Abstract
Nanomaterials play important role in performance of dye-sensitized solar cells. In this paper, highly phase pure anatase TiO2 microspheres were synthesized using a low-cost hydrothermal route. Initially, X-ray diffraction studies and Raman spectroscopic analysis were carried out, and the formation of tetragonal structure of TiO2 with the anatase phase was confirmed. The UV–Vis DRS studies showed the excellent reflectance and optical band-gap energy of 3.29 eV. The well-interconnected spherical nanoparticles with different sizes were examined by Field Emission Scanning Electron Microscopic analysis. The fabricated dye-sensitized solar cell (DSSC) composed of prepared TiO2 microspheres as photoanode exhibited a higher power conversion efficiency (PCE) (η) of 5.4% as compared to commercial P25 with PCE of 3.6%. The higher Jsc (12.03 mA/cm2) in the fabricated DSSC due to efficient dye loading capacity and high light-scattering property was also observed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
According to Nobel laureate Richard Smalley, the big challenge for the next 50 years is mainly in energy conservation and developments towards it to overcome environmental problems [1]. As compared to other renewable energy sources, solar energy proves to be most preferred source due to its continuous and abundant energy supply. The ‘Golden Triangle’ issues were considered to be an important thing to develop photovoltaic (PV) technology. The issue contains decreasing the device cost, enhancing the power conversion efficiency, and improving the long-term stability [2].Among three generations of solar cells, third-generation solar cells have the ability to rectify the aforementioned issues. Till date, the third-generation solar cells such as perovskite solar cells (PSC) (η = 22%) [3], dye-sensitized solar cells (DSSC) (η = 14%) [4], organic solar cells (OSC) (η = 18%) [5], and quantum dot-sensitized solar cells (QDSSC) (η = 16%) [6] were employed due to their superior performance and cost efficiency. More recently, DSSCs have received great attention due to their lower cost, easy fabrication process, lightweight, and high efficiency. Till date, these cells have shown the maximum power conversion efficiency of 14.3% and 14.7% under 100 mW/cm2 and 50 mW/cm2, respectively [4]. The cutting edge of research mainly focuses on all DSSCs components such as semiconductor photoanode, highly electrocatalytic counter electrodes, sensitizers, and electrolytes. To achieve an excellent performance of photoanodes, larger surface area, fast electron transport kinetics, and large light-scattering effects are required [7]. TiO2 is a wide band-gap semiconductor metal oxide and is effectively used in versatile applications like photocatalysis [8], Li-ion battery [9], hydrogen production [10], antireflective coatings [11], gas sensors [12], optical filters [13], water splitting [14], and photoelectrochemical solar energy conversion [15]. Due to its unique features such as the high surface area to absorb dye molecules, long-term stability, ease of availability, cost-affordability, non-toxic nature, compatible optical and electronic properties, it is specifically used as photoanode in DSSCs [16]. As compared to other photoanode materials like ZnO [17], SnO2[18], WO3[19], Nb2O5 [20], SrTiO2 [21], Zn2SnO4 [22] and BaSnO3 [23], the anatase TiO2 photoanode exhibits the highest power conversion efficiency of about 14% [4]. Various nanostructures like nanotubes [24], nanowires [25], nanofibers [26], hollow spheres [27], and microspheres of TiO2 [28,29,30] were effectively used as photoanode material. Among these aforementioned nanostructures, microspheres were extensively used in DSSC due to their larger surface area, large sphere diameter, superior dye adsorption ability, better light-scattering property, and efficient interconnection of nanospheres [31]. Several researchers have adopted various techniques to synthesize the microspheres using high-temperature hydro/solvothermal treatment [32, 33], electrospray preparation [34], low-temperature copolymer synthesis [35], template-free chemically induced self-transformation [36], and electrospun process [37].
In this investigation, the authors have synthesized well-interconnected TiO2 microspheres (TiO2 MS) by hydrothermal method and fabricated the DSSC using prepared TiO2 as photoanode. The detailed structural and morphological properties were studied using powder X-ray diffraction (XRD) analysis, Raman spectroscopic analysis, and field emission scanning electron microscopic (FESEM) analysis. UV–Vis spectroscopic analysis was carried out to study the optical properties, and the optical band-gap energy was found to be 3.29 eV. The fabricated DSSC performance was evaluated, and the PCE (5.4%) from J-V curves, IPCE (56%), and electron lifetime τ (25.24 ms) from EIS were obtained. It was noted that TiO2 MS-based photoanode exhibits superior DSSC performance than P25-based photoanode due to its better light-scattering ability and electron transport kinetics.
2 Experimental section
2.1 Materials used
The following reagents or chemicals were used to prepare TiO2 MS and fabrication of DSSCs without any further purifications. Fluorine-doped tin oxide (FTO, 8Ω/square) substrates, Pluronic 123 (99%), Titanium tetraisopropoxide (99%), Acetylacetone (99%), and Polyoxyethylene (10) octyl phenyl ether (Triton-X-100) were purchased from Sigma Aldrich. Titanium tetrachloride (99%) was purchased from Spectrochem. Cis- diisothiocyanato-bis(2,2’-bipyridyl-4,4’dicarboxylato) ruthenium (II) bis (tetrabutylammonium) (known as N719 dye), Platisol T/SP, and Iodolyte HI-30 were purchased from Solaronix SA, Switzerland, Tetrabutanol, Ethanol (99%), Hydrochloric acid (32%), and Acetonitrile were purchased from Merck.
2.2 Synthesis of TiO2 microspheres
In this typical synthesis procedure, the hydrothermal method was adopted for synthesis of TiO2 MS, and it is as shown in Fig. 1. Initially, 3 mL of absolute ethanol containing 0.2 g of pluronic 123 was stirred for 15 min, and it is termed as solution A for future reference. On the other hand, 1 mL of titanium tetraisopropoxide was added into solution B containing 0.7 mL of concentrated HCl and stirred for 15 min. Then, solution B was successively added into solution A and stirred for 30 min. The resultant solution was kept at ambient temperature without any disturbance for 24 h. After that, 12 mL of ethanol was added into the resultant mixture and transferred into Teflon-lined stainless steel autoclave for hydrothermal reaction in a hot-air oven at 150 °C for 24 h. The obtained product was rinsed several times with deionized water and ethanol to remove unwanted impurities. The resulting material was dried at 80 °C for 24 h in a vacuum oven. The dried material was annealed at 500 °C for 1 h in a muffle furnace.
2.3 Assembly of DSSC
Various steps were followed for assembly of DSSC: Initially, 1 g of prepared TiO2 MS was mixed with 0.1 mL of acetylacetone and grounded well with the aid of agate mortar and pestle. The grounded products were stirred with a solution containing a 1:1 volume ratio of ethanol and water. 0.4 mL of Triton-X was added to the above solution to form a homogenous TiO2 paste. The FTO glass substrate was cleaned with deionized water and ethanol using an ultrasonication bath for 15 min. The cleaned FTO substrate was soaked with 0.2 M of aqueous TiCl4 solution at 70 °C for 30 min and rinsed with ethanol and heated at 500 °C for 30 min (Pre-TiCl4 treatment). After that, the doctor blade technique was adopted to coat TiO2 paste on FTO substrate, and the coated FTO substrate was sintered at 500 °C for 1 h to evaporate the binder materials in the coated films. The thickness of the sample coated on FTO substrate was found to be ~ 14 µm using a thickness profilometer. The post-TiCl4 treatment on sintered TiO2 film was followed like pre-TiCl4 treatment process. A similar process was followed to prepare commercial P25 film on FTO substrate. The resultant films were soaked in 0.5 mM of N719 dye in a 1:1 volume ratio of tertbutanol (10 mL) and acetonitrile (10 mL) solution for 24 h under dark conditions. The platinum (Pt) counter electrode was coated on another FTO substrate by doctor blade technique with Platisol T/SP as the platinum precursor and thermally decomposed at 400 °C for 30 min. At last, the dye-adsorbed photoanode and Pt counter electrodes were sandwiched together. To complete the device fabrication, a small amount of Iodolyte HI-30 as an aqueous electrolyte was injected in-between the two electrodes and the active area of the cell was found to be 0.25 cm2 [38].
2.4 Characterization techniques
The crystallographic information of prepared TiO2 MS was analyzed using a powder X-ray diffraction (EMPYREAN X-RAY DIFFRACTOMETER, PANALYTICAL, NETHERLANDS) instrument having a Cu-Kα radiation wavelength of 1.5406 Å. Micro-Raman measurements were carried out using (RENISHAW InVia Confocal Raman microscope, United Kingdom). Field Emission Scanning Electron Microscope (ZEISS, ΣIGMA, GHBM, GERMANY) was used to analyze the morphological properties of the prepared TiO2 MS. The optical properties of the prepared material were analyzed using UV-Vis DRS (PerkinElmer, Lambda 35 spectrophotometer). The current density–voltage characteristics of fabricated DSSC were measured using Keithley digital source meter 2400 under 100 mW/cm2 illumination coming from a solar simulator (UHE-16 equipped with a 150 W Xenon Arc lamp and an AM 1.5 filter, Sciencetech, Canada). The light intensity was calibrated with a standard crystalline silicon solar cell to carry out the J-V measurements. Incident photon-to-current conversion efficiency (IPCE) data were collected in the wavelength from 350 to 800 nm by using QE system (Enlitech, QE-R). The electrochemical impedance measurement of the fabricated DSSC was analyzed using an electrochemical workstation (FRA/VERSASTAT3, V3-500, Princeton Applied Research, USA) under the illumination at open-circuit voltage.
3 Results and discussions
3.1 X-ray diffraction analysis
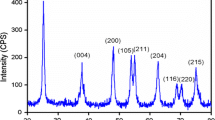
Phase identification, crystalline features, and average crystallite size of hydrothermally synthesized TiO2 MS and commercial TiO2 (P25) were examined by powder XRD analysis, and the graphs are as shown in Fig. 2. From Fig. 2, it is observed that TiO2 MS possesses a tetragonal crystal structure of an anatase phase with preferred (101) orientation at 2θ = 25.32°. All the diffraction peaks are well matched with JCPDS No: 21–1272 (a = b = 3.785, c = 9.513) with a crystallographic space group of I41/amd. The diffraction peaks located at 25.31°, 37.86°, 48.07°, 53.97°, 55.09°, 62.72°, 68.85°, 70.31°, and 75.15° were corresponding to (101), (004), (200), (105), (211), (204), (116), (220), and (215) crystal planes, respectively. No other additional diffraction peaks corresponding to metallic Ti and other impurity phases like rutile and brookite were observed. Hence, it is evident that synthesized material has pure phase and crystalline nature [28]. The commercial TiO2 (P25) has a mixed phase of both anatase and rutile. In Fig. 2, the notations A and R represent anatase and rutile phase, respectively. The average crystallite size was found to be 18 nm with the aid of Debye–Scherrer formula, which is as follows:
where ‘D’ is the average crystallite size (nm), ‘k’ is the shape factor which corresponds to 0.9, ‘λ’ is the wavelength of X-ray used (1.5406 Å), ‘β’ is the full width at half maximum of prominent diffraction peak (degree), and ‘θ’ is the Bragg diffraction angle (degree) [39].
3.2 Raman analysis
Figure 3 shows the Raman spectra of synthesized TiO2 MS. The Raman scattering peaks obtained at 144 cm−1, 197 cm−1, 397 cm−1, 516 cm−1, and 639 cm−1 were ascribed to E1g, E1g, B1g, A1g, and E1g-active vibrational Raman modes. The peaks at 144 cm−1, 197 cm−1, and 639 cm−1correspond to E1g mode which are attributed to the symmetric stretching mode of the O–Ti–O bond. The B1g and A1g modes of peaks at 397 cm−1 and 516 cm−1 mainly correspond to symmetric and antisymmetric vibration-bending modes of O–Ti–O bond, respectively. Here, all the observed characteristics peaks belong to the typical anatase TiO2 phase, and no other additional phases were observed from Raman spectra which are also in good agreement with XRD results [40,41,42].
3.3 UV–visible spectroscopic and dye desorption studies
To study the optical properties of the prepared TiO2 MS, UV–Vis DRS spectroscopic measurements were carried out. Figure 4 shows diffused reflectance spectra of prepared TiO2 microspheres which were recorded in the wavelength range of 300 to 1100 nm. The prepared TiO2 microspheres exhibit excellent reflectance which will enhance the light-scattering property. The optical band-gap energy of prepared TiO2 microspheres was determined using Kubelka–Munk function method. The relation is given as follows:.
where F(R) is the Kubelka–Munk function and R is the reflectance. F(R) is directly proportional to absorption coefficient (K) and inversely proportional to scattering coefficient (S) [43]. To estimate the band-gap energy, the graph plotted between hυ and (hυF(R))2 and the band-gap energy was found to be 3.29 eV which is in good agreement with bulk TiO2.
To estimate the dye loading performance of TiO2 MS and P25, N719 dye-adsorbed photoanodes were immersed in 30 ml of solution containing 0.1 M of NaOH solution and amount of desorbed dye molecules was characterized by UV–Vis spectroscopic analysis. Figure 5 shows the UV–Vis absorbance spectra of N719 dye and desorbed dye solutions from TiO2 MS and P25 in NaOH solution. From this graph, it can be observed that the dye loaded on synthesized TiO2 MS is much higher than P25 which will offer excellent current density.
3.4 FESEM analysis
The surface morphological properties of the prepared TiO2 MS and commercial TiO2 (P25) were characterized by field emission scanning microscopic analysis, and the FESEM micrograph is shown in Fig. 6a and b. From this micrograph, the TiO2 MS exhibits smoothly well-defined interconnected nanospheres of various sizes. These microspheres will provide better pathway for electron transport. There is some variation in the size of the microspheres. This could be due to some incomplete hydrolysis which occurred during the hydrothermal process resulting into the formation of irregular-shaped TiO2 microspheres [44]. This can be tuned or controlled by optimizing experimental process parameters.
3.5 Photovoltaic performance of the fabricated dye-sensitized solar cell
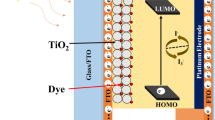
Figure 7 shows the schematic representation of DSSC consisting of semiconductor metal oxide (TiO2 MS) coated on FTO substrate sensitized with N719 ruthenium complex dye for absorbing visible light, I3−/I−-based redox electrolytes for reducing oxidized dye and boosting the redox couple regeneration, a platinum counter electrode for collecting the electrons from the external circuit. According to the principle of DSSC, the voltage generated under the illumination of light radiation corresponds to the difference between the Fermi level of the electron in the binary semiconductor metal oxide and the redox potential of the electrolyte.
Figure 8 demonstrates the current density–voltage (J–V) characteristics of fabricated DSSC using prepared TiO2 MS as photoanode sensitized with N719 dye for 24 h. The obtained solar cell parameters such as open-circuit voltage (Voc), short-circuit current density (Jsc), fill factor (FF), and power conversion efficiency (η) are tabulated in Table 1. The fill factor (FF) and power conversion efficiency (η) can be expressed as follows:
where Voc and Jsc are open-circuit voltage (V) and short-circuit current density (mA/cm2) of DSSC, respectively. Vmax and Imax are the maximum voltage and current, respectively. Pin is the incident light power (100 mW/cm2) [41].
The results obtained for the fabricated device exhibit the short-circuit current density (Jsc) about 12.03 mA/cm2, open-circuit voltage (Voc) about 0.716 V, fill factor (FF) about 0.63, and the power conversion efficiency (η) of 5.4%. At the same time, the device fabricated using P25 exhibits the short-circuit current density (Jsc) of about 9.64 mA/cm2, open-circuit voltage (Voc) of 0.612 V, fill factor about 0.59, and the power conversion efficiency of about (η) 3.6%. Basically, the Jsc of the fabricated DSSC was estimated by the initial number of photo-generated electrons injected into the conduction band of TiO2 photoanode, which is significantly influenced by the light-harvesting performance of the photoanode including the scattering effect of the photoanode structure and the overall dye loading amount [45]. In DSSC, the incident light loss occurs due to the backscattering of incident light in the FTO/TiO2 interface. The small underlayer consisting of small-sized TiO2 nanoparticles or TiCl4 treatments (pre or post) effectively showed the superior photocurrent density and the power conversion efficiency because of greater effect on electronic transport and causes the downward shift in the band edge [45, 46].
Incident photon-to-current conversion efficiency (IPCE) measurements were carried out to understand the performance of photoelectrodes which is shown in Fig. 9. The IPCE can be expressed as follows [47]
where J is current density (mA/cm2), λ is the incident light wavelength (nm), and Ilight is the measured light intensity (mA/cm2). Here, the authors have used wavelength ranging from 350 to 800 nm and the highest IPCE of about 56% was obtained at 520 nm for TiO2 MS-based DSSC and about 39% was obtained at 520 nm for commercial P25. That mainly attributes to superior light scattering deriving from its characteristic photonic reflection effect which results in the higher Jsc in the fabricated device [45].
In DSSC, the electrochemical impedance spectroscopic analysis was used to understand the detailed charge transport and recombination kinetics of electrochemical properties. Figure 10 shows the Nyquist plot of a fabricated device which exhibits three semicircles corresponding to the diffusion of I3−/I− electrolyte in lower-frequency region, charge transport at Pt/electrolyte interface in higher-frequency region, and the electron transport kinetics at dye/TiO2/electrolyte interface in middle-frequency region [48]. Figure 11 demonstrates the corresponding bode plot of the fabricated device and the maximum mid-frequency was observed at 6.31 Hz. The electron lifetime can be found to be 25.24 ms with the aid of the following relation and all EIS parameters are tabulated in Table 2.
where fmax is the peak frequency obtained from the bode plot [48].
Table 2 shows the comparative analysis of TiO2-based DSSC. Hun-Gi Jung et al. have used solvothermal approach to synthesis uniform-size TiO2 microspheres, and they achieved PCE about 4.2% and current density about 8.7 mA/cm2 using solid-state electrolyte [49]. Dubey et al. have achieved PCE about 1.5% and 2.1% for sol–gel and solvothermally derived TiO2 nanoparticles [50]. Zn-doped TiO2 microspheres exhibit PCE of 4.6% with current density of 14.38 mA/cm2 [51]. Fang Xu et al. prepared photoanode using solvothermally derived uniform-sized TiO2 microspheres and achieves PCE about 5.5% and 12.36 mA/cm2 current density [52]. Mono-dispersed TiO2 microspheres exhibit excellent current density about 14 mA/cm2 and PCE about 5.7%, which were synthesized using chemical hydrolysis method [53]. In this present study, we have achieved the power conversion efficiency of 5.4% with excellent current density of 12.03 mA/cm2 using hydrothermally synthesized irregular-sized TiO2 microspheres. Here, the microspheres have huge impact on improvement in higher current density, better dye loading capability, and better pathway to movement of electrons (Table 3).
4 Conclusion
The TiO2 microspheres were successfully synthesized using a one-step hydrothermal route. The pure anatase phase formation of TiO2 MS was confirmed with the aid of XRD and Raman analysis. The average crystallite size was found to be 18 nm by adopting the Debye–Scherrer formula. The UV–Vis DRS results depict better reflectance and the optical band-gap energy was found to be 3.29 eV using Kubelka–Munk function plot. The DSSC was fabricated with the structure of FTO/TiO2 MS – dye/redox couple electrolyte/Pt/FTO, and its efficiency was estimated under standard one sun illumination (100 mW/cm2). The photoanode made up of TiO2 MS exhibits excellent power conversion efficiency of 5.4% than commercial P25 (3.6%). The improved PCE and Jsc could be due to superior light-scattering properties, electron transport properties, and pre-and post-treatment of TiCl4 in Photoanode.
Data availability
Available data are transparent.
References
M.S. Ahmad, A.K. Pandey, N.A. Rahim, Renew. Sust. 77, 89 (2019)
S. Mozaffaria, M.R. Nateghib, M.B. Zarandi, Renew. Sust. 71, 675 (2017)
W.S. Yang, B.W. Park, E.H. Jung, N.J. Jeon, Y.C. Kim, D.U. Lee, S.S. Shin, J. Seo, E.K. Kim, J.H. Noh, S.I. Seok, Science 356, 1376 (2018)
K. Kakiage, Y. Aoyama, T. Yano, K. Oya, J. Fujisawab, M. Hanaya, ChemComm 88, 15894 (2015)
Q. Liu, Y. Jiang, K. Jin, J. Qin, J. Xu, W. Li, J. Xiong, J. Liu, Z. Xiao, K. Sun, S. Yang, X. Zhang, L. Ding, Sci. Bull. 65, 272 (2020)
M. Hao, Y. Bai, S. Zeiske, L. Ren, J. Liu, Y. Yuan, N. Zarrabi, N. Cheng, M. Ghasemi, P. Chen, M. Lyu, D. He, J.H. Yun, Y. Du, Y. Wang, S. Ding, A. Armin, P. Meredith, G. Liu, H.M. Cheng, L. Wang, Nat. Energy 5, 79 (2020)
X. Sun, Y. Liu, Q. Tai, B. Chen, T. Peng, N. Huang, S. Xu, T. Peng, X.Z. Zhao, J. Phys. Chem. C 116, 11859 (2012)
A. Wold, Chem. Mater. 5, 280 (1993)
H.G. Jung, S.W. Oh, J. Ce, N. Jayaprakash, Y.K. Sun, Electrochem. Commun. 11, 756 (2009)
B. Liu, L.M. Liu, X.F. Lang, H.Y. Wang, X.W.D. Lou, E.S. Aydil, Energy Environ. Sci. 7, 2592 (2014)
B.S. Richards, Sol. Energy Mater. Sol. Cells 79, 369 (2003)
B. Karunagaran, P. Uthirakumar, S.J. Chung, S. Velumani, E.-K. Suh, Mater. Charact. 58, 680 (2007)
M. Kitui, M.M. Mwamburi, F. Gaitho, C.M. Maghanga, Int. J. Thin Fil. Sci. Tec. 4, 17 (2015)
S.U.M. Khan, M. Al-Shahry, W.B. Ingler Jr., Science 297, 2243 (2002)
B. O’Regan, M. Gratzel, Nature 353, 737 (1991)
Y.-Y. Yu, B.-X. Lei, M.-L. Xie, G.-L. Huang, W. Sun, Z.-F. Sun, Adv Powder Technol 26, 1072 (2015)
S.S. Kanmani, K. Ramachandran, J. Mater. Sci. 48, 2076 (2013)
E. Ramasamy, J. Lee, J. Phys. Chem. C 114, 22032 (2010)
H. Zheng, Y. Tachibana, K.K. Zadeh, Langmuir 26, 19148 (2010)
J.Z. Ou, R.A. Rani, M.H. Ham, M.R. Field, Y. Zhang, H. Zheng, P. Reece, S. Zhuiykov, S. Sriram, M. Bhaskaran, R.B. Kaner, K.K. Zadeh, ACS Nano 6, 4045 (2012)
P. Jayabal, V. Sasirekha, J. Mayandi, K. Jeganathan, V. Ramakrishnan, J. Alloys, Compd 586, 456 (2014)
J. Chen, L. Lu, W. Wang, J. Phys. Chem. C 116, 10841 (2012)
N. Rajamanickam, P. Soundarrajan, S.M. Senthil Kumar, K. Jayakumar, K. Ramachandran, Electrochim. Acta 296, 771 (2019)
M.R. Venkatraman, N. Muthukumarasamy, P. Balraju, P. Selvakumar, V. Dhayalan, P. Arivalagan, Int. J. Hydrog. Energy 45, 15441 (2020)
X. Feng, K. Zhu, A.J. Frank, C.A. Grimes, T.E. Mallouk, Angew. Chem. 124, 2781 (2012)
M.S. Mahmoud, M.S. Akhtar, I.M.A. Mohamed, R. Hamdan, Y.A. Dakka, N.A.M. Barakat, Mater. Lett. 225, 77 (2018)
X.Y. Ye, Y.H. Gu, H. Chen, Y.F. Cao, Y.Y. Liu, B.X. Lei, W. Sun, Z.F. Sun, Adv. Powder Technol. 30, 2408 (2019)
Z.Q. Li, Y.P. Que, L.E. Mo, W.C. Chen, Y. Ding, Y. Mei Ma, L. Jiang, L.H. Hu, S.Y. Dai, Appl. Mater. Interfaces 7, 10928 (2015)
J.D. Peng, C.M. Tseng, R. Vittal, K.C. Ho, Nano Energy 22, 136 (2016)
P. Ilaiyaraja, T.K. Das, P.S.V. Mocherla, C. Sudakar, Sol. Energy Mater. Sol. Cells 169, 86 (2017)
H. Tao, W. Chen, J. Wang, W. Ke, J. Wan, J. Wu, G. Fang, Electrochim. Acta 137, 17 (2014)
X. Miao, K. Pan, Y. Liao, W. Zhou, Q. Pan, G. Tian, G. Wang, J. Mater. Chem 1, 9853 (2013)
F. Sauvage, D. Chen, P. Comte, F. Huang, L.P. Heiniger, Y.B. Cheng, R.A. Caruso, M. Graetzel, ACS Nano 8, 4420 (2010)
D. Hwang, H. Lee, S.Y. Jang, S.M. Jo, D. Kim, Y. Seo, D.Y. Kim, Appl. Mater. Interfaces 3, 2719 (2011)
Y. Zhou, E.Y. Ding, W.D. Li, Mater. Lett. 61, 5050 (2007)
J. Yu, J. Zhang, Dalton Trans. 25, 5860 (2010)
G. Zhu, L. Pan, J. Yang, X. Liu, H. Sun, Z. Sun, J. Mater. Chem. 22, 24326 (2012)
R. Govindaraj, N. Santhosh, M.S. Pandian, P. Ramasamy, Appl. Surf. Sci. 449, 166 (2018)
S.S. Kanmani, N. Rajkumar, K. Ramachandran, Int. J. Nanosci. 10, 227 (2011)
M.R. Venkatraman, N. Muthukumarasamy, S. Agilan, V. Asokan, D. Velauthapillai, Mater. Res. Bull. 97, 351 (2018)
R. Govindaraj, N. Santhosh, M.S. Pandian, P. Ramasamy, M. Sumita, J. Mater. Sci.: Mater. Electron. 29, 3736 (2018)
X. Tao, P. Ruan, X. Zhang, H. Sun, X. Zhou, Nanoscale 8, 3539 (2015)
T. Amutha, M. Rameshbabu, S.S. Florence, N. Senthilkumar, I.V. Potheher, K. Prabha, Res. Chem. Intermed. 45, 1929 (2019)
T.Z. Ren, Z.Y. Yuan, B.L. Su, Chem. Phys. Lett 170, 374 (2003)
C. Ma, L. Wang, Z. Guo, Y. Lv, W. Chen, H. Ming, P. Ma, J. Wang, Colloids Surf. A 538, 94 (2018)
S.D. Liu, Y.K. Ren, Z. Zhou, W.C. Chen, Z.Q. Li, F.L. Guo, L.-E. Mo, J.H. Wu, L.H. Hu, S.Y. Dai, Adv. Powder Technol. 329, 225 (2019)
N. Rajamanickam, K. Ramachandran, J. Colloid Interface Sci. 580, 407 (2020)
Z. Ge, C. Wang, Z. Chen, T. Wang, T. Chen, R. Shi, S. Yu, J. Liu, Mater. Res. Bull. 135, 111148 (2021)
H.G. Jung, S. Nagarajan, Y.S. Kang, Y.K. Sun, Electrochim. Acta 848, 89 (2013)
R.S. Dubey, K.V. Krishnamurthy, S. Singh, Results Phys. 102390, 14 (2019)
Y. Zhang, L. Wang, B. Liu, J. Zhai, H. Fan, D. Wang, Y. Lin, T. Xie, Electrochim. Acta 6517, 56 (2011)
F. Xu, X. Zhang, Y. Wu, D. Wu, Z. Gao, K. Jiang, J. Alloys, Compd 227, 574 (2013)
Z. Zhu, C. Zhu, H. Liu, Y. Wu, G. Chen, T. Lv, Appl. Surf. Sci. 301, 308 (2014)
Acknowledgements
The authors gratefully acknowledge Sathyabama Institute of Science and Technology for providing access to their Raman and FESEM facilities.
Funding
SSN Trust (partially).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gowthambabu, V., Deshpande, M., Govindaraj, R. et al. Synthesis of anatase TiO2 microspheres and their efficient performance in dye-sensitized solar cell. J Mater Sci: Mater Electron 32, 26306–26317 (2021). https://doi.org/10.1007/s10854-021-06923-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-021-06923-1