Abstract
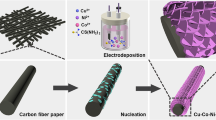
The conversion of insulating polymeric membrane into conducting activated carbon nanofiber (ACF) membrane and the decoration of consequent fibers with flower-like CuO/NiO nanoarchitectures are accomplished, respectively, via the carbonization and electrodeposition processes. The glucose utilization efficacy of CuO/NiO/ACF is accelerated through the diffusion and adsorption of analyte into the nanofibers’ voids and stacked layers, respectively, of ACF and flower-like architectures. The conducting carbon web, binary metal oxide synergism, and porous architecture of CuO/NiO/ACF proliferate the considerable sensitivity (247 µA mM−1 cm−2), low sensing limit (146 nM), and wide linear range (0.00025–5 mM) on glucose sensing along with the real sample analysis. The concordant electrochemical glucose oxidation behavior realized at different bending angles exposes the flexibility of CuO/NiO/ACF. Thus, the free-standing, flexible, binder-less, recyclable, and cost-and time-effective features of CuO/NiO/ACF convenience the glucose detection, affording an innovative technological platform for the evolution of high performance and durable glucose sensors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Diabetes, a chronic top-rated disease afflicts major metabolic complications including heavy stress, cardiovascular disease, depression, anxiety, unconscious syndromes, hyperglycemia, and kidney, nerve, and eye damages in human physiological system, which may eventually lead to death [1]. The growing interest to regulating the glucose level has become a fascinating aspect in medical sectors [2]. The precise and accurate electrochemical technique is headed as a foray for the development of glucose sensors in bio-medical and bio-technology sectors [3]. In this context, the use of direct electron transfer based enzyme-free electrochemical sensor is pronounceable in glucose detection, owing to its productive features including reliability, stability, sensitivity, selectivity, and low cost [4, 5]. However, enzyme-free electrochemical glucose sensors (EFEGSs) suffer with innumerable constrains including high cost of conventional electrodes [glassy carbon electrode (GCE) [6], carbon cloth [7], platinum [8], gold [9], FTO [10], and ITO] [11] and precious metal catalysts, time consuming multi-step processes of cleaning and polishing of electrodes, catalyst modification, and insulating binder utilization [6,7,8,9,10,11]. It collectively hampers the scale-up processes of EFEGSs, urging the design and evolution of time- and cost-efficient free-standing glucose sensor probes.
Hence, a number of research efforts including hydrogel [12], aerogel [13], nickel foam [14], and graphene [15] based free-standing probes were widely devoted to exploring the aspiring configuration for EFEGSs. However, the fabrication of above free-standing electrodes demand the use of sophisticated instruments, expensive processes, toxic chemicals and gases, which confine its applicability in glucose sensors. In this context, polymeric nanofiber membranes are deemed as the proficient glucose sensor probes because of their rational surface area, elevated thermal and physical stabilities, excellent chemical resistance, etc. [16]. Nevertheless, the insulating characteristic of a host polymer limits the electron transfer, prohibiting the glucose electrooxidation kinetics, which would be swamped with the transformation of polymeric frameworks into conductive architectures in the form of activated carbon nanofiber (ACF) membrane.
The modification of carbon nanofibers in ACF with the metal/metal oxide nanomaterials is a coherent and extensible strategy to stimulate the efficient glucose detection. Amid the various transition metal (Pt, Pd, Ag, Au, Ni, Cu, Co, etc.) and metal oxide (ZnO, TiO2, Co3O4, CuO, SnO2, NiO, etc.) nanomaterials [17, 18], CuO nanostructures have established exceptional consideration, owing to its well-known p-type semiconducting behavior (bandgap: approximately 1.35 eV), low cost, abundance in nature, elevated electrical conductivity (3.80 × 10−5 to 2.25 × 10−3 S cm−1), electron affinity (4.05 eV) etc. [19,20,21,22]. Kumar et al. developed the Cu nanoparticles decorated poly(vinylidene fluoride-co-hexafluoropropylene (PVdF-HFP) nanofiber membrane using electrospinning technique and revealed the rational enzyme-free glucose detection properties including the detection limit and linear range, respectively, of 0.011 µM and 0.001 to 6.055 mM. Owing to the insulating host polymeric material of PVdF-HFP, the electrode/electrolyte interfacial properties of resultant electrochemical probe were confined, yielding the sluggish glucose electrooxidation kinetics [23]. On the other side, Zhang et al., developed the CuO/ACFs modified GCE and perceived the detection limit and linear range, respectively, of 0.2 µM and 0.0005 to 11 mM on enzyme-free glucose sensing [24]. However, the ACFs obtained in the form of a powder compels the modification of GCE with the conventional processes, diverging the momentous objective of the use of ACF as a free-standing glucose sensor probe [24]. Beyond the above, the momentous explorations were not accomplished on CuO/ACFs nanocomposites. Furthermore, CuO nanostructures usually suffer with the surface stability issues because of the low oxidation resistance, confining their electrical conductivity [25]. It prohibits the use of CuO nanostructures in commercial practices, which would be tackled with the binary metal oxide and hierarchical architectures. Accordingly, this research effort is intended to transform the non-conducting nanofiber membrane into electrically conducting ACF membrane via the stabilization and carbonization processes. The electrochemical activeness of above ACF membrane toward enzyme-free glucose sensing is promoted via the decoration of ACFs with flower-like CuO/NiO nanostructures. The adequate voids and stacked layers accrued, respectively, in ACFs and flower-like CuO/NiO architectures accelerate the glucose utilization efficacy, while the homogeneously implanted metallic sites on ACFs maximize the electrocatalytic oxidation of glucose. Thus, the free-standing, binder-less, and large surface-volume ratio concerts of as-developed CuO/NiO/ACF probe provides futuristic dimensions for the evolution of high performance and durable EFEGSs at an affordable cost.
2 Experimental
2.1 Chemicals and reagents
Polyacrylonitrile (PAN, M.W. = 1,50,000 g mol−1), N,N-dimethylformamide (DMF, ≥ 99.5%), copper (II) chloride dehydrate (CuCl2·2H2O, AR, ≥ 97%), nickel (II) chloride hexahydrate NiCl2·6H2O, AR, ≥ 97%), ammonium chloride (NH4Cl, AR, ≥ 99%), potassium chloride (KCl, AR, ≥ 95.5%), sodium hydroxide (NaOH, (pellet), AR, ≥ 98%), glucose (GLU, ≥ 99.5%) and other used interfering substrates were acquired from Sigma-Aldrich and used without any further refinement.
2.2 Preparation of ACF membrane
Electrospinning equipment was used to inject the 10 wt% PAN solution at a feeding rate of 0.3 ml h−1 through a stainless-steel nozzle of a plastic syringe. Upon the consistent supply of 12 kV, the ejection of PAN nanofibers was accomplished and the consequent fibers were dropped over the rotating drum collector, which was retained 15 cm away from the stainless-steel nozzle. The as-collected fiber membrane was pre-oxidized at 220 °C for 3 h under ambient atmospheric conditions, consequenced with the carbonization process at 550 °C for 1 h under inert regime.
2.3 Preparation of CuO/NiO/ACF-x membranes
The electrochemical pre-treatment of ACF membrane was implemented using amperometric strategy in 1 M NH4Cl at 2.0 V vs. Ag/AgCl. The pre-treated ACF membrane (1 × 1 cm2—surface area) was located in a mixture of 0.05 M Cu2+ and 0.05 M Ni2+ in 1 M NH4Cl and − 1.2 V vs. Ag/AgCl was applied to the ACF at variant time intermissions for the preparation of CuO/NiO/ACF-x membranes. On the bases of electrodeposition time including 30, 60, 90, 120, and 150 s, the resultant CuO/NiO/ACF membranes are termed, respectively, as CuO/NiO/ACF-30, CuO/NiO/ACF-60, CuO/NiO/ACF-90, CuO/NiO/ACF-120, and CuO/NiO/ACF-150. For comparison, the CuO/ACF-120 membrane was prepared under identical conditions with the use of 0.1 M Cu2+ in 1 M NH4Cl as an electrolyte.
2.4 Material characterizations
The morphological properties of prepared membranes were inspected with Hitachi SU8000 scanning electron microscopy (SEM) and CRYO ARM™ 300 transmission electron microscopy (TEM). The diffraction patterns of prepared ACFs were acquired with Rigaku D/max-2500 X-ray diffraction (XRD) instrument. The structural characterizations of prepared ACFs were acquisitioned with JY-HR800 FT-IR, HORIBA-LabRAMHR Raman, and ESCALAB 250Xi X-ray photo electron spectroscopies (XPS).
2.5 Electrochemical characterizations
The capabilities of prepared ACFs on EFEGS were scrutinized with CHI-650D electrochemical workstation at room temperature in a conventional 3-electrode cell assembly.
3 Results and discussion
3.1 Morphological studies
Morphological features of as-developed fiber membranes are inspected with the use of SEM images (Fig. 1). The smooth, interconnected, and well-organized nanofibers with a mean diameter of 500 nm are witnessed for the as-spun PAN membrane (Fig. 1a). The conversion of PAN into free-standing ACF membrane involves the stabilization and carbonization processes, engaging the conversion of white colored PAN into black colored ACF membrane. The stabilization process accomplished at 220 °C pertains the dehydrogenation and cyclization reactions, constructing the ladder-like structure [26]. The sub-sequenced carbonization process at 550 °C provokes the turbostatic structure with the engagement of aromatic progression and polymerization, yielding the free-standing ACF membrane [27]. The SEM image of ACF membrane establishes the intertwined fibers with the reduced fiber diameter (300 nm), which is due to the removal of polymeric content under the carbonization process (Fig. 1b). The electrodeposition process accomplished at 30 s on ACF membrane yields the scarcely populated CuO/NiO nanoparticles on ACF (CuO/NiO/ACF-30) with a mean diameter of 170 nm (Fig. S1a), while the enhanced population of CuO/NiO nanoparticles along with an increased particle diameter are observed for CuO/NiO/ACF-60 (Fig. S1b). With the 90 s of electrodeposition time, the CuO/NiO nanoparticles are transformed into nanoflakes and their mean length is observed to be 210 nm (Fig. S1c). A further increment in the electrodeposition time of 120 s leads to the enhanced mean length of nanoflakes along with the constitution of 3D rose flower-like morphology and the mean thickness of nanoflakes is observed to be 68 nm (Fig. 1c and d). The self-stacked and intertwined nanoflakes constitute the 3D flower-like architecture with a mean length of 1.3 μm. Upon the supply of current to the counter electrode, the Cu2+/Ni2+ ions are electrochemically processed into CuO/NiO nuclei, followed by the formation of primary particles on ACF membrane. For diminishing the interfacial surface energy, the primary particles are aggregated, preceded with the crystallization and growth of nanoflakes. With the prolonged electrodeposition time, the fresh structures are formed over the primary aggregates, enhancing the population of nanoflakes on ACFs. The occurrence of self-assembly and Ostwald ripening process on nanoflakes stimulate the 3D rose flower-like morphology on ACFs (Fig. S2) [28, 29]. When the electrodeposition time was further increased to 150 s, the flower-like morphologies of CuO/NiO are distorted (CuO/NiO/ACF-150) (Fig. S1d). For comparison, CuO/ACF-120 was developed under identical conditions, displaying the CuO flower-like architectures on ACF (Fig. S1e). The inspected SEM images evidently envisage the uniformly decorated metal oxide nanostructures on carbon fibers with the robust interaction, benefitting the electrochemically active and stable sites for effectual glucose electrooxidation. It is also perceived that the black color of ACF membrane is disrupted upon the electrodeposition process.
TEM image of the nanoflakes of 3D rose flower-like CuO/NiO nanostructures exposes the transparent, smooth, and crumpled morphologies (Fig. 1e). The consequential selected-area diffraction (SAED) pattern realizes polycrystalline structure of CuO/NiO nanoflakes as obvious from the indiscriminately prescribed bright spots and rings (inset of Fig. 1e).
3.2 Diffraction studies
The semi-crystalline structure of PAN membrane is enunciated from the obtained (100) and (002) reflection planes (Fig. 2ia) [30]. The formation of ACF from PAN under the calcination at 220 and 550 °C is witnessed from the vanished and intensified diffraction peak associated with the (100) reflection plane (Fig. 2ib) [31]. The monoclinic structure of CuO in CuO/ACF is certified from the (110), (002), (111), (\(20\overline{2}\)), (020), (202), (220), (311), and (\(22\overline{2}\)) reflection planes, respectively, at 32.6, 35.3, 38.7, 48.6, 53.8, 58.1, 67.9, 72.4, and 75.3° (JCPDS-Card No.: 48-1548) (Fig. 2ic) [32]. Together with the CuO and ACF diffraction peaks, CuO/NiO/ACF reveals the face centered cubic (fcc) structured NiO’s reflection planes including (111), (200), and (311) (JCPDS-Card No.: 71-1179), articulating the generation of CuO/NiO/ACF (Fig. 2i(d)) [33]. The intensified crystalline peaks acquired for the metal oxide nanostructures enunciate the physical integrities of as-developed CuO/ACF and CuO/NiO/ACF membranes.
3.3 Raman studies
PAN membrane depicts the broad Raman band over a wide region, depicting the inadequate patterned atoms (Fig. 2iia) [34]. The transformation of polymeric fibers into ACF is confirmed from the carbonaceous G and D bands, respectively, at 1590 and 1421 cm−1. Furthermore, the 2D band scrutinized at 2783 cm−1 exposes the ordered graphitic structure patterns (Fig. 2iib) [35]. The existence of CuO in CuO/ACF is explored from the Cu(II) vibrational modes of Ag, Bg′, and Bg″, respectively, at 278, 434, and 620 cm−1 (Fig. 2ii(c)) [36]. Together with the CuO and NiO distinctive bands, CuO/NiO/ACF-120 demonstrates the Ni(II) vibrational modes of two phonon excitation (2P) 2TO and longitudinal optical (LO) one phonon mode (1P), respectively, at 712 and 505 cm−1 (Fig. 2iid) [37]. Thus, the perceived Raman spectra clearly anticipate the prevalence of both the carbon and metal oxide units in as-developed CuO/ACF and CuO/NiO/ACF-120 membranes, elucidating their efficacious composite formation.
3.4 XPS studies
CuO/NiO/ACF-120’s XPS spectrum establishes the distinctive C 1s, N 1s, O 1s, Cu 2p, and Ni 2p peaks (Fig. 3a). The core XPS spectrum of C 1s manifests the C–O (286.6 eV), C–C (284.4 eV), and carbidic carbon (282.9 eV) bonds (Fig. 3b) [38]. The heteroatom-N dopant in sp2 carbon atoms of CuO/NiO-120/ACF is enunciated from the substitutional N-doping (396.7 eV) and pyridinic-N bond (398.7 eV) (Fig. 3c) [39]. The O 1s spectrum elucidates the prevalence of lattice oxygen (OL2−) in metal oxide sites, oxygen vacancies (Ov), and adsorbed surface oxygen species, respectively, at 529.1, 530.6, and 532.05 eV (Fig. 3d) [40]. The Cu 2p spectrum exposes the Cu 2p3/2 and Cu 2p1/2 peaks, respectively, at 934.1 and 954.3 eV and the satellite peaks of Cu 2p3/2 and Cu 2p1/2 are comprehended, respectively, at 942.2 and 960.9 eV (Fig. 3e) [41]. The de-convoluted Ni 2P spectrum is fitted with two spin orbit doublets; the prevalence of Ni2+ in as-developed CuO/NiO/ACF-120 is ensured from the bands at 852.8 (Ni 2p3/2) and 871.2 eV (Ni 2p1/2), which are substantially dominant over the distinctive Ni3+ bands found, respectively, at 854.4 (Ni 2p3/2) and 874.2 eV (Ni 2p1/2). The satellite peaks of Ni 2p3/2 and Ni 2p1/2 are established, respectively, at 861.3 and 880.8 eV (Fig. 3f) [42]. The scrutinized XPS spectra not only pronounce the constituents of as-developed CuO/NiO/ACF-120 membrane but also the oxidation states of prevailing metals, which is useful in predicting the glucose electrooxidation mechanism at CuO/NiO/ACF-120 membrane.
3.5 Electrochemical characterizations
The electrochemical behavior of processed PAN and ACF series membranes were investigated with the cyclic voltammetry in 0.1 M NaOH at 50 mV s−1 (Fig. 4a). Owing to the absence of electron mobility channels, PAN membrane demonstrates a lower background current [43], while an improvised background current is witnessed for ACF membrane because of the increased electrical conductivity (0.9 S cm−1) achieved via the formation of graphitic carbon and N-dopant [44]. Upon the decoration of CuO nanoparticles on ACFs (CuO/ACF-120), the well-defined redox peaks with the increased current responses are perceived, owing to the formation of Cu3+/Cu2+ redox pairs and improved electrical conductivity (1.1 S cm−1). The electrooxidation of Cu2+ into Cu3+ is witnessed at under the forward sweep, which is preceded with an electrochemical reduction of Cu3+ into Cu2+ at under the backward sweep (Fig. 4a). The composite formation of CuO with NiO in the form of CuO/NiO/ACF-120 demonstrates the increased redox peak current compared to that of CuO/ACF, which is relevant to the binary metal oxide synergism. With the adsorption of OH− ions and electrochemical oxidation process, the transformation of Cu2+/Ni2+ into Cu3+/Ni3+ is occurred under the forward sweep at 0.53 V vs. Ag/AgCl. The electrochemical conversion of Cu3+/Ni3+ into Cu2+/Ni2+ is witnessed at 0.6 V vs. Ag/AgCl under the backward sweep, which collectively demonstrates the generation of Cu3+/Ni3+/Cu2+/Ni2+redox pairs. CuO/NiO/ACF-120 demonstrates the maximum redox peak current because of its elevated surface area and maximized electrical conductivity (3.3 S cm−1) accomplished via the stacked and interconnected nanoflakes and the engaged electrochemical process is exemplified in Fig. 4b.
a CV responses of ACFs in 0.1 M NaOH at 50 mV s−1, b electrochemical mechanism engaged at CuO/NiO/ACF-120 under alkaline regime, and c CV responses of CuO/NiO/ACF-120 membrane in 0.1 M NaOH under variant sweep rates and the inset of c visualizes the calibration plot of square root of sweep rate vs. peak current
The redox behaviors of CuO/NiO/ACF-120 in 0.1 M NaOH are detected with discrete sweep rates of 10–100 mV s−1. The step-by-step increment of both the oxidation peak current (Ipa) and reduction peak current Ipcs are recognized with an enhanced sweep rate. Moreover, the linear scale witnessed between the Ipas/Ipcs and square root of the sweep rate (Fig. 4c) infers the diffusion-controlled progression for the accomplished electrochemical reaction.
The electrocatalytic activeness of processed free-standing membranes for glucose oxidation was scrutinized with the voltammograms in 0.5 mM glucose/0.1 M NaOH at 50 mV s−1 (Fig. 5a). Owing to the electrochemical inertness accelerated via the absence of active sites and electrical conductivity, PAN membrane does not manifest glucose oxidation and demonstrates the background current [45]. The removal of electrochemically inert sites and N-doping process via the calcination process stimulate the moderate Ipa toward glucose oxidation at ACF membrane. The variation in spin distribution and atomic charge generated via the unpaired electron cloud of N atoms on electron deficient carbon atoms facilitate the active sites of ACF [46]. Moreover, an enhancement in the surface disorders and defects with the N-doping process promotes the electrical conductivity, accelerating the moderate glucose oxidation kinetics [47]. With an integration of CuO nanostructures on ACF, an apparent enhancement in the Ipa on glucose oxidation is witnessed for CuO/ACF at 0.45 V vs. Ag/AgCl, related to the electrocatalytic behavior of Cu3+ active centers. The GOR kinetics is further promoted with the binary metal oxide formation of CuO with NiO as evident from the increased Ipas for CuO/NiO/ACF-x series membranes compared to that of CuO/ACF-120. The mulitlayered ACF exposes a number of cavities and voids among the nanofibers, accelerating the diffusion and accommodation of an electrolyte and maximizes the glucose accessibility to the active centers [48]. The complete carbonization of PAN, N-doping process on carbon lattices, and binary metal oxide synergism accelerate the electrical conductivity of CuO/NiO/ACF-x, minimizing the mass transport resistance of electrons/ions. Moreover, the binder-free electrodeposition of CuO/NiO nanostructures on ACF guarantees the robust adherence of electrochemically active sites with the substrate, limiting the internal resistance of a system [49]. The electrochemically progressive and robust CuO and NiO sites are accountable for the sensitive and stable glucose oxidation kinetics. Owing to the unfilled d-orbitals and unpaired d-electrons, the Cu3+/Ni3+ active centers of CuO/NiO/ACF may generate bonds with glucose and or endiolate anions produced from the deprotonation of glucose under alkaline regime. In general, the free energy of adsorption is strongly reliant upon the number of unpaired ‘d’ electrons per metal atom and their energy levels. In this line, the glucose and surroundings (adjacent metal atoms in CuO/NiO) are deemed as ligands to the catalytic center of central metallic ion and the properties of metal-adsorbate bond is moderated with the surroundings [4, 50]. In this work, the structural and morphological features of CuO/NiO/ACF and their higher metal oxidation states accelerate an interaction of as-developed probe with glucose and or endiolate anions and the elucidative confirmation in exemplifying the affianced interaction is under progress. With the cumulative endeavors of unique morphological and structural features, maximum electrolyte utilization, continual electron conduction paths, accelerated active sites, and multifacet constituents, CuO/NiO/ACF-x series membranes escalate the electrooxidation of glucose. Amid the processed CuO/NiO/ACF-x series, CuO/NiO/ACF-120 reveals the maximum glucose oxidation response. Cu3+/Ni3+ centers engendered at CuO/NiO/ACF-120 under an alkaline regime electrocatalytically oxidizes glucose at 0.55 V vs. Ag/AgCl with an elevated Ipa of 0.0807 mA (Fig. S3). Under an alkaline regime, glucose is initially deprotonated into endiolate anions. The resultant endiol form directly contacts with the Cu3+/Ni3+ centers and electrooxidized into glucanolactone via the two electron transfer process [51] and the research endeavors interconnected in elucidating the above mechanism are under progress. Upon the electrooxidation of glucose into glucanolactone, the higher oxidation states of metallic centers (Cu3+/Ni3+) are transformed into lower oxidation states (Cu2+/Ni2+) and the implicated glucose sensing mechanism is pictorially represented in Fig. 5b.
a CV responses of ACFs in the prevalence of 0.5 mM glucose in 0.1 M NaOH at 50 mV s−1, b glucose electrooxidation mechanism engaged at CuO/NiO/ACF-120 under alkaline regime, c CV responses of CuO/NiO/ACF-120 with diverse glucose concentrations at 50 mV s−1, and d CV responses of CuO/NiO/ACF-120 as a function of sweep rate in 0.5 mM glucose/0.1 M NaOH and the inset of d visualizes the square root of sweep rate vs. peak current
Compared to the 1D nanoparticles and 2D nanoflakes, 3D flower-like morphology constituted with the regularly stacked petals functions as an effectual reservoir for the electrolyte accommodation. The highly interconnected 2D petals accelerate the diffusion paths for efficacious electrolyte penetration, facilitating the accessibility of an electrolyte with the active centers via the enhancement of mass transport of ions/electrons with lower resistance [52].
Additionally, the increasing glucose concentration of 0.5–2.5 mM establishes the substantial improvement in Ipa, intercepting the antifouling concerns of CuO/NiO/ACF-120 under severe electrochemical regimes (Fig. 5c).
The implicated electrochemical reaction kinetics at CuO/NiO/ACF-120 is visualized with the aid of various sweep rates (10–100 mV s−1) against 0.5 mM glucose in 0.1 M NaOH (Fig. 5d). A linear relationship visualized between the Ipa vs. square root of sweep rate declares the diffusion administered process.
The flexibleness of constructed CuO/NiO/ACF-120 on glucose sensing performances was analyzed at the discrete bending spots with the angles of 0° to 180° (Fig. 6a). CuO/NiO/ACF-120’s surface area was retained to 2 × 2 cm2 for investigating its flexible glucose sensing performances. The relevant voltammograms clearly illustrate an inconsiderable variation in the Ipa response at the broad/narrow bending angles (Fig. 6a and b), approving the excellent flexibility of constructed CuO/NiO/ACF-120.
3.6 Amperometric behavior of CuO/NiO/ACF-120 toward glucose detection
The authentic glucose sensing capableness of CuO/NiO/ACF-120 was investigated by monitoring its amperometric consequences against various glucose concentrations in 0.1 M NaOH at 0.55 V vs. Ag/AgCl. The progressive response of CuO/NiO/ACF-120 against glucose is recorded in the form of staircase-like amperogram and the step-by-step increment in amperogram current is speculated with an escalation in analyte concentration (Fig. 7a and inset of Fig. 7a). The hierarchical porous architecture and cavities exerted among the nanofibers and petals of flower-like structure manifest the maximum glucose adsorption [53], upholding the analyte utilization efficacy at CuO/NiO/ACF-120. The interconnected electron conduction channels accomplished via the well-connected nanofibers, heteroatom dopant, and direct electrodeposition of CuO/NiO flower-like nanoarchitectures on ACFs deprived of the practice of a non-conducting binder lower the internal resistance of CuO/NiO/ACF-120 and accelerates the swift electron transport [54]. The binary metal oxide synergism and surface defects of N-dopant efficiently electrooxidize glucose at CuO/NiO/ACF-120 with the considerable glucose sensing performances including the lower detection limit and sensitivity, respectively, of 146 nM and 247 µA mM−1 cm−2. Moreover, CuO/NiO/ACF-120’s current responses act linearly to the broad glucose concentration (0.00025 to 5 mM) (Fig. 7b). The realized glucose detection performances of CuO/NiO/ACF-120 surpass the execution of similar glucose sensors reported till date (Table S1), ameliorating the work function of CuO/NiO/ACF-120 in enzyme-free glucose detection. Generally, the translation of fabricated electrochemical probes into commercial glucose sensors is inhibited with the prevailing constrictions of custom probes such as burdensome polishing, pre-treatment, and catalyst modification, adoption of an insulating binder, steep resistivity, leaching of catalysts, inferior stability, and high expenditure. The aforesaid constrains of usual probes (Table S1) are efficaciously conquered with the directly electrodeposited CuO/NiO flower-like nanostructures on ACFs. Despite the CuO/NiO/ACF-120’s inferior glucose sensing performances than those of few efforts (Table S1), the uncomplicated, affordable, and highly replicable features authenticate it’s constructive applications.
a CuO/NiO/ACF-120’s amperometric behavior toward the succeeding glucose inclusion at 0.55 V vs. Ag/AgCl in 0.1 M NaOH and the inset of a shows amperometric behavior of CuO/NiO/ACF-120 toward 250 nM to 40 µM glucose, b calibration plot of CuO/NiO/ACF-120’s current behavior against the analyte concentration, c CuO/NiO/ACF-120’s glucose amperometric responses in the presence and absence of 0.1 M NaCl at 0.55 V vs. Ag/AgCl, and d interference test of CuO/NiO/ACF-120 against several interfering substrates and glucose under alkaline regimes at 0.55 V vs. Ag/AgCl
Generally, the competent interaction of metal oxide active sites with Cl− ions may lead to the metal–Cl complex, which may reduce the original electrochemical activity of metal oxide nanostructures [55]. In this line, the revelation of developed sensor probe’s capability against Cl− poisoning is essential for practical applications. Accordingly, the amperometric experimentation was established for CuO/NiO/ACF-120 against the gradually mixed glucose at various concentrations with 0.1 M NaCl under alkaline regimes (Fig. 7c and inset of Fig. 7c). The amperograms scrutinized under the presence and lack of Cl− ions are indistinguishable with each other, sustaining the superior Cl− poisoning resistance of CuO/NiO/ACF-120.
The competence in neglecting the intrusive impacts of electrochemically active materials as that of target analyte is considered as a substantial factor in determining the performance of enzyme-free electrochemical glucose sensors [56]. In this line, the anti-interference competence of fabricated CuO/NiO/ACF-120 was scrutinized with the sequential injections of 0.1 mM concentration of prevailing physiologically intervening molecules including UA, U, CA, DA, KCl, NaCl, AA, and AP along with the 0.5 mM glucose in alkaline regime at 0.55 V vs. Ag/AgCl (Fig. 7d). The acquired amperogram postulates that the aforesaid intervening species do not interfere the glucose sensing capability of CuO/NiO/ACF-120. An iso-electric point of CuO/NiO at alkaline regime is estimated as ~ 9.75–10.25, enumerating the negative charges [57]. On the other side, the proton loss stimulated for AA and UA under alkaline regime profound negative charges, manifesting the concrete repelling effect against CuO/NiO with negative charges at CuO/NiO/ACF-120. The fabricated probe has also exposed exquisite anti-interference capability against the reducing sugars of Fru, Gal, Man, Suc, Xyl, and Lac, which are ordinarily found in human blood. Interestingly, the pre-incorporated intervening ions and molecules do not disturb the CuO/NiO/ACF-120’s glucose amperometric consequences, disclosing the superior anti-interference capability of CuO/NiO/ACF-120 on glucose sensing.
3.7 Stability and reproducibility
The consistency of fabricated CuO/NiO/ACF-120 in EFEGS is apprehended through the assessment of amperometric i–t behavior for 70 days in 2 mM glucose/0.1 M NaOH at 0.55 V vs. Ag/AgCl. CuO/NiO/ACF-120 perpetuates 93.1% of its original current response at 70th day of operation (Fig. S4), stating the prodigious firmness of constructed electrochemical probe. The reproducibility of nine indistinguishably fabricated CuO/NiO/ACF-120s was ascertained with their amperomertic i–t behaviors under similar regimes and the obtained superior relative standard deviation (RSD) of 2.5% manifests good reproducibility of constructed probes. CuO/NiO/ACF-120 displays a RSD of 2.3% for nine continual glucose electrooxidation processes, affirming the superior repeatability of a system.
3.8 Real sample analysis
The actualization of CuO/NiO/ACF-120 in real sample analysis is propounded by scrutinizing its electrochemical glucose sensing ability in human serum. The analyte at a pre-established concentration was moderately mixed with the human serum and the externally added analyte’s concentration was scrutinized using amperometric test at 0.55 V vs. Ag/AgCl. CuO/NiO/ACF establishes the substantial RSD and recovery, respectively, in the range of 2.14–2.84% and 98.1–103.8% (Table 1), which are in line with the analytical performances of commercial gluconometer, attesting its apprehension in real sample analysis.
4 Conclusions
PAN membrane fabricated from the electrospinning process was subjected for the stabilization and carbonization processes, followed by the electrodeposition for the decoration of CuO/NiO flower-like architectures on ACFs. The influences of electrodeposition time over the formation of various CuO/NiO morphologies and the growth and formation mechanisms of above nanostructures are elucidated in detail. The significances of fiber diameter, carbonaceous architecture, and binary metal oxide synergism on glucose detection are elaborated with the aid of various electroanalytical techniques, guiding the innovative attributes for the procession of flexible EFEGS probes. The porous architectures of ACFs and flower-like frameworks of CuO/NiO nanostructures promote the glucose utilization efficiency, while the carbonaceous architectures and heteroatom doping facilitate the electron transportation behavior, which collectively endorses the glucose electrooxidation kinetics at CuO/NiO/ACF-120. Thus, the front-most work functions of glucose detection probes including sensitive, selective, and stable features are accomplished with the developed CuO/NiO/ACFs and the farther endeavors affiliated with the evolution of miniaturized, transportable, flexible, and affordable EFEGS with CuO/NiO/ACFs are under progression.
References
J. Tsai, E.S. Ford, C. Li, G. Zhao, L.S. Balluz, Physical activity and optimal self-rated health of adults with and without diabetes. BMC Public Health 10, 365–373 (2010)
C. Rivet, H. Lee, A. Hirsch, S. Hamilton, H. Lu, Microfluidics for medical diagnostics and biosensors. Chem. Eng. Sci. 66, 1490–1507 (2011)
S.A. Zaidi, J.H. Shin, Recent developments in nanostructures based elecrochemcial glucose sensors. Talanta 149, 30–42 (2016)
G.G. Kumar, G. Amala, S.M. Gowtham, Recent advancements, key challenges and solutions in non-enzymatic electrochemical glucose sensors based on graphene platforms. RSC Adv. 7, 36949–36976 (2017)
P.P. Tomanin, P.V. Cherepanov, Q.A. Besford, A.J. Christofferson, A. Amodio, C.F. McConville, I. Yarovsky, F. Caruso, F. Cavalieri, Cobalt phosphate nanostructures for non-enzymatic glucose sensing at physiological pH. ACS Appl. Mater. Inter. 10, 42786–42795 (2018)
L. Zhao, G. Wu, Z. Cai, T. Zhao, Q. Yao, X. Chen, Ultrasensitive non-enzymatic glucose sensing at near-neutral pH values via anodic stripping voltammetry using a glassy Carbon electrode modified with Pt3Pd nanoparticles and reduced graphene oxide. Microchim. Acta 182, 2055–2060 (2015)
X. Wang, Y. Zheng, J. Yuan, J. Shen, J. Hu, A.J. Wang, L. Wu, L. Niu, Three-dimensional NiCo layered double hydroxide nanosheets array on carbon cloth, facile preparation and its application in highly sensitive enzymeless glucose detection. Electrochim. Acta 224, 628–635 (2017)
G. Wang, X. He, L. Wang, A. Gu, Y. Huang, B. Fang, B. Geng, X. Zhang, Non-enzymatic electrochemical sensing of glucose. Microchim. Acta 180, 161–186 (2013)
Y. Bai, W. Yang, Y. Sun, C. Sun, Enzyme-free glucose sensor based on a three-dimensional gold film electrode. Sens. Actuators B Chem. 134, 471–476 (2008)
J. Zhang, X. Xiao, Q. He, L. Huang, S. Li, F. Wang, A nonenzymatic glucose sensor based on a copper nanoparticle-zinc oxide nanorod array. Anal. Lett. 47, 1147–1161 (2014)
T. Wang, Y. Yu, H. Tian, J. Hu, A novel non-enzymatic glucose sensor based on cobalt nanoparticles implantation-modified indium tin oxide electrode. Electroanalysis 26, 1–9 (2014)
Y.L.T. Ngo, L. Sui, W. Ahn, J.S. Chung, S.H. Hur, NiMnO4 spinel binary nanostructure decorated on three-dimensional reduced graphene oxide hydrogel for bifunctional materials for non-enzymatic glucose sensor. Nanoscale 9, 19318–19327 (2017)
J. Yang, W. Tan, C. Chen, Y. Tao, Y. Qin, Y. Kong, Nonenzymatic glucose sensing by CuO nanoparticles decorated nitrogen-doped graphene aerogel. Mater. Sci. Eng. C 78, 210–217 (2017)
M. Ma, W. Zhu, D. Zhao, Y. Ma, N. Hu, Y. Suo, J. Wang, Surface engineering of nickel selenide nanosheets array on nickel foam: an integrated anode for glucose sensing. Sens. Actuators B Chem. 278, 110-116[J] (2019)
M. Zhang, A. Halder, C. Hou, J. Ulstrup, Q. Chi, Free-standing and flexible graphene papers as disposable non-enzymatic electrochemical sensors. Bioelectrochemistry 109, 87–94 (2016)
A. Senthamizhan, B. Balusamy, T. Uyar, Glucose sensors based on electrospun nanofibers: a review. Anal. Bioanal. Chem. 408, 1285–1306 (2016)
D.W. Twang, S. Lee, M. Seo, T.D. Chung, Recent advances in electrochemical non-enzymatic glucose sensors—a review. Anal. Chim. Acta 1033, 1–34 (2018)
K. Tian, M. Prestgard, A. Tiwari, A review of recent advances in nonenzymatic glucose sensors. Mater. Sci. Eng. 41, 100–118 (2018)
Q. Bao, C.M. Li, L. Liao, H. Yang, W. Wang, C. Ke, Q. Song, H. Bao, T. Yu, K.P. Loh, J. Guo, Electrical transport and photovoltaic effects of core–shell CuO/C60 nanowire hetero structure. Nanotechnology 20, 065203–065210 (2009)
Y. Zhong, T. Shi, Z. Liu, S. Cheng, Y. Huang, X. Taol, G. Liao, Z. Tang, Ultrasensitive non-enzymatic glucose sensors based on different copper oxide nanostructures by in-situ growth. Sens. Actuators B Chem. 236, 326–333 (2016)
P. Thongbai, T. Yamwong, S. Maensiri, Correlation between giant dielectric response and electrical conductivity of CuO ceramic. Solid State Commun. 147, 385–387 (2008)
Y.B. Zhang, J. Yin, L. Li, L.X. Zhang, L.J. Bie, Enhanced ethanol gas-sensing properties of flower-like p-CuO/n-ZnO heterojunction nanorods. Sens. Actuators B Chem. 202, 500–507 (2014)
T.R. Kumar, K.J. Babu, D.J. Yoo, A.R. Kim, G.G. Kumar, Binder free and free-standing electrospun membrane architecture for sensitive and selective non-enzymatic glucose sensors. RSC Adv. 5, 41457–41467 (2015)
J. Zhang, X. Zhu, H. Dong, X. Zhang, W. Wang, Z. Chen, In situ growth cupric oxide nanoparticles on carbon nanofibers for sensitive non enzymatic sensing of glucose. Electrochim. Acta 105, 433–438 (2013)
Y. Zhong, T. Shi, Z. Liu, S. Cheng, Y. Huang, X. Tao, G. Liao, Z. Tang, Ultrasensitive non-enzymatic glucose sensors based on different copper oxide nanostructures by in-situ growth. Sens. Actuators B Chem. 236, 326–333 (2016)
S.M. Saufi, A.F. Ismail, Development and characterization of polyacrylonitrile (PAN) based carbon hollow fiber membrane. Membr. Sci. Technol. 24, 843–854 (2002)
B. Zhang, Y. Yu, Z.L. Xu, S. Abouali, M. Akbari, Y.B. He, F. Kang, J.K. Kim, Correlation between atomic structure and electrochemical performance of anodes made from electrospun carbon nanofiber films. Adv. Energy Mater. 4, 1301448–1301456 (2013)
M. Figiela, M. Wysokowski, M. Galinski, T. Jesionowski, I. Stepniak, Synthesis and characterization of novel copper oxide-chitosan nanocomposites for non-enzymatic glucose sensing. Sens. Actuators B Chem. 272, 296–307 (2018)
S.K. Shinde, D.P. Dubal, G.S. Ghodake, V.J. Fulari, Hierarchical 3D-flower-like CuO nanosructure on copper foil for supercapacitors. RSC Adv. 5, 1–19 (2015)
P. Tamilarasan, S. Ramaprabhu, Graphene based all-solid-state supercapacitors with ionic liquid incorporated polyacrylonitrile electrolyte. Energy 51, 374–381 (2013)
Y. Huang, F. Cui, Y. Zhao, J. Lian, J. Bao, H. Li, Controlled growth of ultrathin NiMoO4 nanosheets on carbon nanofiber membrane as advanced electrodes for asymmetric supercapacitors. J. Alloys Compd. 753, 176–185 (2018)
X. Duan, H. Huang, S. Xiao, J. Deng, G. Zhou, Q. Li, T. Wang, 3D herarchical CuO mesocrystals from ionic liquid precursor: towards better electrochemical performance for Li-ion Batteries. J. Mater. Chem. A 4, 8402–8411 (2016)
C. Dong, X. Xiao, G. Chen, H. Guan, Y. Wang, I. Djerdj, Porous NiO nanosheets self-grown on alumina tube using a novel flash synthesis and their gas sensing properties. RSC Adv. 5, 4880–4885 (2015)
R. Zhao, Y. Wang, X. Li, B. Sun, Y. Li, H. Ji, J. Qiu, C. Wang, Surface activated hydrothermal carbon-coated electrospun PAN fiber membrane with enhanced adsorption properties for herbicide. ACS Sustain. Chem. Eng. 4, 2584–2592 (2016)
J.H. Zhou, Z.J. Sui, J. Zhu, P. Li, D. Chen, Y.C. Dai, W.K. Yuan, Characterization of surface oxygen complexes on carbon nanofibers by TPD, XPS and FT-IR. Carbon 45, 785–796 (2007)
G.H. Tsai, P.H. Fei, C.M. Lin, S.L. Shiu, CuO and CuO/Graphene nanostructured thin films as counter electrodes for Pt-Free dye-sensitized solar cells. Coatings 8, 21–33 (2018)
J. Saravanan, R. Ramasamy, H.A. Therese, G. Amala, G.G. Kumar, Electrospun CuO/NiO composite nanofibers for sensitive and selective non-enzymatic nitrite sensors. New J. Chem. 41, 14766–14771 (2017)
Y. Wang, F. Qu, J. Liu, Y. Wang, J. Zhou, S. Ruan, Enhanced H2S sensing characteristics of CuO-NiO core-shell microspheres sensors. Sens. Actuators B Chem. 209, 515–523 (2015)
E.A.D.S. Filho, E.F. Pieretti, R.T. Bento, M.F. Pillis, Effect of nitrogen-doping on the surface chemistry and corrosion stability of TiO2 films. J. Mater. Res. Technol. 9(1)), 922–934 (2020)
S. Zhu, J. Lei, Y. Qin, L. Zhang, L. Lu, Spinal oxide CoFe2O4 grown on Ni foam as an efficient electrocatalyst for oxygen evolution reaction. RSC Adv. 9, 13269–13274 (2019)
D.P. Dubal, G.S. Gund, R. Holze, H.S. Jadhav, C.D. Lokhande, C.J. Park, Surfactant-assisted morphological tuning of hierarchical CuO thin films for electrochemical supercapacitors. Dalton Trans. 42, 6459–6467 (2013)
Z. Qiu, H. Gong, G. Zheng, S. Yuan, H. Zhang, X. Zhu, H. Zhou, B. Cao, Enhanced physical properties of pulsed laser deposited NiO films via annealing and lithium doping for improving perovskite solar cell efficiency. J. Mater. Chem. C 5, 7084–7094 (2017)
E. Mirzael, J. Ai, M. Sorouri, H. Ghanbari, J. Verdi, R.F. Majidi, Functionalization of PAN based electrospun carbon nanofibers by acid oxidation: study of structural, electrical and mechanical properties. Fuller. Nanotubes Carbon Nanostruct. 3, 1–30 (2015)
Y. Hou, S. Cui, Z. Wen, X. Guo, X. Feng, J. Chen, Strongly coupled 3D hybrids of N-doped porous carbon nanosheet/CoNi alloy-encapsulated carbon nanotubes for enhanced Electrocatalysis. Small 11, 5940–5948 (2015)
Y. Chen, H. Zhang, H. Xue, X. Hu, G. Wang, C. Wang, Construction of a non-enzymatic glucose sensor based on copolymer P4VP-co-PAN and Fe2O3 nanoparticles. Mater. Sci. Eng. 35, 420–425 (2014)
Z. Sun, Y. Jiang, L. Zeng, X. Zhang, S. Hu, L. Huang, Controllable local electronic migration induced charge separation and red-shift emission in carbon nitride for enhanced photocatalysis and potential phototherapy. Chem. Commun. 55, 6002–6005 (2019)
D. Geng, S. Yang, Y. Zhang, J. Yang, J. Liu, R. Li, T.K. Sham, X. Sun, S. Ye, S. Knights, Nitrogen doping effects on the structure of graphene. Appl. Surf. Sci. 257, 9193–9198 (2011)
Q. Wang, Y. Ma, X. Jiang, N. Yang, Y. Coffinier, H. Belkhalfa, N. Dokhane, M. Li, R. Boukherroub, S. Szunerits, Electrophoretic deposition of carbon nanofibers/Co(OH)2 nanocomposites: application for non-enzymatic glucose sensing. Elecroanalysis 28, 119–125 (2016)
V. Archana, Y. Xia, R. Fang, G.G. Kumar, Hierarchical CuO/NiO-carbon nanocomposite derived from metal organic framework on cello tape for the flexible and high performance nonenzymatic electrochemical glucose sensors. ACS Sustain. Chem. Eng. 7, 6707–6719 (2019)
D. Pletcher, Electrocatalysis: present and future. J. Appl. Electrochem. 14, 403–415 (1984)
S.K. Meher, G.R. Rao, Archetypal sandwich-structured CuO for high performance non-enzymatic sensing of glucose. Nanoscale 5, 2089–2099 (2013)
L. Liu, Z. Wang, J. Yang, G. Liu, J. Li, S. Chen, Q. Guo, NiCo2O4 nanoneedle-decorated electrospun carbon nanofiber nanohybrids for sensitive non-enzymatic glucose sensors. Sens. Actuators B Chem. 258, 920–928 (2018)
K.J. Babu, S. Sheet, Y.S. Lee, G.G. Kumar, Three-dimensional dendrite Cu-Co/reduced graphene oxide architectures on a disposable pencil graphite electrode as an electrochemical sensor for nonenzymatic glucose detection. ACS Sustain. Chem. Eng. 6, 1909–1918 (2018)
R. Qiu, X.L. Zhang, R. Qiao, Y. Li, Y. Kim, Y.S. Kang, CuNi dendritic material: synthesis, mechanism discussion, and application as glucose sensor. Chem. Mater. 19, 4174–4180 (2007)
K.J. Babu, T. Rajkumar, D.J. Yoo, P. Siew-Moi, G.G. Kumar, Electrodeposited nickel cobalt sulphide flower-like architectures on disposable cellulose filter paper for enzyme-free glucose sensor applications. ACS Sustain. Chem. Eng. 6, 16982–16989 (2018)
S. Shakir, J. Saravanan, N. Rizan, K.J. Babu, Md.A. Aziz, P.S. Moi, V. Periasamy, G.G. Kumar, Fabrication of capillary force induced DNA template Ag nanopatterns for sensitive and selective enzyme-free glucose sensors. Sens. Actuators B Chem. 256, 820–827 (2018)
P. Salazar, V. Rico, A.R. Gonzalez-Elipe, Nickel–copper bilayer nanoporous electrode prepared by physical vapor deposition at oblique angles for the non-enzymatic determination of glucose. Sens. Actuators B Chem. 226, 436–443 (2016)
Acknowledgements
This research effort was supported by the Council of Scientific and Industrial Research (CSIR), New Delhi Major Project Grant No.: 01(2997)19/EMR-II and Department of Science & Technology (DST), New Delhi Major Project Grant No.: DST/TMD/HFC/2K18/52. The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through Research Group Project under grant number R.G.P.2/96/42.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Saravanan, J., Pannipara, M., Al-Sehemi, A.G. et al. Flower-like CuO/NiO nanostructures decorated activated carbon nanofiber membranes for flexible, sensitive, and selective enzyme-free glucose detection. J Mater Sci: Mater Electron 32, 24775–24789 (2021). https://doi.org/10.1007/s10854-021-06927-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-021-06927-x