Abstract
Mn added ZnS (Zn0.97Mn0.03S) and Mn–Cr-doped ZnS (Zn0.95Mn0.03Cr0.02S) nanostructures were synthesized by co-precipitation process. XRD pattern confirmed the cubic phase with highest intensity along (111) orientation. The shrinkage of crystallite size from 36 Å (Zn0.97Mn0.03S) to 26 Å (Zn0.95Mn0.03Cr0.02S) and the influence of Cr/Mn on microstructural, optical and photoluminescence properties in ZnS were investigated. The substitution of Cr in Zn0.97Mn0.03S lattice not only diminished the crystallite size and also produced more defect-associated luminescent activation centres. The elevated micro-strain from 9.71 × 10–3 (Zn0.97Mn0.03S) to 13.11 × 10–3 (Zn0.95Mn0.03Cr0.02S) by Cr substitution is due to the decrease of size and the higher micro-strain at Cr = 2% is owing to the drop off of activation energy which is originated from higher electro-negativity of Cr ions than Zn2+ ions. The enhanced lattice parameters by Cr doping may be due to the coexistence of both Cr3+ ions and Cr2+ ions where the existence of Cr2+ ions is higher than Cr3+ ions and substitute Zn2+ basic ions with the ionic radius of 0.74 Å in the Zn–Mn–S host lattice. The presence of Zn2+, Mn2+ and Cr3+ ions in Zn–Mn–Cr–S lattice was confirmed by XPS spectra. SEM/TEM micrographs explored the microstructure and confirmed the sized reduction by Cr doping. The elevation in band gap from 3.50 eV (Zn0.97Mn0.03S) to 3.63 eV (Zn0.95Mn0.03Cr0.02S, ∆Eg ~ 0.13 eV) by Cr addition was explained by Burstein–Moss effect and reduced crystallite size. The tuning of band gap and crystallite size of basic ZnS nanostructure by Mn/Cr substitution encourages these materials for modern electronic applications. FTIR spectra established the occurrence of Mn/Cr in Zn–S lattice by their characteristic bondings. The elevated yellowish-orange emission at 594 nm in Mn/Cr substituted ZnS is due to the exchange communication among the s–p electron states of Cr3+, Mn2+ and Zn2+ ions in Zn–S lattice. The inclusion of Mn /Cr provides an efficient control over modification of various emissions which suggests their applications in organic LED materials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Recently, group II–VI semiconducting materials exhibit great scientific and technological significance within different family of semiconductors because of their numerous practical applications in science and technology [1,2,3,4]. Quantum dots (QDs) are semiconducting material of nano-scale dimensions with distinctive properties determined by its size. Yang et al. [5] investigated N and S heavily doped carbon QDs (CQDs) with size around 1–6 nm which had better photoluminescence (PL) performance and better sensitive to Cu2+ and Hg2+, respectively. Selenium added graphene QDs [6] with size around 1–5 nm possessed a quantum field of 0.29 and a PL lifetime of 3.44 nm which guaranteed a better selectivity for the fluorescent switch. Wang et al. [7] reported the controlled PL behavior in sulfur-doped GQDs by tuning the S concentrations. Huang and their co-workers [8] developed a weak electrolyte-based electrochemical method to enhance the oxidation and cutting process and achieved a better yield of GQDs.
Porous based graphene (PGN) showed an outstanding performance in PGN-based detectable molecule separation or other biomedical applications [9]. Zhu and their co-workers [10] demonstrated that graphene oxide can be oxidized and cut into GQDs by hydroxyl radicals. Back‐gated field‐effect transistors made of single‐layer C3N exhibited an on–off current ratio reaching 5.5 × 1010 [11]. The challenges of developing solid‐state PL CQDs induced a great curiosity among research people [12]. Nitrogen-based CD probes can also distinguish tumor cells from normal cells and be used to evaluate their proliferation activity [13].
ZnCo2O4–Co3O4 nano-composite was prepared using a green reducer and stabilizer (Phoenix dactylifera extract) [14]. The nano-composite was used as a photo-catalyst for different dyes like Rhodamine B, Acid Black 1 and phenol red under visible illumination. The photo-catalytic yield was extremely efficient. The results described that 100, 95.7 and 100% of Rhodamine B, Acid Black 1 and phenol red as target contaminants were degraded utilizing ZnCo2O4–Co3O4 under 75 min of visible radiation. Moreover, the ZnCo2O4–Co3O4 composite possessed excellent stability and re-cyclability without any obvious decrease in the catalytic yield until 9 runs. Eco-friendly and green synthesized route is used prepare RGO/Cu nanocomposite by the reduction of GO and Cu2+ [15]. The photo-catalytic results revealed that RGO/Cu is an effective catalyst for reducing organic pollutants. The present sample removes the dyes like Rhodamine B and MB as 91.0 and 72.0%, respectively.
The oxide, PbWO4, was prepared using sono-chemical route with maltose as a capping agent [16]. Usage of UV radiation using PbWO4 gave the photo-catalytic yield of 93%. Holmium oxide nanoparticles were synthesized using precipitation route with triethyle-netetramine as a precipitant [17]. The capping agent plays an effective role in shape, size and photo-catalytic control. The photo-catalytic activity of holmium oxide has been compared in the degradation of methyl violet pollutant under UV irradiation. Dy2Ce2O7 nanostructure was developed using salt-assistant combustion way [18]. The size and morphology of the synthesized oxide was controlled by a kind of amino acid. The photo-catalytic activity of Dy2Ce2O7 was evaluated by degradation of eosin Y dye as water contaminant under UV illumination.
Among the different semiconducting materials, ZnS is the significant one due to its unique properties [19] and applications [20,21,22]. The two most common forms of ZnS are zinc blende and wurtzite structure where the energy gap varied between 3.72 and 3.77 eV [23, 24]. Moreover, ZnS have the elevated transmittance in the higher wavelength and significantly huge exciton binding energy and so helpful for manipulating well-organized optoelectronic device applications [25,26,27]. By its unique features and characteristics, ZnS can be employed in an extensive spectrum of different applications like solar cells, photo-voltaics, sensors, electroluminescence devices, modern emitting diodes, and lasers [28, 29].
The intentional doping of metal ions into ZnS is fundamental to modify the optical nature of semiconducting materials by building innovative quantum states between two bands of semiconductors [30, 31]. ZnS is believed as a suitable host material for optically dynamic elements like transition metal (TM) ions, which can fascinatingly provoke an impressive change in energy level structures, surface activities, visual and electrical characteristics and stimulates its transition probabilities [32,33,34]. In opto-electronic applications, it is essential that the doping element enclose a deep energy level and elevated voltage stability. Therefore, TM ions can be chosen as a suitable element due to the extraordinary properties accessible by an inclusion into ZnS basic lattice [34, 35].
The modified and improved property of ZnS by the addition of TM element has created an immense curiosity in the possibility to alter and elevate their optical and micro-structural properties for their scientific and industrial applications [36]. Among the various possible TM ions, Mn2+ is considered as the doping agents owing to its extraordinary luminescence characters using in optical sensors, phosphors, lasers, fluorescence bio-imaging and displays [37, 38]. The ionic radius and the ionic charge of Mn2+ ion is very close to Zn2+ ion. Moreover, Mn2+ occupies the various sites of ZnS lattice and stimulates the considerable modification in its optical, structural, microstructure, electronic properties [39,40,41]. Up to now, an immense deal of hard work has been paid to inspect the optical nature and structure of Mn-doped ZnS semiconducting material due to its excellent thermal and photo-stability.
Bhargava et al. [42] achieved the superior quantum efficiencies and better lifetime shortening by Mn in Zn–S lattice. Kripal [43] studied the luminescence and conductivity nature of Mn substituted ZnS nanostructures and they noticed that the photo-sensitivity was enhanced by Mn2+ into ZnS system. A noticeable increase in visible transmittance and a small decline in resistivity by Mn2+ in ZnS were found by Jrad [44]. Goudarzi et al. [45] described that the insertion of Mn occupied the substitutional position of Zn and modified the energy level of ZnS which promotes the elevated luminescence properties. The luminescence and photo-catalytic studies carried out by Nasser [46] in Mn/ZnS described that the inclusion of Mn2+ stimulates the charge separation and activate the photo-catalytic activity of Mn-doped ZnS. From the literature it is understood that the higher percentage of Mn produced the secondary phase generation. To avoid the secondary phase generation, Mn level is optimized as 3% (Zn0.97Mn0.3S).
The addition of two or more elements through ZnS causes a significant progression in PL, energy gap, size and charge transport properties. Doping at higher percentage with no secondary phase or metallic cluster is achieved by the addition of two or more suitable TM ions [47]. In this work, Cr is chosen as the 2nd doping element because it can easily enters into ZnS lattice [48] and enhanced the stability of ZnS [49]. The higher doping percentage without secondary phase is achieved by Cr doping. The Cr percentage is limited to 2% and it is attained by fixing the appropriate of quantity of precursors. Moreover, the addition of Cr3+ through ZnS stimulates the different special defect sites in the lattice owing to the dissimilarities among Cr3+ and Zn2+ ions. Poornaprakash reported the disappearance of blue emission bands and appearance of RTFM by Cr addition in ZnS [50]. Recently, Aqeel et al. [51] described that Cr added ZnS performed the superior photo-catalytic behavior and hence they may use as an effective one for the elimination of environmental pollutants.
Lattice contraction and the enhancement of energy levels were reported by Reddy et al. [52] in ZnS with increase of Cr concentrations. Yang et al. [53] noticed the strong emissions around 515–560 nm region in Co2+, Cu2+ dual doped ZnS nanostructure. The PL intensity of dual-doped material is considerably superior than ZnS. Liu et al. [54] reported the red radiation emission in Mn–Cd dual-doped ZnS nanostructures. Yang et al. [55] described the PL characteristics of Ni2+/Mn2+-doped ZnS. The similar PL studies were made on Mn, Pr co-doped ZnS [56], Co–Cu-doped ZnS [57], Cu–Mn-doped ZnS nanostructures [58].
The optical, structural, and magnetic studies on Mn single-doped ZnS [36,37,38,39,40,41,42,43,44,45,46], Cr single added ZnS [45,46,47,48] and some other TM dual-doped ZnS [53,54,55,56,57,58] were reported in the literature. But, the comprehensive investigation of optical, morphological and emission characters on Cr, Mn dual-doped ZnS is almost scanty. In this work, Zn0.97Mn0.03S and Zn0.95Mn0.03Cr0.02S quantum dots (QDs) were synthesized by co-precipitation route. In this work, co-precipitation is chosen to prepare Mn/Cr-doped ZnS QDs because it is a simple, economical and industrially viable technique [59,60,61,62,63] to prepare technologically important ZnS-based materials. The structural, spectral, morphological and PL properties of the Mn2+/Cr3+-doped ZnS QDs are characterized systematically to achieve the better insight about the crystal nature, morphology, bonding nature, energy gap and emission properties.
2 Experimental details
2.1 Preparation of Zn0.97Mn0.03S and Zn0.95Mn0.03Cr0.02S QDs
The preparation of Zn0.97Mn0.03S and Zn0.95Mn0.03Cr0.02S QDs has been carried out by co-precipitation route. Zinc acetate (Zn(CH3COO)2·2H2O), Na2S, manganese acetate (Mn(CH3COO)2·2H2O) and chromium acetate dihydrate (Cr (CH3COO)2·2H2O) are employed as resource materials for Zn2+, S2−, Mn2+ and Cr3+ ions, respectively. The ultra-pure de-ionized water used as solvent. All the chemicals were bought from M/s. Merck and utilized without additional purification since they possessed analytical grade (AR) with 99.99% purity. The molar quantities were taken as per the targeted doping concentration i.e. the doping composition of Mn2+ is fixed as 3% and Cr3+ fixed as 2% to prepare Zn0.95Mn0.03Cr0.02S QDs. The source materials were weighed as per the expected compositions and dissolved in 100 ml ultra-pure de-ionized water. Separate solutions of manganese acetate, chromium chloride, zinc acetate and Na2S were prepared. The preparative technique is reported in the literature [64].
For the synthesis of Zn0.95Mn0.03Cr0.02S QDs, 0.95 M Zn(CH3COO)2·2H2O and 0.03 M Zn(CH3COO)2·2H2O were slowly dissolved one by one in 100 ml water under constant stirring to obtain clear and homogeneous solution. After getting homogeneous solution, 0.02 M chromium acetate dihydrate was mixed at constant stirring. A separate solution made by mixing 1 M sodium sulfide in 100 ml water is dissolved with the previous solution. The solution was mixed drop wise to the initial solution under constant stirring of 1000 rpm for 8 h at 60 °C as in Fig. 1.
The preparation process is depending on the slow release of Zn2+, Mn2+, Cr3+ and S2− ions in the final solution. The ions condense on an ion–ion basis in the solution. Aqueous ammonia solution was mixed to keep the pH of the mixer solution as 9. After 8 h, a light greenish white precipitate was deposited at the bottom portion of the flask. The final precipitates after complete reaction have been filtered out using fine filter paper and washed several time using methanol and de-ionized double distilled water to purify the samples i.e., eliminate the un-wanted impurities. The resultant product was dehydrated by keeping it into a furnace at 60 °C for 7 h. The dried output was taken out and crushed well with help of agar mortar. All the chemical reaction process was carried out at room temperature.
2.2 Characterization techniques
X-ray diffractometer (XRD) was employed to explore crystal structure of Zn0.97Mn0.03S and Zn0.95Mn0.03Cr0.02S QDs. The diffracted patterns were recorded by RigaKuC/max-2500 diffractometer using CuKa radiation (λ = 1.5406 Å) at 40 kV and 30 mA from 2θ = 20° to 70°. The morphology of the prepared QDs were recorded using scanning electron microscope (Make: JEOLJSM, Model: 6390) and transmission electron microscopy (Make: Philips, Model: CM200-FEG).
The efficiency of the doping ions in the ZnS was verified. The presence of the metal elements was confirmed by X-ray photoelectron spectroscopy (XPS) (Perkin–Elmer PHI 1600 ESCA) with a monochromatic Mg Kα X-ray source (hυ = 1486.6 eV) and manual point control object with a scanning rate of 0.5 eV. The FTIR studies were done by Fourier transform infra-red (FTIR) spectrometer (Model: Perkin Elmer, Make: Spectrum RX I) from 400 to 4000 cm−1. Pellets were made by mixing the nanoparticles with KBr at 1 wt%.
The UV–visible absorption studies were made to examine their optical properties using UV–visible spectrometer (Model: lambda 35, Make: Perkin Elmer) from 310 to 550 nm at room temperature. Room-temperature PL studies were carried out (Model: Hitachi, Make: F-2500) from 345 to 700 nm at excitation wavelength of 330 nm and power of 150 W to study the emission properties.
3 Results and discussion
3.1 XRD—structural analysis
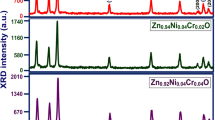
Figure 2 represents a typical XRD pattern of Zn0.97Mn0.03S and Zn0.95Mn0.03Cr0.02S QDs. The obtained XRD patterns exhibit three strong and wide peaks which reflect the nano-crystalline nature of the material [65]. It has been noticed from Fig. 2 that the orientation along (111) plane is preferred one than the orientations such as (220) and (311) because of its high intensity. The obtained major XRD peaks matched well with the JCPDS card No. 80-0020 with lattice parameter a ≈ 0.5311 Å. The well-broadened XRD peaks clearly showed the cubic structure of the sample. The similar cubic phase was noticed by Sakthivel et al. [65] in Mn and Cr-doped ZnS.
The absence of secondary or other impurity phases associated to Cr/Mn metals or its oxides confirmed that both Mn2+ and Cr3+ ions are properly substituted into the ZnS lattice. No peaks corresponding to strange elements or impurities found in Fig. 2 verified the phase purity of the synthesized samples and also confirmed that the substitution of Mn2+ and Cr3+ in Zn–S lattice not changed the original cubic structure of the material. Table 1 presents the values of peak intensity, peak position (2θ), full width at half maximum (FWHM, β) value, d value, lattice parameter 'a', crystallite size (D) and micro-strain (ε) along (111) plane of Zn0.97Mn0.03S and Zn0.95Mn0.03Cr0.02S nanostructures.
Generally, the FWHM of any XRD orientation is inversely proportional to the crystallite size. In the present investigation, Cr addition into Zn0.97Mn0.03S enhances the FWHM from 2.3° to 3.1° (Zn0.95Mn0.03Cr0.02S). The increase of FWHM along (111) orientation by the addition of Cr is responsible for the shrinkage of crystallite size as illustrated in Table 1. The peak position along (111) plane of Zn0.97Mn0.03S QDs is shifted to smaller 2θ side by the inclusion of Cr in the Zn–Mn–S lattice. But, Kaur et al. [66] reported that the peak shift is along higher 2θ side by Cr addition in ZnS nanostructure. The peak position shift along smaller 2θ side by Cr addition may possibly owing to the dissimilarity among the ionic radii between Cr, Zn, and Mn ions [67]. The size of the QDs are estimated by Scherrer relation [68],
Here, λ is the X-wavelength used, β is the angular peak width at half maximum along (111) plane and θ is the Bragg’s diffraction angle. Similarly, the Micro-strain (ε) provoked in the Zn–Mn–Cr–S lattice is calculated with the help of the relation [69],
The derived crystallite size from the Debye relation (seen from Table 1) is diminished from 36 Å (Zn0.97Mn0.03S) to 26 Å (Zn0.95Mn0.03Cr0.02S) by the inclusion of tiny quantity of Cr (Cr = 2%). The elevated FWHM by Cr doping supports the present shrinkage of size. The value of micro-strain obtained from the above relation is increased from 9.71 × 10–3 (Zn0.97Mn0.03S) to 13.11 × 10–3 (Zn0.95Mn0.03Cr0.02S). The current enhancement in micro-strain by Cr substitution is also responsible for the decrease of size and the higher micro-strain at Cr = 2% is due to the drop off of activation energy which is originated from higher electro-negativity of Cr ions than Zn2+ ions [70]. The drop off of the activation energy and the enhanced micro-strain [71] by the Cr inclusion in Zn-Mn–Cr–S lattice deteriorate the crystal and hence suppress the crystal size.
In general, the alteration in lattice constants and inter-planar distance depends upon the nature of dopants and its concentrations, the defect states and strain/stress induced by the dopant in the host lattice and the variation in ionic radii between the dopant and Zn2+ [72]. The lattice parameter ‘a’ is attained by the relation,
Here, d is lattice spacing, h, k, l are miller indices. Figure 3 shows the alteration in lattice parameter ‘a’ and crystallite size for Zn0.97Mn0.03S and Zn0.95Mn0.03Cr0.02S QDs. Both the lattice parameter 'a' and inter-planner distance ‘d’ are increased by Cr incorporation in Zn–Mn-S lattice. Generally, two forms of Cr ions are possible, first one is Cr3+ ion which has ionic radius of ≈ 0.63 Å and the second one is Cr2+ ion which possesses the ionic radius of ≈ 0.89 Å. The noticed increment in lattice parameters as shown in Table 1 and Fig. 3 may be due to the coexistence of both Cr3+ ions and Cr2+ ions where the existence of Cr2+ ions is higher than Cr3+ ions and substitute Zn2+ basic ions with the ionic radius of 0.74 Å in the Zn–Mn–S host lattice [73].
3.2 SEM and TEM—morphological study
Figure 4a represents the SEM image i.e., the typical surface microstructure of Zn0.97Mn0.03S QDs. The morphology of Zn0.97Mn0.03S QDs shows uneven sized and irregular shaped particles which may be owing to the agglomeration between the grains. Figure 4b shows the surface morphology of Zn0.95Mn0.03Cr0.02S QDs with a huge distribution of tiny sized grains on the surface with cluster form. The addition of Cr into Zn–Mn–S lattice creates a strong agglomeration with small sized particles. The real size of the particles/grains cannot be estimated from the current SEM images due to its limitation by the agglomeration and the resolution of the instrument.
TEM micrographs have been taken to get the better understanding about the grain size and shape of the particles. The typical TEM images of Zn0.97Mn0.03S and Cr-doped Zn0.97Mn0.03S QDs are listed in Fig. 5. Figure 5a displays the micro-structural image of Zn0.97Mn0.03S QDs which possesses the mixture of spherical, square, and hexagonal shaped structure with un-even gain size. The shape and size of the particles/grains in the Mn-doped ZnS QDs are irregular and chaotic nature. Figure 5b shows the morphology of Zn0.95Mn0.03Cr0.02S QDs. It is detected from Fig. 4b that during the incorporation of Cr in Zn0.97Mn0.03S, the morphology of the agglomerations be likely to be more spherical with considerable reduction in size. TEM micrograph of Zn0.95Mn0.03Cr0.02S QDs displays the small sized grains distributed over the microstructures due to the drop off of its surface energy by Cr addition.
TEM is employed to explore the minerals at elevated magnifications, with significant added information presented by selected-area electron diffraction (SAED) patterns. SAED patterns are particularly helpful for the examination of minute crystals and partially disordered material with few hundred Å. SAED pattern of Zn0.97Mn0.03S and Zn0.95Mn0.03Cr0.02S nanostructures are illustrated in Fig. 5c and d, respectively. The three rings corresponding to (h k l) orientations agreed well with the XRD pattern. Among the three orientations, namely, (111), (22) and (311), the (111) orientation is very bright which verifies the prime peak value of XRD. Adjacent orientations are similar successive peaks of XRD pattern. All SAED patterns convey the cubic phase of the prepared samples, which is in better agreement with the noticed XRD pattern.
3.3 EDX—compositional analysis
EDX examination has been taken out to evaluate the basic element composition and the basic elements like Zn, S, Mn and Cr of the samples [74] with an appropriate stoichiometric composition. The basic and fundamental elemental mapping analysis of Mn/Cr doped ZnS by EDX spectra is presented in Fig. 6. The inset of Fig. 6 exhibits the atomic % of the elements like Zn, Mn, Cr and S in Mn/Cr doped ZnS nanostructures. The quantitative examination of atomic % of the elemental composition in of Zn0.97Mn0.03S and Zn0.95Mn0.03Cr0.02S nanostructures using EDX investigation is presented in Table 2.
Mn/(Zn + Mn + Cr) ratio is found to be 2.94% and 3.05% for Zn0.97Mn0.03S and Zn0.95Mn0.03Cr0.02S nanostructures, respectively. Moreover, Cr/(Zn + Mn + Cr) ratio derived in Zn0.95Mn0.03Cr0.02S is ≈ 2.04%. The atomic % of Zn gradually decreased and also Cr level enhanced by Cr substitution which evidently indicates that the Cr ions are properly substituted into Zn–Mn–S lattice. The experiential results are almost equivalent to their stoichiometry with the least amount variation from the real values.
3.4 X-ray photoelectron spectroscopy (XPS)
XPS is useful to explore the doping elements and verify the valence state of Zn, Mn and Cr ions in Zn–S lattice at their equivalent binding energy. Figure 7 presents the derived XPS spectra of the Zn 2p, Mn 2p and Cr 2p core levels. The presence of Mn and Cr is confirmed by Mn 2p and Cr 2p XPS peaks.
Figure 7a shows the overall XPS spectra of Mn, Cr doped ZnS which contains the peaks corresponding to S2−, Cr3+, Mn2+ and Zn2+. The higher resolution spectra of XPS spectra of Cr 2p have been revealed in the inset of Fig. 7a where the peak of Cr 2p3/2 is located at 577 eV [75]. The location of Cr 2p3/2 peak is different from those of 574.2 eV corresponding to Cr metal, 579.0 eV for Cr6+ and 576.0 eV for Cr2+, however it is close to the location of Cr 2p3/2 (576.7 eV) in Cr2O3 [48]. It recommends that the Cr elements are included into the Zn–S lattice as Cr3+ ions. The intensity of Cr 2p1/2 peak is higher than Cr 2p3/2. The main reason is the presence of XPS photoelectron corresponding to Cr 2p1/2 situated at 585.2 eV [76].
Figure 7b shows the Zn 2p spectra of Mn/Cr-doped ZnS which exhibits two peaks, first peak around 1022 eV corresponding to 2p3/2 and the another peak around 1046 eV corresponding to 2p1/2. The peak locations corresponding to 2p3/2 and 2p1/2 state of Zn confirms the existence of Zn2+, which accurately equivalent to the binding energy of Zn–S bonding [77]. The peak corresponding to S 2p state with binding energy around 162 eV as in Fig. 7 confirms the occurrence of Zn2+ in Zn–S lattice [78]. The existence of Mn is confirmed from the Mn 2p spectra within the binding energy range of 630–660 eV as displayed in Fig. 6c. It is noticed from Fig. 6c that the intensity of Mn 2p states weak. The observed peaks in the region of 642 eV and 654.2 eV related to the Mn 2p spectra verify the existence of Mn in the Zn–Cr–S lattice and these Mn2+ ions could play as substitutes for the Zn atoms or as interstitial one [53].
3.5 Fourier transform infrared (FTIR) studies
The FTIR spectra of Zn0.97Mn0.03S and Zn0.95Mn0.03Cr0.02S nanostructures are presented in Fig. 8 from 400 cm−1 to 4000 cm−1 and their wave number assignments are listed in Table 3. The wide and the broad absorption bands in the synthesized samples within the wave number range of 3350–3450 cm−1 are associated to O–H stretching vibration of water [4]. The strong absorption bands ~ 1574–1598 cm−1 are accountable for O–H bending vibration [79]. The bands around 1122 cm−1 and 1023–1026 cm−1 are correlated to the substitution of Cr/Mn in Zn location and the micro-structural Mn/Cr in Zn–S matrix [4]. The weak band located around 939 cm−1 represents the imperfection/defects stimulated by Cr substitution in Zn–Mn–S matrix which is not present in Zn0.97Mn0.03S [63]. The characteristic bands about 674–676 cm−1 and 492–519 cm−1 are corresponding to Zn–S stretching vibrations [80]. The bands corresponding to Zn–S show the better agreement with the earlier studies [81]. A close resemblance of the two FTIR spectra (without and with Cr-doped Zn0.97Mn0.03S) specifies that Cr3+ ions have substituted into ZnS.
3.6 Optical absorption and band gap estimation
Generally, the optical characters of the QDs were studied by the optical absorption performance of the materials [82,83,84,85]. The absorption of Zn0.97Mn0.03S and Zn0.95Mn0.03Cr0.02S nanostructures between the wavelength around 310–550 nm is illustrated as shown in Fig. 9a. Mn-doped ZnS (Zn0.97Mn0.03S) nanostructures exhibit a higher absorption along UV wavelength region i.e., below 360 nm and exhibit a sudden fall off at upper wavelength region. The inclusion of Cr3+ into Zn0.97Mn0.03S diminishes the absorption intensity.
During the introduction of Cr into Zn0.97Mn0.03S, both Cr3+ and Cr2+ ions may co-exist in the host lattice and the existence of Cr2+ is higher than Cr3+ ions which has higher ionic radius. Therefore, the substitution of Cr2+ ions with higher ionic radius induces the lattice disorder and defects within the host lattice [73]. The present defect formation is one of the reasons to decrease the absorption intensity. One more reason for the decrease of absorption intensity may originates from the scattering of light along the grain boundaries and cluster of Cr in the lattice [86]. The similar decrease of intensity is observed by Reddy et al. [86] in Cu- and Cr-doped ZnS nanoparticles. In addition to the shrinkage of absorption intensity, the inclusion of Cr3+ ions induce the absorption edge moving along the higher frequency region i.e., the higher energy region (blue shift). The current blue shift of absorption edge and the shrinkage of absorption intensity are also supported by the size decrement which is presented in Table 1. Moreover, the blue shift of absorption edge indicates the increase of energy gap of the material.
The energy gap of Mn-doped and Mn–Cr co-doped ZnS nanostructures have been derived with the help of the plot among (αhυ)2 versus hυ [87] as revealed in Fig. 9b. The energy gap of pure ZnS from literature is found to be 3.67 eV at ambient temperature [88]. The energy gap of Mn = 3% doped ZnS (Zn0.97Mn0.03S) obtained from Fig. 7b is 3.5 eV which is ~ 0.17 eV (∆Eg ~ 0.17 eV) smaller than ZnS. The 3d electrons existing in Mn interact with the electrons in Zn which creates the dislocation/imperfections in the structural bonding of ZnS [36]. The induced interaction among the ‘s’ and ‘p’ electrons existing in ZnS and ‘3d’ electrons in Mn2+ ions is accountable for the present decrease in band gap [89]. The incorporation of Mn into Zn–S also creates some new energy levels nearer to the valence level of ZnS which is also the additional probable reason for the present decrease in band gap [90].
The rapid increase of band gap from 3.50 eV (Zn0.97Mn0.03S) to 3.63 eV (Zn0.95Mn0.03Cr0.02S, ∆Eg ~ 0.13 eV) is achieved by adding Cr where, Cr3+ ions with smaller ionic radius can be restore Zn2+ ions in the substitutional sites. The substitution of Cr in Zn–Mn–S lattice releases additional free carriers which alter the Fermi level and shift nearer to the conduction level and widen the energy gap. The enhanced energy gap (blue shift) during Cr doping is explained by the Burstein–Moss shift [91, 92]. The decrease of size is also play a main role in the elevation of band gap [93].
3.7 Photoluminescence (PL) spectra
PL is the main optical characteristics for different PL nano-materials, like nano-sized particles and QDs [94,95,96,97]. Figure 10a illustrates the PL spectra of ZnS from 350 to 650 nm at room temperature. ZnS shows a wide PL peak from 370 to 550 nm as explained by Faita et al. [98]. The addition of Mn and Cr strongly modified the intensity and peak position. Figure 10b and c reveals the PL emissions of without and with Cr-doped Zn0.97Mn0.03S nanostructures from 345 to 700 nm. Figure 10b contains a strong near violet (NV) emission at 388 nm. Even though, no major change in peak position of UV band is noticed, peak intensity is decreased by Cr = 2% doping. Generally, the luminescence in the visible region arises from impurity or native defect-associated transitions [99]. The present NV band is originated from the nano-sized particles and the stimulated defects like Zn/S interstitials. Here, various peaks are combined into a single and broad band at 388 nm [100]. The similar shrinkage of PL intensity was described by Nasser et al. [36] in Mn–ZnS and they explained that the reducing band-band recombination rate is accountable for the reduced PL intensity.
Figure 10c illustrates the PL spectra of Zn0.97Mn0.03S and Zn0.95Mn0.03Cr0.02S nanostructures between 500 and 700 nm. Both samples exhibit a band corresponding to yellowish-orange wavelength at 594 nm. Figure 10c reflected that PL intensity is shifted to higher side by Cr addition in Zn0.97Mn0.03S. A band at 594 nm is due to the exchange communication among the s–p electron states of Cr3+, Mn2+ and Zn2+ ions in Zn–S lattice [101,102,103]. Figure 11 represents the energy level diagram of Cr-doped Zn0.97Mn0.03S to describe the different emissions such as NV and yellowish-orange. The dominant visible emission band emerged ~ 594 nm is initiated from the electronic switch over from 4T1 level to 6A1 level in the 3d configuration of Mn2+/Cr3+ ions [104, 105]. The emissions around 580–600 nm was also noticed by Kole et al. [106] and Karar et al. [107]. It is implied from Fig. 10 that the charge carriers may relax to the defect positions, from which they recombine via the d-orbital of Cr3+ or be transferred to Mn2+ states that lead to the characteristic Cr3+ and Mn2+ emissions [108]. The obtained elevated yellowish-orange wavelength emission in Mn/Cr substituted ZnS highlights their applications optical devices like organic LEDs and solar cells.
4 Conclusions
Following are the conclusions drawn from the present investigations:
-
▪Mn added ZnS (Zn0.97Mn0.03S) and Mn–Cr doped ZnS (Zn0.95Mn0.03Cr0.02S) nanostructures were synthesized by co-precipitation process.
-
▪XRD pattern confirmed the cubic phase with highest intensity along (111) orientation.
-
▪The shrinkage of crystallite size from 36 Å (Zn0.97Mn0.03S) to 26 Å (Zn0.95Mn0.03Cr0.02S) and the influence of Cr/Mn on microstructural, optical and PLproperties in ZnS were reported.
-
▪The substitution of Cr in Zn0.97Mn0.03S lattice not only diminished the crystallite size and also produced more defect-associated luminescent activation centres.
-
▪The elevated micro-strain from 9.71 × 10–3 (Zn0.97Mn0.03S) to 13.11 × 10–3 (Zn0.95Mn0.03Cr0.02S) by Cr substitution is due to the decrease of size and the higher micro-strain at Cr = 2% is owing to the drop off of activation energy which is originated from higher electro-negativity of Cr ions than Zn2+ ions.
-
▪The enhanced lattice parameters by Cr doping may be due to the coexistence of both Cr3+ ions and Cr2+ ions where the existence of Cr2+ ions is higher than Cr3+ ions and substitute Zn2+ basic ions with the ionic radius of 0.74 Å in the Zn–Mn–S host lattice.
-
▪SEM/TEM micrographs explored the microstructure and confirmed the sized reduction by Cr doping.
-
▪The presence of Zn2+, Mn2+ and Cr3+ ions in Zn–Mn–Cr–S lattice was confirmed by XPS spectra.
-
▪The elevation in band gap from 3.50 eV (Zn0.97Mn0.03S) to 3.63 eV (Zn0.95Mn0.03Cr0.02S, ∆Eg ~ 0.13 eV) by Cr addition was explained by Burstein–Moss effect and reduced crystallite size.
-
▪The tuning of band gap and crystallite size of basic ZnS nanostructure by Mn/Cr substitution encourages these materials for modern electronic applications.
-
▪FTIR spectra established the occurrence of Mn/Cr in Zn–S lattice by their characteristic bondings.
-
▪The elevated yellowish-orange emission at 594 nm in Mn/Cr substituted ZnS is due to the exchange communication among the s–p electron states of Cr3+, Mn2+ and Zn2+ ions in Zn–S lattice.
-
▪The inclusion of Mn/Cr provides an efficient control over modification of various emissions which suggests their applications in organic LED materials.
References
E.G. Alvarez-Coronadoa, L.A. Gonzáleza, J.C. Rendón-Ángelesa, M.A. Meléndez-Lirab, R. Ramírez-Bon, Mat. Sci. Semicon. Proc. 81, 68–74 (2018)
J.A. Ruffner, M.D. Hilmel, V. Mizrahi, G.I. Stegeman, U. Gibson, J. Appl. Opt. 28, 5209–5214 (1989)
F. Göde, C. Gümüs, M. Zor, J. Cryst. Growth 299, 136–141 (2007)
S. Ummartyotin, N. Bunnak, J. Juntaro, M. Sain, H. Manuspiya, Solid State Sci. 14, 299–304 (2012)
S. Yang, J. Sun, X. Li, W. Zhou, Z. Wang, P. He, G. Ding, X. Xie, Z. Kang, M. Jiang, J. Mater. Chem. A 2, 8660–8667 (2014)
S. Yang, J. Sun, P. He, X. Deng, Z. Wang, C. Hu, G. Ding, X. Xie, Chem. Mater. 27, 2004–2011 (2015)
G. Wang, Q. Guo, D. Chen, Z. Liu, X. Zheng, A. Xu, S. Yang, G. Ding, ACS Appl. Mater. Interfaces 10, 5750–5759 (2018)
H. Huang, S. Yang, Q. Li, Y. Yang, G. Wang, X. You, B. Mao, H. Wang, Y. Ma, P. He, Z. Liu, G. Ding, X. Xie, Langmuir 34, 250–258 (2018)
S. Yang, Y. Yang, P. He, G. Wang, G. Ding, X. Xie, Langmuir 33, 913–919 (2017)
C. Zhu, S. Yang, G. Wang, R. Mo, P. He, J. Sun, Z. Di, Z. Kang, N. Yuan, J. Ding, G. Ding, X. Xie, J. Mater. Chem. B 3, 6871–6876 (2015)
S. Yang, W. Li, C. Ye, G. Wang, H. Tian, C. Zhu, P. He, G. Ding, X. Xie, Y. Liu, Y. Lifshitz, S. Lee, Z. Kang, M. Jiang, Adv. Mater. 29, 1605625 (2017)
A. Xu, G. Wang, Y. Li, H. Dong, S. Yang, P. He, G. Ding, Small 16, 2004621 (2020)
J. Li, S. Yang, Z. Liu, G. Wang, P. He, W. Wei, M. Yang, Y. Deng, P. Gu, X. Xie, Z. Kang, G. Ding, H. Zhou, X. Fan, Adv. Mater. 33, 2005096 (2021)
S. Zinatloo-Ajabshir, S. AliHeidari-Asil, M. Salavati-Niasari, Sep. Purif. Technol. 267, 118667 (2021). https://doi.org/10.1016/j.seppur.2021.118667
H. Safajou, M. Ghanbari, O. Amiri, H. Khojasteh, F. Namvar, S. Zinatloo-Ajabshir, M. Salavati-Niasari, Int. J. Hydrog. Energy 46, 20534–20546 (2021)
S. Zinatloo-Ajabshir, M. Baladi, M. Salavati-Niasari, Ultrason. Sonochem. 72, 105420 (2021)
S. Zinatloo-Ajabshir, S. Mortazavi-Derazkola, M. Salavati-Niasari, J. Mol. Liq. 231, 306–313 (2017)
Z. Salehi, S. Zinatloo-Ajabshir, M. Salavati-Niasari, J. Mol. Liq. 222, 218–224 (2016)
S. Wageh, L. Shu-Man, F. Tian You, X. Rong, J. Lumin. 102–103, 768–773 (2003)
K. Hedayat, J. Nanostruct. 5, 395–401 (2015)
S.A. Rosli, Z.A. Zubir, N.M.A. Aziz, Adv. Mater. Res. 364, 434–438 (2012)
R. Seodi, A. Shabaka, W.H. Eisa, B. Anies, N.M. Farage, Phys. B 405, 919–924 (2010)
J. Borah, K. Sarma, Acta Phys. Pol. A 114, 713–719 (2008)
H. Haddad, A. Chelouche, D. Talantikite, H. Merzouk, F. Boudjouan, D. Djouadi, Thin Solid Films 589, 451–456 (2015)
N.S. Karan, S. Sarkar, D.D. Sarma, P. Kundu, N. Ravishankar, N. Pradhan, J. Am. Chem. Soc. 133, 1666–1669 (2011)
J.K. Salem, T.M. Hammad, S. Kuhn, M.A. Draaz, N.K. Hejazy, R. Hempelmann, J. Mater. Sci. Mater. Electron. 25, 2177–2182 (2014)
J. Diaz-Reyes, R. Castillo-Ojeda, R. Sánchez-Espíndola, M. Galván-Arellano, O. Zaca-Morán, Curr. Appl. Phys. 15, 103–109 (2015)
X. Wang, H. Huang, B. Liang, Z. Liu, D. Chen, G. Shen, Crit. Rev. Solid State Mater. Sci. 38, 57–90 (2013)
W. Daranfed, M. Aida, A. Hafdallah, H. Lekiket, Thin Solid Films 518, 1082–1084 (2009)
S.C. Erwin, L. Zu, M.I. Haftel, A.L. Efros, T.A. Kennedy, D.J. Norris, Nature 436(7047), 91–94 (2005)
D. Bera, L. Qian, T.K. Tseng, P.H. Holloway, Materials 3(4), 2260–2345 (2010)
Y. Huang, Y. Cai, H. Liu, Particuology 9, 533–536 (2011)
C. Falcony, M. Garcia, A. Ortiz, J. Alonso, J. Appl. Phys. 72, 1525–1527 (1992)
J. Cao, D. Han, B. Wang, L. Fan, H. Fu, M. Wei, B. Feng, X. Liu, J. Yang, J. Solid State Chem. 200, 317–322 (2013)
R. Viswanath, H.B. Naik, G.Y. Kumar, P.P. Kumar, G.A. Kumar, R. Praveen, J. Lumin. 153, 446–452 (2014)
S. Ebrahimi, B. Yarmand, N. Naderi, Thin Solid Films 676, 31–41 (2019)
X. Ma, J. Song, Z. Yu, Thin Solid Films 519, 5043–5045 (2011)
M. Geszke-Moritz, H. Piotrowska, M. Murias, L. Balan, M. Moritz, J. Lulek, R. Schneider, J. Mater. Chem. B 1, 698–706 (2013)
H. Labiadh, B. Sellami, A. Khazri, W. Saidani, S. Khemais, Opt. Mater. 64, 179–186 (2017)
J. Huang, L. Wang, K. Tang, M. Lin, Y. Yu, X. Lu, Y. Xia, Surf. Coat. Technol. 228, S397–S400 (2013)
A.B. Cruz, Q. Shen, T. Toyoda, Thin Solid Films 499, 104–109 (2006)
R. Bhargava, D. Gallagher, X. Hong, A. Nurmikko, Phys. Rev. Lett. 72, 416 (1994)
R. Kripal, A.K. Gupta, S.K. Mishra, R.K. Srivastava, A.C. Pandey, S. Prakash, Spectrochim. Acta A. 76, 523–530 (2010)
A. Jrad, W. Naffouti, T.B. Nasr, S. Ammar, N. Turki-Kamoun, J. Mater. Sci. Mater. Electron. 28, 1463–1471 (2017)
A. Goudarzi, G.M. Aval, S.S. Park, M.-C. Choi, R. Sahraei, M.H. Ullah, A. Avane, C.-S. Ha, Chem. Mater. 21, 2375–2385 (2009)
R. Nasser, H. Elhouichet, M. Férid, Appl. Surf. Sci. 351, 1122–1130 (2015)
K.C. Sebastian, M. Chawda, L. Jonny, D. Bodas, Mater. Lett. 64, 2269–2272 (2010)
B.Q. Wang, J. Iqbal, X.D. Shan, G.W. Huang, H.G. Fu, R.H. Yu, D.P. Yu, Mater. Chem. Phys. 113, 103–106 (2009)
K. Sato, H. Yoshida, J. Appl. Phys. 39, L555–L558 (2000)
B. Poornaprakash, K. Naveen Kumar, U. Chalapathi, M. Reddeppa, P.T. Poojitha, Si.-H. Park, J. Mater. Sci. Mater. Electron. 27, 6474–6479 (2016)
M. Aqeel, M. Ikram, A. Asghar, A. Haider, A. Ul-Hamid, M. Naz, M. Imran, S. Ali, Appl. Nanosci. 10, 2045–2055 (2020)
D.A. Reddy, A. Divya, G. Murali, R.P. Vijayalakshmi, B.K. Reddy, Phys. B 406, 1944–1949 (2011)
P. Yang, M. Lu, G. Zhou, D. Yuan, D. Xu, Inorg. Chem. Commun. 4, 734–737 (2001)
J.Z. Liu, P.X. Yan, G.H. Yue, J.B. Chang, D.M. Qu, R.F. Zhuo, J. Phys. D: Appl. Phys. 39, 2352–2356 (2006)
P. Yang, M. Lu, D. Xu, D. Yuan, C. Song, S. Liu, X. Cheng, Opt. Mater. 24, 497–502 (2003)
J.W. Lee, K. Cho, H. Kim, J.-H. Kim, B. Park, T. Noh, S.H. Kim, S. Kim, Jpn. J. Appl. Phys. 44, 7694–7697 (2005)
L. Ma, W. Chen, Nanotechnology 21, 385604–385611 (2010)
W. Wang, F. Huang, Y. Xia, A. Wang, J. Lumin. 128, 610–614 (2008)
S. Zinatloo-Ajabshir, M. Mousavi-Kamazani, Ceram. Int. 47, 23702–23724 (2021)
S. Zinatloo-Ajabshir, M. Mousavi-Kamazani, Int. J. Appl. Ceram. Technol. 13, 108–115 (2016)
S. Zinatloo-Ajabshir, M. Mousavi-Kamazani, J. Mater. Sci. Mater. Electron. 26, 5812–5821 (2015)
F. Beshkar, S. Zinatloo-Ajabshir, M. Salavati-Niasari, Chem. Eng. J. 279, 605–614 (2015)
S. Zinatloo-Ajabshir, M. Salavati-Niasari, J. Mater. Sci. Mater. Electron. 27, 3918–3928 (2016)
S. Muthukumaran, R. Gopalakrishnan, Phys. B: Cond. Matter. 407, 3448–3456 (2012)
P. Sakthivel, S. Muthukumaran, J. Phys. Chem. Solids 120, 183–189 (2018)
P. Kaur, S. Kumar, A. Singh, C.L. Chen, C.L. Dong, T.S. Chan, K.P. Lee, C. Srivastava, S.M. Rao, M.K. Wu, Superlattices Microstruct. 83, 785–795 (2015)
L.B. Duan, X.R. Zhao, J.M. Liu, T. Wang, G.H. Rao, Appl. Phys. A 99, 679–683 (2010)
P. Sakthivel, S. Muthukumaran, Opt. Laser Technol. 103, 109–117 (2018)
D. Anbuselvan, S. Muthukumaran, Opt. Mater. 42, 124–131 (2015)
L.P. Ravaro, D.I. Santos, L.V.A. Scalvi, J. Phys. Chem. Solids 70, 1312–1316 (2009)
R. Bhargava, P.K. Sharma, S. Kumar, A.C. Pandey, N. Kumar, J. Solid State Chem. 183, 1400–1408 (2010)
U. Ozgur, Y. Alivov, C. Liu, A. Teke, M.A. Reshchikov, S. Dogan, V.A. Cho, H. Morkoc, J. Appl. Phys. 98, 041301 (2005)
S. Yılmaz, M. Parlak, S. Ozcan, M. Altunbas, E. McGlynn, E. Bacaksız, Appl. Surf. Sci. 257, 9293–9298 (2011)
Sk. Johny Basha, V. Khidhirbrahmendra, J. Madhavi, U.S. Udayachandran Thampy, Ch. Venkata Reddy, R.V. Ravikumar, J. Sci. Adv. Mater. Dev. 4, 260–266 (2019)
Y. Liu, Y. Yang, J. Yang, Q. Guan, H. Liu, L. Yang, Y. Zhang, Y. Wang, M. Wei, X. Liu, L. Fei, X. Cheng, J. Solid State Chem. 184, 1273–1278 (2011)
P. Prathap, N. Revathi, Y.P. Venkata Subbaiah, K.T. Ramakrishna Reddy, J. Phys.: Condens. Matter. 20, 5205 (2008)
S.W. Shin, S.R. Kang, J.H. Yun, A.V. Moholkar, J.H. Moon, J.Y. Lee, J.H. Kim, Sol. Energy Mater. Sol. Cells 95, 856–863 (2011)
B. Asenjo, A.M. Chaparro, M.T. Gutirez, J. Herrero, J. Klaer, Sol. Energy Mater. Sol. Cells 92, 302–306 (2008)
K. Nakamoto, Infrared and Raman Spectra of Inorganic and Coordination Compounds, Parts-A and B (Wiley, New York, 1997)
B.S. Rema Devi, R. Raveendran, A.V. Vaidyan, Pramana—J. Phys. 68, 679–687 (2007)
Y. Tong, Z. Jiang, C. Wang, Z.Y. Xin, Z. Hong, S. Liu, Mater. Lett. 62, 3385–3387 (2008)
P. Lakshmi Praveen, D.P. Ojha, J. Mol. Liq. 197, 106–113 (2014)
P. Lakshmi Praveen, D.P. Ojha, J. Mol. Model. 18, 1513–1521 (2012)
P. Lakshmi Praveen, Mol. Cryst. Liq. Cryst. 667, 44–53 (2018)
P. Lakshmi Praveen, D.S. Ramakrishna, D.P. Ojha, Mol. Cryst. Liq. Cryst. 643, 76–82 (2017)
D.A. Reddy, G. Murali, B. Poornaprakash, R.P. Vijayalakshmi, B.K. Reddy, Appl. Surf. Sci. 258, 5206–5211 (2012)
D. Theyvaraju, S. Muthukumaran, Phys. E. 74, 93–100 (2015)
A. Firdous, M. Aslam Baba, D. Singh, A.H. Bhat, Appl. Nanosci. 5, 201–206 (2015)
P.K. Ghosh, S.F. Ahmed, S. Jana, K.K. Chattopadhyay, Opt. Mater. 29, 1584–1590 (2007)
V. Mote, Y. Purushotham, B. Dole, Cerâmica 59, 614–619 (2013)
J. Yang, X. Zhao, X. Shan, H. Fan, L. Yang, Y. Zhang, J. Alloys Compd. 556, 1–5 (2013)
S. Kumar, S. Chatterjee, K.K. Chattopadhyay, A.K. Ghosh, J. Phys. Chem. C 116, 16700–16708 (2012)
S.T. Tan, B.J. Chen, X.W. Sun, W.J. Fan, H.S. Kwok, X.H. Zhang, S.J. Chua, J. Appl. Phys. 98, 013505 (2005)
K.R. Reddy, K.P. Lee, J.Y. Kim, Y. Lee, J. Nanosci. Nanotechnol. 8, 5632–5639 (2008)
K.R. Reddy, H.M. Jeong, Y. Lee, A.V. Raghu, J. Polym. Sci. A: Polym. Chem. 48, 1477–1484 (2010)
M. Hassan, E. Haque, K.R. Reddy, A.I. Minett, J. Chen, V.G. Gomes, Nanoscale 6(2014), 11988–11991 (1994)
A.P. Alivisatos, Semiconductor clusters, nanocrystals, and quantum dots. Science 27, 1933–1937 (1996)
F.L. Faita, K. Ersching, C.M. Poffo, L.C. Benetti, D.M. Trichês, S.M. Souza, A.D.C. Viegas, J.C. de Lima, J. Alloys Compd. 590, 176–183 (2014)
Q. Pan, D. Yang, Y. Zhao, Z. Ma, G. Dong, J. Qiu, J. Alloys Compd. 579, 300–304 (2013)
B. Bhattacharjee, C.-H. Lu, Thin Solid Films 514, 132–137 (2006)
X. Zeng, J. Zhang, F. Huang, J. Appl. Phys. 111, 123525 (2012)
J. Kaur, M. Sharma, O.P. Pandey, Opt. Mater. 47, 7–17 (2015)
B. Xia, I.W. Lenggoro, K. Okuyama, Chem. Mater. 14, 4969–4974 (2002)
B. Dong, L. Cao, G. Su, W. Liu, J. Colloid Interface Sci. 367, 178–182 (2012)
A. Datta, S. Biswas, S. Kar, Nanosci. Nanotechnol. 10, 3670–3676 (2007)
A. Kole, C. Tiwary, P. Kumbhakar, J. Appl. Phys. 113, 114308 (2013)
N. Karar, F. Singh, B. Mehta, J. Appl. Phys. 95, 656–660 (2004)
Y. Wang, M. Yang, Y. Ren, J. Fan, Anal. Chim. Acta 1040, 136–142 (2018)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Binu, P.J., Ganesh, R.C. & Muthukumaran, S. Crystal structure, energy gap and photoluminescence investigation of Mn2+/Cr3+-doped ZnS nanostructures by precipitation method. J Mater Sci: Mater Electron 32, 23174–23188 (2021). https://doi.org/10.1007/s10854-021-06803-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-021-06803-8