of any integral component after Ni substitution treatment. The appearance of the characteristic line of the Si element is due to the glass holder of the samples. The ratio of silicon and oxygen produced by the glass holder for samples was corrected and deleted from the device measurements, which depends on the atomic ratio of each element in SiO2.
Table 3 presents three methods for measuring the proportions of the elements in the LSMO and LSMNO samples. The first method is to calculate the atomic ratios of the elements in a theoretical way, and the second method is done according to the characteristic lines in the spectrum of the EDX device. However, the third method is to calculate the ratio of the elements as the ratio of the atomic weight in the compound while keeping the stoichiometric chemical weight of the compound constant. The obtained results show that the ratio La/Sr is changed when adding nickel. This is due to the fact that the process is accompanied by amourphoucity increase which leads to the irregular atomic concentration. Especially, we see excess of oxygen on the surface.
3.3 Study of magnetization versus magnetic field
To evidence the effect of Ni addition on the LSMO perovskites, we will briefly report the valence of Ni when substituting Mn for large variety of manganites. In the case La0.7Pb0.3Mn1−xNixO3 in Full Ni concentration range (x = 0–0.5), XPS results confirm Ni2+ ionic nature. Similarly, via an EPR study of Ni-doped LaCaMnO Perovskite, Rubinstein [50] et al. found that Nickel exist as Ni2+ ions when replacing Mn. Same conclusion is also evidenced in the case of La0.67Sr0.33Mn1−xNixO3 for lower Ni concentrations (x ≤ 0.2) by an XPS study [19]. In the same context [14], it is demonstrated via an XPS study of La0.8Sr0.2Mn1−xNixO3 that when substituting Ni by Mn, the ratio of Ni3+/Ni2+ decreases greatly for lower Ni concentration and also ionic entities of Mn are only Mn3+ and Mn4+. However, as explained in reference [13], when there are oxygen vacancies, due to the formation of imbalance in the atomic ratio between O and Mn atoms a new adaptive situation produces Mn2+ ions. According to our synthesis route when supposing that there are no oxygen vacancies, the above published results permit to conclude that the preponderant ionic species are Ni2+, Mn3+, and Mn4+.
Figure 6 shows the magnetization versus magnetic field for both materials. It is noted that the magnetization starts to saturate approximately at a magnetic field 2.5 KPo with nearly 0.563 μB and 0.357 μB, respectively, for LSMO and LSMNO. Then, the magnetization slightly continues to increase giving rise to a long tail, where their values reach 0.610 μB and 0.376 μB for higher magnetic field of 10 KPo. The long tail of magnetization in both cases is due to the competition between the ferromagnetic and antiferromagnetic moments coupling as demonstrated in the case of La0.7Sr0.3Mn1−xNixO3 [51]. Therefore, before adding Nickel, the most origin ferro–antiferro competition arises essentially from ferromagnetic Mn3+/Mn4+ moment and the antiferromagnetic Mn3+/Mn3+ (and/or Mn4+/Mn4+) moments. However, after nickel addition, the new ferromagnetic Mn4+/Ni2+ moment would be created. At the same time additional positive exchange interactions between Ni2+/Mn3+ and Ni2+/Ni2+ [52] would be created. To support the magnetization decrease, we refer to the global neutrality of both materials according to the material composition, where the charge conservation can be checked from the general electronic formula: La3+0.2Sr2+0.8(Mn3+0.2−y, Mn4+0.8−x+y) Nix2+O32−. Indeed, in the case of the reference material (La3+0.2Sr2+0.8(Mn3+0.2, Mn4+0.8)O32−), both ionic entities Mn3+ and Mn4+ can contribute to the ferromagnetic coupling. However, when nickel is added at x = 0.1(y = 2x) (La3+0.2Sr2+0.8(Mn4+0.9) Ni0.12+O32−, only Mn4+ ions can exist, giving rise to the suppression of the Mn3+/Mn4+ moment known as ferromagnetic components [52]. Therefore, the initial ferromagnetic moment (Mn3+/Mn4+) is replaced by Mn4+/Ni2+ moment. The suppression of antiferromagnetic Mn3+/Mn3+ moment is accompanied by the appearance of both Ni2+/Mn3+ and Ni2+/Ni2+ moments. Based on these results and according to the lower Ni concentration, the magnetization might be decreased. It is also reported that the decrease of magnetization saturation is related to a large grain boundary volume present in nano-crystalline influencing the number of atoms in ordered states [53].
The inset of Fig. 6 displays a hysteresis loop plot, which shows the remanent magnetization values Mr = 0.034 μB and 0.019 μB, respectively, for LSMO and LSMNO. Their corresponding coercive fields are approximately 22.8 Oe and 44.5 Oe. Therefore, the weak fraction of Ni substituting Mn induces the increase of coercive field and a net decrease of remanent magnetization. However, compared to other perovskites [25], Hc remains very weak, supporting their use as memory device. According to previous study [53, 54], the remanent magnetization depends on its saturation value. However, some other parameters lead to the variation of Mr and Hc, such as texture, nano-crystallites size, anisotropy, and phases present in the material. In our case, anisotropy caused by grain size decrease and the increase of amorphousity caused by Ni2+ cations lead to the magnetic moment arrangement and consequently the increase of coercivity.
3.4 Conductance analysis
The variation of the conductance G with frequency from 102 Hz to 5 MHZ in the temperature ranging from 300 to 420 K is shown in Fig. 7a and b, respectively, for LSMO and LSMNO samples. At lower frequency (less than 105 Hz), the conductance is approximately constant and correspond to the long range translational motion of ions [55]. In this frequency range, dominated by dc conductance, the electric field cannot affect the hopping conduction mechanism. For both cases, the Gdc values (plateau) increase with temperature rising, indicating that the process is thermally activated where the mobile ion can hop successfully to its nearest vacant site. However, at higher frequencies the conductance increase sharply with frequency and correspond to the Gac. In the full frequency range, the evolution of the conductance can be written as the single power Jonscher law [56]:
In this expression, A represents the pre-exponential factor that determinates polarazability and n is a frequency factor, providing information degree of interaction between mobile carriers and lattices [57, 58]. The fitting data relative in all measurement temperatures are summarized in Table 4.
From these results, and based on continuous increase of Gdc in the full temperature range, we present the Arrhenius plot of the Gdc (T) according to (Eq. 8) in Fig. 8. In this equation, G0 is the pre-exponential factor, Ea is the activation energy, KB is the Boltzman constant, and T is the temperature. The activation energies required to activate the mobile charge carriers in both cases are calculated from the linear fit. In the case of LSMO, the activation energy until temperature range is 164 meV. However, in the case LSMNO, two temperature ranges are selected (T < 400 K) and (T > 400 K). Their typical corresponding activation energies are, respectively, 65 meV and 322 meV. We think that both energies are attributed to grain and grain size contributions. The obtained values of activation energies are in good agreement with other perovskites-type ferroelectric oxides [59].
In all cases, the Gdc is assisted by trapping of polaron in the potential well of height Ea. When temperature gets higher, one side of the well will be lowered, allowing polarons jumping into the nearest neighboring site. This thermal process leads to the conductivity exponential rise, confirming thermally activated conduction.
Figures 9a and b illustrate, respectively, the evolution of frequency factor n as a function of temperature for both LSMO and LSMNO perovskites and the fitting parameters at the temperature of 420 K.
a Frequency dependence of the factor (n) and b illustration of the fitting parameters at the temperature of 420 K for LSMO and LMSNO: (■) Experimental, (—) Fit using Eq. 7
The temperature dependence of the frequency factor n permits to give specification of the Gac conduction mechanisms [58]. As shown in Fig. 9a, the frequency factor in both cases is higher than 1 and shows a different variation. Based on the literature, the values of this factor can be either less or higher than 1, depending on the material nature. When n reaches values greater than 1, it is proposed [60] that the charge carriers undergo a motion encompassing localized hopping between neighboring sites and if n is less than 1, the motion involves a translational motion with a sudden hopping. Moreover, it has been recently reported [61] that (n > 1) means a motion of mobile charge carriers from site to site with tunneling effect as proposed by Gilory et al. [62]. In our case, for the LSMO, the n value shows globally a monotonic increase with temperature supporting the non-overlapping small polaron tunneling (NSPT) model for conductivity. However, in the case of LSMNO, the frequency factor n decreases until reaching a minimum and then increases with rising temperature, matching well with the overlapping large polaron tunneling (OLPT) model [58]. Therefore, the interaction between individual charges and lattice distortion couldn’t give overlapping in the case of LSMO. However, adding Nickel leads to give a higher spatial extension of the polaron wells by comparison to the interatomic distances, giving rise to the overlapping of two neighboring sites.
4 Conclusion
Perovskite oxide LSMO and LSMNO powders were successfully prepared by the standard solid-state reaction method in order to carry out the effect of lower Nickel substituted Mn on structural, magnetic, and conductive properties. EDX experimental results are in good accordance with those obtained theoretically, supporting the full integral reaction of all standard elements. From FESEM results, both materials contain agglomerations of nanoparticles constituted by grains and grain boundaries, with further reduced particle size when adding Ni element. Whereas X-ray analyses show that both materials crystallize in single-phase rhombohedral structure with 3RC group, Ni adding leads to some structural modifications such as the nano-crystallite size decrease and the increase of the amorphoucity. Lattice parameters and internal micro-strain are consequently increased. Furthermore, from magnetic measurements, we support the existence of Nickel as Ni2+ replacing Mn3+ with approximately same radius ions which lead to reduce the saturation and remanent magnetizations and nearly twice value of the coercive field. Both ferromagnetic and antiferromagnetic moments coupling are present; however, Ni addition leads to the suppression of the Mn3+/Mn4+ moment known as ferromagnetic components. Moreover, from conductance measurements, the lower frequency direct conductivity is thermally activated for which the activation energy deceases from 164 to 65 meV after Nickel addition. However, at higher frequencies, the conduction is assisted by non-overlapping small polaron tunneling (NSPT) model for LSMO and, the overlapping large polaron tunneling (OLPT) model for LSMNO. Nickel addition leads, therefore, to reducing the wall height and gives the resulting polaron a more spatial extension exceeding the interatomic distances.
References
L.W. Martin, Y.-H. Chu, R. Ramesh, Advances in the growth and characterization of magnetic, ferroelectric, and multiferroic oxide thin films. Mater. Sci. Eng. R Rep. 68, 89 (2010). https://doi.org/10.1016/j.mser.2010.03.001
M.A. Subramanian, B.H. Toby, A.P. Ramirez, W.J. Marshall, A.W. Sleight, G.H. Kwe, Colossal magnetoresistance without Mn3+/Mn4+ double exchange in the stoichiometric pyrochlore TI2Mn2O7. Science 273, 81 (1996). https://doi.org/10.1126/science.273.5271.81
M. Baibich, J. Broto, A. Fert, F. Nguyen Van Dau, F. Petroff, P. Etienne, G. Creuzet, A. Friederich, J. Chazelas, Giant magnetoresistance of (001)Fe/(001)Cr magnetic superlattices. J. Phys. Rev. Lett. 61, 2472 (1988). https://doi.org/10.1103/PhysRevLett.61.2472
L. Balcells, R. Enrich, J. Mora, A. Calleja, J. Fontcuberta, Manganese perovskites: thick-film based position sensors fabrication. Appl. Phys. Lett. 69, 1486 (1996). https://doi.org/10.1063/1.116916
S. Jin, T. Tiefel, M. McCormack, R. Fastnacht, R. Ramesh, L.H. Chen, Thousandfold, change in resistivity in magnetoresistive La-Ca-Mn-O films. Science 264, 413 (1994). https://doi.org/10.1126/science.264.5157.413
W. Xia, Z. Pei, K. Leng, X. Zhu, Research progress in rare earth-doped perovskite manganite oxide nanostructures. Nanoscale Res. Lett. 15, 9 (2020). https://doi.org/10.1186/s11671-019-3243-0
M. Egilmez, K. Chow, J. Jung, Anisotropic magnetoresistance in perovskite manganites. Mod. Phys. Lett. B. 25, 697 (2011). https://doi.org/10.1142/S0217984911026176
G. George, S.L. Jackson, C.Q. Luo, D. Fang, D. Luo, D. Hu, J. Wen, Z. Luo, Effect of doping on the performance of high-crystalline SrMnO3 perovskite nanofibers as a supercapacitor electrode. Ceram Int 44, 21982 (2018). https://doi.org/10.1016/j.ceramint.2018.08.313
R. Bindu, K. Maiti, R. Rawat, S. Khalid, Electronic and structural transition in La0.2Sr0.8MnO3. Appl. Phys. Lett. 92, 121906 (2008). https://doi.org/10.1063/1.2898885
C. Doroftei, L. Leontie, Nanocrystalline SrMnO3 perovskite prepared by sol–gel self-combustion method for sensor applications. J. Sol-Gel Sci. Technol 97, 146 (2021). https://doi.org/10.1007/s10971-020-05419-4
A. Kumar, M. Kumar, R.P. Singh, P.K. Singh, Opto-electronic, magnetic, thermodynamic and thermoelectric properties of cubic perovskite SrMnO3: a first principle based spin polarized calculation. Solid State Commun. 324, 114139 (2021). https://doi.org/10.1016/j.ssc.2020.114139
G.H. Jonker, J.H. Van Santen, Ferromagnetic compounds of manganese with perovskite structure. Physica 16, 337 (1950). https://doi.org/10.1016/0031-8914(50)90033-4
J. Zhao, C. Liu, J. Li, R. Wu, J. Wang, H. Qian, H. Guo, J. Li, K. Ibrahim, Oxygen vacancy induced electronic structure variation in the La0.2Sr0.8MnO3 thin film. AIP Adv. 9, 055208 (2019). https://doi.org/10.1063/1.5088738
Z. Wang, Y. You, J. Yuan, Y.X. Yin, Y.T. Li, S. Xin, D. Zhang, Nickel-doped La0.8Sr0.2Mn1-xNixO3 nanoparticles containing abundant oxygen vacancies as an optimized bifunctional catalyst for oxygen cathode in rechargeable lithium-air batteries. ACS Appl. Mater. Interfaces 8, 6520 (2016). https://doi.org/10.1021/acsami.6b00296
E. Oumezzine, S. Hcini, E.-K. Hlil, E. Dhahri, M. Oumezzine, Effect of Ni-doping on structural, magnetic and magnetocaloric properties of La0.6Pr0.1Ba0.3Mn1-xNixO3 nanocrystalline manganites synthesized by Pechini sol–gel method. J. Alloy. Compd. 615, 553 (2014). https://doi.org/10.1016/j.jallcom.2014.07.001
C.P. Reshmi, S.S. Pillai, K.G. Suresh, M.R. Va, Room temperature magnetocaloric properties of Ni substituted La0.67Sr0.33MnO3. Solid State Sci. 19, 130 (2013). https://doi.org/10.1016/j.solidstatesciences.2013.02.019Ge
B.D. Cullity, S.R. Stock, Elements of X-Ray Diffraction, 3rd edn. (Education Limited, London, Pearson, 2014), pp. 102–104
A.A.S. Akl, A.S. Hassanien, Microstructure and crystal imperfections of nanosized CdSxSe1-x thermally evaporated thin films. Superlatt Microstr. 85, 67 (2015). https://doi.org/10.1016/j.spmi.2015.05.011
Z.H. Wang, J.W. Cai, B.G. Shen, X. Chen, W.S. Zhan, Exchange interaction, spin cluster and transport behaviour in perovskites La0.67Sr0.33(Mn1-xNix)O3 (x ≤ 0.2). J. Phys. Condens. Matter. 12, 601 (2000). https://doi.org/10.1088/0953-8984/12/5/308
S. Kuharuangrong, Effects of Ni on the electrical conductivity and microstructure of La0.82Sr0.16MnO3. Ceram. Int. 30, 273 (2004). https://doi.org/10.1016/S0272-8842(03)00099-3
T. Ishihara, J.A. Kilner, M. Honda, Y. Takita, Oxygen surface exchange and diffusion in the new perovskite oxide ion conductor LaGaO3. J. Am. Chem. Soc. 119, 2747 (1997). https://doi.org/10.1021/ja964128l
S. Pala, E. Bose, B.K. Chaudhuri, H.D. Yang, S. Neeleshwar, Y.Y. Chen, Effect of Ni doping in rare-earth manganite La0.7Pb0.3Mn1-xNixO3 (x=0.0–0.5). J. Magn. Magn. Mater. 293, 872 (2005). https://doi.org/10.1016/j.jmmm.2004.12.005
A. Hammouche, E.J.L. Schouler, M. Henault, Electrical and thermal properties of Sr doped lanthanum manganites. Solid State Ion. (1988). https://doi.org/10.1016/0167-2738(88)90358-X
A.A. Akl, S.A. Mahmoud, S.M. Al-Shomar, A.S. Hassanien, Improving microstructural properties and minimizing crystal imperfections of nanocrystalline Cu2O thin films of different solution molarities for solar cell applications. Mater. Sci. Semicond. Process. 74, 183 (2018). https://doi.org/10.1016/j.mssp.2017.10.007
N. Hamdaoui, Y. Azizian-Kalandaragh, M. Khlifi, L. Beji, Structural, magnetic and dielectric properties of Ni0.6Mg0.4Fe2O4 ferromagnetic ferrite prepared by sol-gel method. Ceram. Int. 45, 16458 (2019). https://doi.org/10.1016/j.ceramint.2019.05.177
F. Gaâbel, M. Khlif, N. Hamdaoui, L. Beji, K. Taibi, J. Dhahri, Microstructural, structural and dielectric analysis of Ni-doped CaCu3Ti4O12 ceramic with low dielectric loss. J. Mater. Sci. Mater. Electron. 30, 14823 (2019). https://doi.org/10.1007/s10854-019-01886-w
S.M. Salili, A. Ataie, M.R. Barati, Z. Sadighi, Characterization of mechano-thermally synthesized Curie temperature-adjusted La0.8Sr0.2MnO3 nanoparticles coated with (3-aminopropyl) triethoxysilane. Mater. Charact. 106, 78 (2015). https://doi.org/10.1016/j.matchar.2015.05.025
A. Peterlin, Molecular model of drawing polyethylene and polypropylene, Molecular model of drawing polyethylene and polypropylene. J. Mater Sci. 6, 490 (1971). https://doi.org/10.1007/BF00550305
P.B. Bowden, R.J. Young, Deformation mechanisms in crystalline polymers. J. Mater Sci. 9, 2034 (1974). https://doi.org/10.1007/BF00540553
A. Silambarasu, A. Manikandan, K. Balakrishnan, Room-temperature superparamagnetism and enhanced photocatalytic activity of magnetically reusable spinel ZnFe2O4 nanocatalysts. J. Supercond. Nov. Magn. 30, 2631 (2017). https://doi.org/10.1007/s10948-017-4061-1
A. Manikandan, E. Hema, M. Durka, K. Seevakan, T. Alagesan, S.A. Antony, Room temperature ferromagnetism of magnetically recyclable photocatalyst of Cu1−xMnxFe2O4-TiO2 (0.0≤x ≤0.5) nanocomposites. J. Supercond. Nov. Magn. 28, 1783 (2015). https://doi.org/10.1007/s10948-014-2945-x
T. Dippong, O. Cadar, E.A. Levei, I.G. Deac, F. Goga, G. Borodi, L.B. Tudoran, Influence of polyol structure and molecular weight on the shape and properties of Ni0.5Co0.5Fe2O4 nanoparticles obtained by sol-gel synthesis. Ceram. Int. 45, 7458 (2019). https://doi.org/10.1016/j.ceramint.2019.01.037
J.M. Haudin, in: Plastic Deformation of Amorphous and Semi-crystalline Materials, ed. by B. Escaig and C. G'Sell (Les Editions de Physique, Les Ulis, France, 1982), p. 291–311.
L. Lin, A.S. Argon, Structure and plastic deformation of polyethylene. J. Mater. Sci. 29, 294 (1994). https://doi.org/10.1007/BF01162485
J. Jia, D. Raabe. Crystallinity and crystallographic texture in isotactic polypropylene during deformation and heating. (ArXivLabs, 2008), http://arxiv.org/abs/0811.2412. Accessed 14 Nov 2008.
H. Klug, L. Alexander, X-Ray Diffraction Procedures for Poly-Crystalline and Amorphous Materials, 2nd edn. (Wiley, New York, 1974), pp. 136–142
A. Weidinger, P.H. Hermans, On the determination of the crystalline fraction of isotactic polypropylene from x-ray diffraction. Macromol. Chem. Phys. 50, 98 (1961). https://doi.org/10.1002/macp.1961.020500107
A. Alaa, Akl, Microstructure and electrical properties of iron oxide thin films deposited by spray pyrolysis. Appl. Surf. Sci. 221, 319 (2004). https://doi.org/10.1016/S0169-4332(03)00951-6
J. Zhang, J. Liu, Q. Peng, X. Wang, Y. Li, Nearly monodisperse Cu2O and CuO nanospheres: preparation and applications for sensitive gas sensors. Chem. Mater. 18, 867 (2006). https://doi.org/10.1021/cm052256f
Z. Zhang, C. Zhong, Y. Deng, L. Liu, Y. Wu, W. Hu, The manufacture of porous cuprous oxide film with photocatalytic properties via an electrochemical–chemical combination method. RSC Adv. 3, 6763 (2013). https://doi.org/10.1039/C3RA40855H
S.M. Al-Shomar, M.A.Y. Barakat, S.A. Mahmoud, A.A. Akl, Microstructure, crystal imperfections and ultrasonic studies of sprayed nanosized Cu2-xSxO and Cu2-yCryO thin films. Dig. J. Nanomater. Biostructures 13, 885 (2018)
S. Eisermann, A. Kronenberger, A. Laufer, J. Bieber, G. Haas, S. Lautenschlager, G. Homm, P.J. Klar, B.K. Meyer, Copper oxide thin films by chemical vapor deposition: synthesis, characterization and electrical properties. Phys. Status Solidi A 209, 531 (2011). https://doi.org/10.1002/pssa.201127493
A.O. Musa, T. Akomolafe, M.J. Carter, Production of cuprous oxide, a solar cell material, by thermal oxidation and a study of its physical and electrical properties. Sol. Energy Mater. Sol. Cells 51, 305 (1998). https://doi.org/10.1016/S0927-0248(97)00233-X
A.A.S. Akl, M. Elhadi, Estimation of crystallite size, lattice parameter, internal strain and crystal impurification of nanocrystalline Al3Ni20Bx alloy by Williamson-Hall method. J. of Ovonic Res. 16, 323 (2020)
J.I. Langford, A.J.C. Wilson, Scherrer after sixty years: a survey and some new results in the determination of crystallite size. J. Appl. Cryst. 11, 102 (1978). https://doi.org/10.1107/S0021889878012844
H. Song, W. Kim, S.-J. Kwon, Magnetic and electronic properties of transition metal substituted perovskite manganites La0.7Ca0.3Mn0.95X0.05O3 (X=Fe Co, Ni). J. Appl. Phys. 89, 3398 (2001). https://doi.org/10.1063/1.1350417
O. Toulemonde, F. Suder, A. Barnabe, A. Maignan, C. Martin, B. Raveau, Charge states of transition metal in “Cr, Co and Ni” doped Ln0.5Ca0.5MnO3 CMR manganites. Eur. Phys. J. B 4, 159 (1998). https://doi.org/10.1007/s100510050364
A.E.-M.A. Mohamed, B. Hernando, A.M. Ahmed, Magnetic, magnetocaloric and thermoelectric properties of nickel doped manganites. J. Alloy. Compd. 692, 381 (2017). https://doi.org/10.1016/j.jallcom.2016.09.050
N. Hamdaoui, Y. Azizian-Kalandaragh, M. Khlifi, L. Beji, Cd-doping effect on morphologic, structural, magnetic and electrical properties of Ni0.6-xCdxMg0.4Fe2O4 spinel ferrite (0 ≤ x ≤ 0.4). J. Alloy. Compd. 803, 964 (2019). https://doi.org/10.1016/j.jallcom.2019.06.339
M. Rubinstein, D.J. Gillespie, J.E. Snyder, T.M. Tritt, Effects of Gd Co, and Ni doping in La2/3Ca1/3MnO3: resistivity, thermopower, and paramagnetic resonance. Phys. Rev. B 56, 5412 (1997). https://doi.org/10.1103/PhysRevB.56.5412
J.W. Feng, L. Hwang, Ferromagnetic cluster behaviors and magnetoresistance in Ni-doped LaSrMnO3 systems. Appl. phys. Lett. 75(1592), 1592–1594 (1999). https://doi.org/10.1063/1.124764
O. Toulemonde, F. Studer, B. Raveau, Magnetic interactions studies of Co and Ni-doped manganites using soft XMCD. Solid State Commun. 118, 107 (2001). https://doi.org/10.1016/S0038-1098(01)00020-5
M. Desai, S. Prasad, N. Venkataramani, I. Samajdar, A.K. Nigam, Anomalous variation of coercivity with annealing in nanocrystalline NiZn ferrite films. J. Appl. Phys. 91, 592 (2002). https://doi.org/10.1063/1.1447504
V.M. Kalita, A.A.F. Lozenko, S.M. Ryabchenko, A.A. Timopheeev, R.A. Trotsenko, Magnetic properties of La0.7Sr0.3MnO3 nanopowders. Low Temp. Phys. 34, 436 (2008). https://doi.org/10.1063/1.2920124
W. Li, R.W. Schwartz, Ac conductivity relaxation processes in CaCu3Ti4O12 ceramics: grain boundary and domain boundary effects. Appl. Phys. Lett. 89, 242906 (2006). https://doi.org/10.1063/1.2405382
A.K. Jonscher, The ‘universal’ dielectric response. Nature 267, 673 (1977). https://doi.org/10.1038/267673a0
K.M. Sangwan, N. Ahlawat, S. Rani, S. Rani, R.S. Kundu, Influence of Mn doping on electrical conductivity of lead free BaZrTiO3 perovskite ceramic. Ceram. Int. 44, 10315 (2018). https://doi.org/10.1016/j.ceramint.2018.03.039
F. Gaâbel, M. Khlifi, N. Hamdaoui, K. Taibi, J. Dhahri, Conduction mechanisms study in CaCu2.8Ni0.2Ti4O12 ceramics sintered at different temperatures. J. Alloy. Compd. 828, 154373 (2020). https://doi.org/10.1016/j.jallcom.2020.154373
M. Sindhu, N. Ahlawat, S. Sanghi, R. Kumari, A. Agarwal, Crystal structure refinement and investigation of electrically heterogeneous microstructure of single phased Sr substituted BaTiO3 Ceramics. J. Alloy. Compd. 575, 109 (2013). https://doi.org/10.1016/j.jallcom.2013.04.026
K. Funke, Jump relaxation in solid electrolytes. Prog. Solid State Chem. 22, 111 (1993). https://doi.org/10.1016/0079-6786(93)90002-9
A. Dhahri, E. Dhahri, E.K. Hlil, Electrical conductivity and dielectric behaviour of nanocrystalline La0.6Gd0.1Sr0.3Mn0.75Si0.25O3. RSC Adv. 8, 9103 (2018). https://doi.org/10.1039/C8RA00037A
K.S. Gilroy, W.A. Phillips, An asymmetric double-well potential model for structural relaxation processes in amorphous materials. Philos. Mag. Lett. 43, 735 (1981). https://doi.org/10.1080/01418638108222343
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hamdaoui, N., Tlili, D., Azizian-Kalandaragh, Y. et al. Effect of Ni-doping on the structural, magnetic, and electronic properties of La0.2Sr0.8MnO3 perovskite. J Mater Sci: Mater Electron 32, 26984–26997 (2021). https://doi.org/10.1007/s10854-021-07072-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-021-07072-1