Abstract
In this paper, a hydrothermal method was applied to synthesize the nanosheet-like pure ZnO and 0.5%, 1 and 3% Co-doped ZnO (Co-ZnO). The pristine and Co-doped ZnO flower-like particles were assembled by porous nanosheets, with the uniform diameter about 18 μm. The N2-BET test found that Co doping significantly increased the specific surface area of the material which was conducive to gas diffusion and adsorption. HRTEM presented that 1% Co-ZnO nanosheets were composed of coral-like nanoparticles. The lattice distances 0.259 nm and 0.276 nm correspond to (002) and (100) crystal plane of ZnO. The gas sensing properties reveal that the 1% Co-doped ZnO present an outstanding enhanced sensitive performance comparing with pure ZnO to ethanol. To 100 ppm target gas, the response increased from 103 to 279.8 and the optimal operating temperature decreased from 369 to 348 °C, and the recovery time decreased from 40 to 18 s. The increased surface carrier concentration which promoted oxygen adsorption by Co was considered to be the key factor to improve the performance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In recent years, as environmental pollution intensifies, the researchers have deeply studied the monitoring and health detection of organic volatile gases. Metal oxide semiconductor (MOS) had been broadly applied in gas monitoring because of low cost, fast dynamic process, high sensitivity, and stable structure [1,2,3,4].
As an important n-type semiconductor, ZnO has been extensively studied in gas sensing field. At present, the commonly recognized sensing mechanism is that oxygen molecules in air adsorb to the surface of ZnO material which capture electrons becoming oxygen ions. While ZnO was in a reducing gas atmosphere, gas molecules would react with adsorbed oxygen ions and carriers were released back into ZnO. The conductivity of ZnO changes during the adsorption and desorption process of oxygen molecules, so as to detect the change of gas composition [5,6,7]. Studies show that carrier concentration, adsorbed oxygen concentration and the specific surface area of the material will greatly affect the sensing performance of the material [8]. The response of MOS sensor can be improved by morphology control, precious metal, transition metal and non-metal doping [9,10,11,12,13].
However pure ZnO materials have poor selectivity, and appropriate element doping can not only effectively improve selectivity, but also improve gas response of materials [14,15,16]. For example, Wang et al. had successfully synthesized In-doped ZnO materials which exhibited better sensing properties such as response and selectivity [8]. Gitae Namgung et al. had studied an Al-doped ZnO nanowire, which showed a unique gas-sensitive property for NO2 gas [17]. What’s more, thanks to the catalytic effect of precious metals, appropriate addition of Au, Ag, Pt and other nanoparticles can promote the ionization of oxygen and improve the performance of the device [18,19,20,21,22]. On the other hand, changing the material morphology is an effective method so as to improve the performance. Many nanostructures, such as nanowires [11, 23, 24], nanorods [12, 25], nanosheets [26], nanotubes [10] and nanospheres [11], had been successfully synthesized and applied.
Taking into account the unique properties of cobalt as a transition metal and the atomic radius close to that of Zn, this work studied the effect of Co doping on the gas-sensing characteristics of ZnO. In recent years, the research on Co-doped ZnO is rare, and the performance of the device is also poor. In this work, a one-step hydrothermal method was used to successfully synthesize three-dimensional porous ZnO materials of which the electrical properties were adjusted by controlling the doping of Co element. And the gas sensitive properties of the material were improved effectively. The sensor performance of ethanol under different concentrations of Co doping was studied, and the doping concentration of Co element was optimized. The experimental results show that the appropriate concentration of Co doping can effectively improve the sensor response, and reduce the operating temperature and recovery time, and improve the selectivity. And the preeminent performance was attributed to high electron concentration which was led to by Co impurity.
2 Experimental
2.1 Preparation of ZnO nanosheets
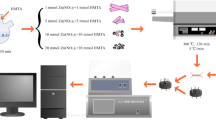
All reagents used in this experiment were of analytical grade and used without further purification. ZnO and Co-ZnO were synthesized by hydrothermal method. In our procedure, 3.5 g Zn(CH3COO)2⋅2H2O was dissolved in 50 mL deionized water and then 1.8 g CO(NH2)2 was add in the solution with magnetic stirring. After stirred for 20 min, the mixture was transferred into a Teflon-lined autoclave and kept at 120 °C for 5 h. The white products were collected by centrifugation and washed with deionized water and ethanol. The products were dried at 70 °C for 8 h, and subsequently were calcined at 500 °C for 2 h in the air atmosphere. The obtained powder was exhibited in Fig. 1.
2.2 Preparation of Co-doped ZnO nanosheets
As Fig. 1 shown, the Co-doped ZnO (Co-ZnO) was prepared via a similar method to ZnO. The first step was to get the mixture of Zn(CH3COO)2·2H2O and CO(NH2)2, a certain proportion of Co(NO3)2·6H2O (Co:Zn = 0.5%, 1%, 3%) was add into the mixture subsequently. The following steps were same to that above. The finally obtained green powders were Co-ZnO.
2.3 Characterization
The crystal structure of as-prepared compounds was analyzed through the X-ray diffraction (XRD, Rigaku D/max-2400) with Cu Kα radiation. The field emission scanning electron microscopy (FESEM, Hitachi S4800), transmission electron microscopy (TEM, FEI Tecnai G2F30) and high-resolution transmission electron microscopy (HRTEM, FEI Tecnai G2F30) were applied to observe structure and morphology of samples at 300 kV.
2.4 Fabrication and measurement of gas sensors
Ground the sample and mixed well with ethanol. Then the mixture was evenly coated on the surface of the ceramic tube. This ceramic tube with a length of 4 mm, an inner diameter of 0.8 mm, and an outer diameter of 1 mm had a pair of gold electrodes at both ends of the tube. The device and heating were welded on a pedestal after annealed at 450 °C for 2 h. The sensor sensing properties were tested by a WS-30B test bench. In this work, the static volumetric method was used to get an accurate target gas.
The environment humidity value was held as 5 RH% (Relative Humidity) during testing. Ra and Rg denoted the sensor resistance in air and in target gas respectively, and the response was defined as Ra/Rg. Response time and recovery time were defined as the time it takes for the sensor to change 90% in resistance from one steady state to another steady state.
3 Results and discussion
3.1 Structural and morphology characteristics
Firstly, the SEM and HRTEM were applied to characterized morphologies of ZnO and Co-doped ZnO. As Fig. 2 revealed, ZnO and Co-ZnO particles are uniformly spherical, with a particle diameter of about 18 μm. After further magnification, as shown in Fig. 2e–h, the spherical particles are formed by the accumulation of smaller-sized flakes, and the surface of the flakes is densely packed with micropores. Intuitively, this porous structure facilitates gas diffusion and adsorption. Comparing four pictures Fig. 2a–d, obviously, the differential incorporation of Co elements did not affect the overall morphology of the particles. Figure 21 characterizes that the material contains Zn, O, and Co elements. Figure 2i–k depicts the uniform distribution of the elements in the material as we would expect.
As shown in Fig. 3, HRTEM characterization was performed for further analysis of Co-ZnO sample. Figure 3a, b correspond to 1% Co-ZnO sample. Obviously, 1% Co-ZnO nanosheets were composed of coral-like nanoparticles. The lattice distances 0.259 nm and 0.276 nm correspond to (002) and (100) crystal plane of the hexagonal phase ZnO, respectively. Moreover, the lattice structure of Co oxide is not observed in Fig. 3b, which proves that Co atoms exist in the composite in the form of substitutive impurity. Combining Fig. 3c, d the TEM and HRTEM pattern of ZnO show that the experimentally prepared materials have good crystallinity and purity.
The crystal structures of the ZnO and Co-ZnO materials were analyzed by XRD. Figure 4 exhibits that the diffraction peaks are consistent with the hexagonal phase of ZnO (JCPDS NO. 36-1451) for all samples. Nevertheless, there is nothing about the characteristic peaks of Co oxide which prove on the other hand that Co element does not exist in the form of oxide corresponding to the TEM result.
Both TEM and SEM analysis show that the prepared material has a porous structure. In order to study the structural differences of different materials in more detail, we performed the nitrogen adsorption/desorption isotherms characterization, and the results are shown in Fig. 5. For ZnO, 0.5% Co-ZnO, 1% Co-ZnO, and 3% Co-ZnO, their BET surface area are 9.4381 m²/g, 11.5912 m²/g, 20.0565 m²/g, 19.1457 m²/g, respectively. In addition, the BJH desorption average pore diameter of the four materials are 468.593 Å, 400.549 Å, 373.807 Å, and 338.053 Å, respectively. As the Co doping concentration increases, the specific surface area of the porous structure material increases significantly, and the structure pore size decreases. This change tends to be flat after the Co concentration is higher than 1%. On the other hand, the pore size distribution diagram shows that the pore size distribution is more concentrated after the Co element is doped, indicating that the uniformity of the micropores has been improved. And a larger specific surface area and a more uniform structure are beneficial to sensor performance.
3.2 Gas sensitive characteristics
As is known to all, for a semiconductor device the operating temperature greatly influences it’s electrical properties. Showing in Fig. 6a, the response to 100 ppm ethanol of sensors based on pure ZnO, 0.5%, 1%, and 3% Co-ZnO in different operating temperature conditions were tested to ascertain the optimal operating temperature. Obviously, the best working temperature corresponds to the coordinates of the highest point of the curve. And the optimal operating temperature of the ZnO device was 369 °C, which was higher than devices based on 0.5%, 1%, and 3% Co-ZnO. Sensors based on 0.5%, 1%, and 3% presented the optimal operating temperature at 328 °C, 348 °C, 286 °C respectively. The graph exhibited that in the low temperature range (T < 300 °C) and high temperature (T > 400 °C), the response was slowly changing with temperature. By contrast, the sensor based on 3% Co-ZnO showed an insensitivity response-temperature function.
a Response of the pure ZnO and 0.5%, 1%, and 3% Co-ZnO to 100 ppm ethanol as a function operating temperature; b dynamic response curves of pure ZnO, 0.5%, 1%, and 3% Co-ZnO at diferent concentrations of ethanol; c response curves of devices based on pure ZnO, 0.5%, 1%, and 3% Co-ZnO to different concentrations; d ten-cycle switching test of pure ZnO, 0.5%, 1%, and 3% Co-ZnO to 100 ppm ethanol respectively
Figure 6b exhibits the dynamic response-recovery curve of the sensors based on pure ZnO, 0.5%, 1%, and 3% Co-ZnO, corresponding to different ethanol concentrations (10 ppm to 800 ppm) at the optimal operating temperature respectively. When in a low concentration ethanol condition, the response of different sensors showed a smaller gap. As the ethanol concentration increased, the sensor response based on 0.5%, 1%, and 3% Co-ZnO were all higher than ZnO sensors. Among them, the device based on 1% Co-ZnO presented the best properties of which the response to 800 ppm ethanol was high to 1885. Figure 6c displays the response-concentration curve at their respective optimal operating temperature for all sensors. Initially, the response changes rapidly with ethanol concentration, and then the curve tends to be flat. Compared with other curves, the curve corresponding to 1% Co-ZnO has a larger slope and a faster increase rate.
As shown in Fig. 6d, the ten-cycle switching test of sensors for ethanol at the optimal operating temperature presents good stability. Figure 7a–d presented the response and recovery time of sensors corresponding to pure ZnO, 0.5%, 1 and 3% Co-ZnO to 100 ppm ethanol at the optimal working temperature severally. The response/recovery time for pure ZnO, 0.5%, 1 and 3% Co-ZnO are 5 s/40 s, 4 s/21 s, 4 s/18 s, 5 s/21 s respectively. Stick out a mile, with the Co doping, the recovery time decreases from 40 to 18 s distinctly. While the response time almost changes nothing.
The gas selectivity is another valuable property, this work has researched sensors’ selectivity to 100 ppm ethanol, acetone, methanol, formaldehyde and xylene at the optimal operating temperature. As the result, Fig. 8 revealed that all sensors had the maximum response to ethanol, and the 1% Co-ZnO device showed the best selectivity.
Table 1 lists the research results of ZnO ethanol sensors in recent years. Comparing operating temperature, response-recovery time, and response, our sample exhibits good response-recovery characteristic and excellent response property.
3.3 Sensing mechanism
It is concluded that urea made a significant impact in the formation of multi-space ZnO nanosheet precursors. In the hydrothermal environment, urea would hydrolyze and release OH− and CO32− and reactions are as follows [4]:
In this work, both ZnO and Co-ZnO show typical n-type semiconductor properties. The carrier concentration in the material is changed when the target gas is absorbed and desorbed from the material surface, which affects the electrical properties of the material. Oxygen molecules in air adsorb to the material surface, a physical process that does not involve charge transfer. With the temperature increasing, oxygen molecules capture electrons in the surface of material and are ionized into oxygen ions. As Fig. 9b exhibited, with the increase of temperature, oxygen ions further ionize, thus reducing the concentration of surface carriers, generating an electron depletion layer [39,40,41]. In terms of ZnO, the electron depletion layer reduces the conductivity of the material, while the contact surface of the nanoparticles creates a potential barrier that hinders electron flow [42, 43]. As shown in Fig. 10, the electric barrier screens out low-energy electrons and the material conductance decreases macroscopically. While the ethanol vapor passed through, the ethanol molecules diffused to the material and reacted with oxygen ions to be oxidized into water and carbon dioxide, meanwhile the captured electrons were released back to bulk as carriers, as indicated in Fig. 9c and Eqs. 5, 6 respectively. In contrast to the adsorption process, the material conductance increases during the desorption of oxygen.
Deducing from the reaction process, the carrier concentration and oxygen adsorption sites dominate the entire gas sensing process. In this study, porous structure and large specific surface area are conducive to gas diffusion while providing a large number of oxygen adsorption sites. In addition, cobalt doping greatly improves the gas sensitivity performance could be owing to the influence of the electron density and oxygen vacancy concentration. First of all, after cobalt is formed as a substitute impurity in ZnO, it exhibits the characteristics of donor impurity, ionizes free electrons and increases the electron concentration in ZnO. The high electron concentration will promote oxygen molecules’ ionization. Second, due to the different radii between Co3+ and Zn2+ ions, a large number of structural defects will occur in ZnO. According to the defect reaction,\({\text{Co}}^{{3 + }} \left( {{\text{ions}}} \right) + {\text{ZnO}} \to {\text{CoZn}} \cdot + {\text{V}}_{{\text{O}}} + {\text{e}}^{ - }\), in which CoZn· and VO represent the electron donor defects and oxygen vacancies of the material respectively, Co ions can replace ZnO to form an oxygen vacancy [32]. Oxygen vacancy will promote the adsorption of O2, thus forming more adsorbed oxygen, leading to a thicker depletion layer and a higher sensing response.
4 Conclusions
In conclusion, this work prepared pristine and Co doped ZnO through a hydrothermal method and successfully applied it to ethanol detecting. The porous nanosheet-like structure favors gas diffusion and adsorption, and the Co doping greatly increased the response. In terms of 100 ppm ethanol vapor, the 1% Co-ZnO response was high to 279.8, which was 2.7 times than ZnO devices. And after Co doping, the recovery time decreased from 40 to 18 s. In addition, the selectivity of the sensor has improved. Studies have found that Co doping increased the specific surface area of the material and improved the pore size distribution, which played a key role in improving the performance of the sensor. Not only that, the study found that the donor impurity Co increased the internal electron concentration and oxygen vacancy concentration of ZnO, and promoted oxygen molecules adsorption and ionization, thus enhancing sensing properties. This work provides some valuable guidance for using Co to adjust the properties of ZnO to improve sensor characteristics.
References
R.S. Ganesh, M. Navaneethan, V.L. Patil, S. Ponnusamy, C. Muthamizhchelvan, S. Kawasaki, P.S. Patil, Y. Hayakawa, Sensitivity enhancement of ammonia gas sensor based on Ag/ZnO flower and nanoellipsoids at low temperature. Sens. Actuators B 255, 672 (2018)
H. Tian, H. Fan, J. Ma, Z. Liu, L. Ma, S. Lei, J. Fang, C. Long, Pt-decorated zinc oxide nanorod arrays with graphitic carbon nitride nanosheets for highly efficient dual-functional gas sensing. J. Hazard. Mater. 341, 102 (2018)
Y. Wang, X.N. Meng, J.L. Cao, Rapid detection of low concentration CO using Pt-loaded ZnO nanosheets. J. Hazard. Mater. 381, 120944 (2020)
X.H. Liu, J. Zhang, L.W. Wang, T.L. Yang, X.Z. Guo, S.H. Wu, S.R. Wang, 3D hierarchically porous ZnO structures and their functionalization by Au nanoparticles for gas sensors. J. Mater. Chem. 21, 349 (2011)
P. Bharathi, M.K. Mohan, V. Shalini, S. Harish, M. Navaneethan, J. Archana, M.G. Kumar, P. Dhivya, S. Ponnusamy, M. Shimomura, Y. Hayakawa, Growth and influence of Gd doping on ZnO nanostructures for enhanced optical, structural properties and gas sensing applications. Appl. Surf. Sci. 499, 143857 (2020)
M. Drobek, J.H. Kim, M. Bechelany, C. Vallicari, A. Julbe, S.S. Kim, MOF-based membrane encapsulated ZnO nanowires for enhanced gas sensor selectivity. ACS Appl. Mater. Interfaces. 8, 8323 (2016)
O. Lupan, V.V. Ursaki, G. Chai, L. Chow, G.A. Emelchenko, I.M. Tiginyanu, A.N. Gruzintsev, A.N. Redkin, Selective hydrogen gas nanosensor using individual ZnO nanowire with fast response at room temperature. Sens. Actuators B 144, 56 (2010)
L.W. Wang, Y.F. Kang, X.H. Liu, S.M. Zhang, W.P. Huang, S.R. Wang, ZnO nanorod gas sensor for ethanol detection. Sens. Actuators B 162, 237 (2012)
K. Zhang, S.W. Qin, P.G. Tang, Y.J. Feng, D.Q. Li, Ultra-sensitive ethanol gas sensors based on nanosheet-assembled hierarchical ZnO-In2O3 heterostructures. J. Hazard. Mater. 391, 122191 (2020)
T.J. Hsueh, S.J. Chang, C.L. Hsu, Y.R. Lin, I.C. Chen, ZnO nanotube ethanol gas sensors. J. Electrochem. Soc. 155, K152 (2008)
W.W. Guo, T.M. Liu, R. Sun, Y. Chen, W. Zeng, Z.C. Wang, Hollow, porous, and yttrium functionalized ZnO nanospheres with enhanced gas-sensing performances. Sens. Actuators B 178, 53 (2013)
H. Meixner, U. Lampe, Metal oxide sensors. Sens. Actuators B 33, 198 (1996)
Y.S. Xu, L.L. Zheng, C. Yang, W. Zheng, X.H. Liu, J. Zhang, Oxygen vacancies enabled porous SnO2 Thin films for highly sensitive detection of triethylamine at room temperature. Acs Appl. Mater. Interfaces. 12, 20704 (2020)
N. Barsan, D. Koziej, U. Weimar, Metal oxide-based gas sensor research: how to? Sens. Actuators B. 121, 18 (2007)
J. Zhang, X.H. Liu, G. Neri, N. Pinna, Nanostructured materials for room-temperature gas sensors. Adv. Mater. 28, 795 (2016)
J.W. Fang, H.Q. Fan, H.L. Tian, G.Z. Dong, Morphology control of ZnO nanostructures for high efficient dye-sensitized solar cells. Mater. Charact. 108, 51 (2015)
Y.F. Qu, J.J. Ding, H.W. Fu, J.H. Peng, H.X. Chen, Adsorption of CO, NO, and NH3 on ZnO monolayer decorated with noble metal (Ag, Au). Appl. Surf. Sci. 508, 145202 (2020)
Y.A. Zhang, Q. Xiang, J.Q. Xu, High performance chemical sensors constructed by noble metal nanoparticles decorated ZnO nanowires. Sens. Lett. 9, 332 (2011)
J. Wang, C.Y. Hu, Y. Xia, S. Komarneni, Highly sensitive, fast and reversible NO2 sensors at room-temperature utilizing nonplasmonic electrons of ZnO/Pd hybrids. Ceram. Int. 46, 8462 (2020)
S. Aslanzadeh, Transition metal doped ZnO nanoclusters for carbon monoxide detection: DFT studies. J. Mol. Model. 22, 160 (2016)
N. Han, Y.J. Tian, X.F. Wu, Y.F. Chen, Improving humidity selectivity in formaldehyde gas sensing by a two-sensor array made of Ga-doped ZnO. Sens. Actuators B 138, 228 (2009)
H. Nanto, T. Minami, S. Takata, Zinc-oxide thin-film ammonia gas sensors with high-sensitivity and excellent selectivity. J. Appl. Phys. 60, 482 (1986)
A.T. Guntner, V. Koren, K. Chikkadi, M. Righettoni, S.E. Pratsinis, E-nose sensing of low-ppb formaldehyde in gas mixtures at high relative humidity for breath screening of lung cancer? ACS Sens. 1, 528 (2016)
Z.H. Wang, Z.W. Tian, D.M. Han, F.B. Gu, Highly sensitive and selective ethanol sensor fabricated with in-doped 3DOM ZnO. ACS Appl. Mater. Interfaces. 8, 5466 (2016)
G. Namgung, Q.T.H. Ta, W. Yang, J.S. Noh, Diffusion-driven Al-doping of ZnO nanorods and stretchable gas sensors made of doped ZnO nanorods/Ag nanowires bilayers. ACS Appl. Mater. Interfaces 11, 1411 (2019)
S. Park, G.J. Sun, C. Jin, H.W. Kim, S. Lee, C. Lee, Synergistic effects of a combination of Cr2O3-functionalization and UV-irradiation techniques on the ethanol gas sensing performance of ZnO nanorod gas sensors. ACS Appl. Mater. Interfaces. 8, 2805 (2016)
P. Wang, S.Z. Wang, Y.R. Kang, Z.S. Sun, X.D. Wang, Y. Meng, M.H. Hong, W.F. Xie, Cauliflower-shaped Bi2O3-ZnO heterojunction with superior sensing performance towards ethanol. J. Alloys Compd. 854, 157152 (2021)
H.R. Yousefi, B. Hashemi, A. Mirzaei, H. Roshan, M.H. Sheikhi, Effect of Ag on the ZnO nanoparticles properties as an ethanol vapor sensor. Mater. Sci. Semicond. Process. 117, 105172 (2020)
Z. Rafiee, H. Roshan, M.H. Sheikhi, Low concentration ethanol sensor based on graphene/ZnO nanowires. Ceram. Int. 47, 5311 (2021)
S. Wang, F.C. Jia, X.M. Wang, L.Q. Hu, Y.P. Sun, G.C. Yin, T. Zhou, Z.Y. Feng, P. Kumar, B. Liu, Fabrication of ZnO nanoparticles modified by uniformly dispersed Ag nanoparticles: enhancement of gas sensing performance. ACS Omega. 5, 5209 (2020)
D.Z. Zhang, G.K. Dong, Y.H. Cao, Y. Zhang, Ethanol gas sensing properties of lead sulfide quantum dots-decorated zinc oxide nanorods prepared by hydrothermal process combining with successive ionic-layer adsorption and reaction method. J. Colloid Interface Sci. 528, 184 (2018)
J.J. Xu, S.J. Li, L. Li, L.Y. Chen, Y.F. Zhu, Facile fabrication and superior gas sensing properties of spongelike Co-doped ZnO microspheres for ethanol sensors. Ceram. Int. 44, 16773 (2018)
A. Umar, M.A. Khan, R. Kumar, H. Algarni, Ag-doped ZnO nanoparticles for enhanced ethanol gas sensing application. J. Nanosci. Nanotechnol. 18, 3557 (2018)
A.B. Khatibani, M. Abbasi, Effect of Fe and Co doping on ethanol sensing property of powder-based ZnO nanostructures prepared by sol-gel method. J. Sol-Gel. Sci. Technol. 86, 255 (2018)
Y. Wei, X.D. Wang, G.Y. Yi, L.X. Zhou, J.L. Cao, G. Sun, Z.H. Chen, H. Bala, Z.Y. Zhang, Hydrothermal synthesis of Ag modified ZnO nanorods and their enhanced ethanol-sensing properties. Mater. Sci. Semicond. Process. 75, 327 (2018)
Y.F. Mo, F. Shi, S.W. Qin, P.G. Tang, Y.J. Feng, Y.Y. Zhao, D.Q. Li, Facile fabrication of mesoporous hierarchical Co-doped ZnO for highly sensitive ethanol detection. Ind. Eng. Chem. Res. 58, 8061 (2019)
Y.L. Chu, S.J. Young, L.W. Ji, T.T. Chu, K.T. Lam, Y.J. Hsiao, I.T. Tang, T.H. Kuo, Characteristics of gas sensors based on Co-doped ZnO nanorod arrays. J. Electrochem. Soc. 167, 117503 (2020)
X. Zhang, W.Y. Lan, J.L. Xu, Y.T. Luo, J. Pan, C.Y. Liao, L.Y. Yang, W.H. Tan, X.T. Huang, ZIF-8 derived hierarchical hollow ZnO nanocages with quantum dots for sensitive ethanol gas detection. Sens. Actuators B 289, 144 (2019)
Q.Q. Jia, H.M. Ji, Y. Zhang, Y.L. Chen, X.H. Sun, Z.G. Jin, Rapid and selective detection of acetone using hierarchical ZnO gas sensor for hazardous odor markers application. J. Hazard. Mater. 276, 262 (2014)
D.R. Miller, S.A. Akbar, P.A. Morris, Nanoscale metal oxide-based heterojunctions for gas sensing: a review. Sens. Actuators B 204, 250 (2014)
F.B. Gu, H.T. Wang, D.M. Han, Z.H. Wang, Enhancing the sensing performance of SnO2 inverse opal thin films by In and Au doping. Sens. Actuators B 245, 1023 (2017)
S. Seal, S. Shukla, Nanocrystalline SnO gas sensors in view of surface reactions and modifications. JOM 54, 35 (2002)
M.E. Franke, T.J. Koplin, U. Simon, Metal and metal oxide nanoparticles in chemiresistors: does the nanoscale matter? Small 2, 36 (2006)
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Nos. 61974057, 50272026), Applied Research and Development Project of Gansu Academy of Sciences (No. 2018JK-02) and Technological Project of Chengguan District of Lanzhou (No. 2019RCCX0007).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in, or the review of, the manuscript entitled, “Effect of Co-doping on the Performance of Nanosheet-like ZnO Ethanol Gas Sensor”.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, X., Li, H., Huang, D. et al. Effect of Co-doping on the performance of nanosheet-like ZnO ethanol gas sensor. J Mater Sci: Mater Electron 32, 26529–26538 (2021). https://doi.org/10.1007/s10854-021-07029-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-021-07029-4