Abstract
We report the hydrothermal synthesis of visible light absorbing and direct energy bandgap kesterite-structured Cu2ZnSnS4 (CZTS) nanoparticles (NP) for photocatalytic and photoelectrochemical (PEC) applications. The crystal structure, morphology, composition, and optical properties of CZTS nanoparticles were analyzed using X-ray diffraction (XRD), scanning electron microscopy (SEM), and UV–Vis microscopy techniques. The tetragonal kesterite phase CZTS nanoparticles with compact spherical morphology with an energy bandgap of about 1.51 eV have been synthesized. The CZTS nanoparticles were employed as a photocatalyst for the degradation of Rhodamine B (RhB) dye. The study reveals that the CZTS photocatalysts with compact spherical shape showed photoactivity around 55% under indoor visible light illumination. The CZTS photoelectrodes were fabricated on fluorine-doped tin oxide (FTO) substrate using a doctor blade coating method. The detailed photoelectrochemical (PEC) studies demonstrate that CZTS photoelectrode showed 2.98% of photoconversion efficiency.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Dyes are an important class of synthetic colored organic compounds that are used in many industries [1]. Consequently, they have become common industrial environmental pollutants during their synthesis and dyeing process. Therefore, it is essential to investigate an appropriate and environmentally friendly treatment for the disposal of these dyes. A number of biological and chemical treatments have been increasingly explored and developed to treat the wastewater. Among the current pollution control technology biodegradation of dye by various microbes is emerged as an effective and promising approach [2]. However, the adsorption of dyes occurs only on the surface of the biosorbent, without undergoing degradation [3]. Some of the biological treatments such as aerobic and anaerobic processes are also not effective for certain dyes [4]. Physical methods like ion exchange, adsorption, and filtration are also not effective because they simply transfer the dyes to another phase rather than degrading them [5]. Photocatalysis is an advanced oxidation process (AOP) in which photocatalyst accelerates the photo-generated electrons when interact with the light, offers a solution to those limitations. The AOP methods are relatively high efficient, cost-effective, and a green approach for the complete mineralization of hazardous pollutants [6].
Several photocatalysts have been tested for the degradation of organic pollutants. TiO2 and ZnO are excellent photocatalysts for the photocatalytic degradation of organic pollutants. However, the main drawback of these materials is the absorption region which is limited only to the ultraviolet region. Therefore, for the effective utilization of the solar spectrum, there is a need to develop semiconductor materials with bandgap in the visible range. The copper zinc tin sulfide (CZTS) has attracted intense attention because of its significant advantages such as less toxicity, low cost, and earth-abundant constituent elements [7]. The CZTS exists in two phases, which are kesterite and stannite with space group Γ4 and Γ42m [8], respectively. These phases belong to tetragonal structures, with a cubic closely packed array of sulfur as an anion and cations located at one-half of the tetragonal void, with a stacking similar to zinc blend structure with lattice parameters a = 5.46 Å and c = 10.93 Å [8]. Especially, kesterite phase CZTS is a p-type semiconductor material that shows an excellent optical absorption about 104 cm−1 and a direct bandgap (Eg) of about 1.45–1.7 eV [9,10,11,12], which makes this material well suited for the applications such as solar cells photocatalysis [11] and the gas sensors [13]. The kesterite phase CZTS shows excellent theoretical photoconversion efficiency as high as 32.2% [14].
Various physical and chemical methods have been reported to deposit CZTS thin films which include sputtering [15], thermal evaporation [16, 17], electrodeposition [18], and spray pyrolysis [19, 20]. The solution-based methods are the most successful approach to synthesize CZTS nanoparticles [21, 22]. However, due to several issues such as toxicity and environmentally harmful reaction solvent (oleylamine, hydrazine), these methods have limitations for the scalable manufacturing processes. Therefore, a green, reliable and non-toxic solvent-based solution process for CZTS synthesis is always favorable for its large-scale production [23, 24].
Herein, low cost and environmentally friendly hydrothermal route was used to synthesize the CZTS nanoparticles using water as a solvent. Some of the researchers have indeed used hydrothermal method for the synthesis of CZTS nanoparticles [25,26,27,28,29,30,31,32]. However, most of these studies were focused on the photovoltaic applications [25,26,27,28,29,30,31,32]. Therefore, in the present study we have synthesized CZTS nanoparticles using hydrothermal method and the films were fabricated on the FTO substrate using doctor blade technique. The structural, optical, and morphological properties of CZTS nanoparticles were investigated. Further, the performance of CZTS photoelectrode was analyzed using PEC measurements under indoor visible light irradiation.
2 Experimental section
2.1 Chemicals and materials
Cupric sulfate pentahydrate (CuSO4·5H2O), zinc sulfate (ZnSO4·7H2O), stannous chloride (SnCl2·2H2O), thiourea CS·(NH2)2, ethylcellulose, terpineol, ethanol, fluorine-doped tin oxide substrate (FTO), and sodium sulfate (Na2SO4) were purchased from S.D. Chem. Ltd., which were used without further purification.
2.2 Hydrothermal synthesis of CZTS nanoparticles
For the synthesis of CZTS nanoparticles, 0.01 M copper sulfate, 0.005 M zinc sulfate, 0.005 M tin chloride, and 0.08 M thiourea were dissolved in water. The resultant solution was stirred for 30 min and then transferred into a Teflon-lined stainless steel autoclave filled up to 75% of its capacity. The autoclave was kept in a hot air oven maintained at 180 °C for 24 h. After that, the oven is kept for cooling naturally to room temperature. The precipitate was collected, washed with distilled water and ethanol several times, and dried in air at 200 °C for 2 h [23, 24].
2.3 Fabrication of CTS/FTO photoelectrode
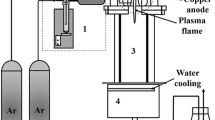
The CZTS thin films were deposited on the fluorine-doped tin oxide (SnO2:F) (FTO) glass substrate using the doctor blade coating method. The FTO substrate was ultrasonically cleaned with distilled water. Initially, 10 mg of CZTS sample and 3 mg of ethyl cellulose were mixed and crushed together. A premixed solution of ethanol and terpineol (10:1) was added dropwise in the mixture containing CZTS and ethyl cellulose to form suitable viscous CZTS paste. The resulting paste was coated on the ultrasonic cleaned FTO substrate and annealed at 250 °C for 30 min. The CZTS/FTO electrodes were used as a photoelectrode to study the performance of the PEC cell. The schematics of the formation mechanism of CZTS nanoparticles using the hydrothermal process and the doctor blade coating method are depicted in Fig. 1.
2.4 Characterization techniques
X-ray diffraction (XRD) pattern was recorded using X-ray diffractometer (XPERT-PRO MPD), with CuKα radiations of wavelength 1.5404 Å in 2θ range between 20° and 70°. The Raman spectrum of the sample was recorded using a Raman spectrometer in the range 600 to 2100 cm−1. The He-Ne laser with wavelength of 532 nm was used as an excitation source. The chemical composition and the morphology of CZTS nanoparticles were characterized by different characterization techniques such as energy-dispersive X-ray spectroscopy (EDS), scanning electron microscopy (SEM, JEOL JSM-IT300), transmission electron microscopy (TEM), selected area electron diffraction (SAED, CM20FEG, 200 kV), and high electron transmission microscopy (HRTEM, JEOL JEM-2100F, 200 kV). The surface area and pore diameter of CZTS nanoparticles were calculated by the Brunauer–Emmett–Teller (BET) equation. The optical properties of the material were studied by UV–Vis (PerkinElmer) absorbance spectrophotometer at room temperature.
2.5 Photocatalytic activity measurement
The photocatalytic reaction was carried in a 750 ml glass cylindrical photochemical reactor. In a typical process, 10 mg of CZTS nanoparticles were mixed in 50 ml of 10 ppm aqueous solution of RhB dye. The solution was kept in dark for 24 h to achieve equilibrium. To study visible light degradation, the sample was illuminated with a 125 W mercury lamp and changes in concentration of dye were measured using UV–Vis absorption spectra at regular intervals of time. The absorption peak observed at 554 nm was used to determine the degradation of the RhB dye. The photodegradation efficiency of CZTS nanoparticles was calculated using the following formula [33]:
where \({A}_{0}\) is the initial absorbance of dye solution before the addition of a catalyst and \({A}_{\mathrm{t}}\) is the absorbance at time t after the addition of the catalyst. The reaction rate constant ‘k’ was determined using the following equation assuming the first order reaction kinetics:
3 Results and discussion
3.1 Structural analysis
Figure 2a depicts the XRD pattern of CZTS nanoparticles synthesized using the hydrothermal method and air annealed at 200 °C for 2 h. All the observed diffraction patterns match with the JCPDS-26-0575 reference of the tetragonal kesterite phase of CZTS [26]. The diffraction peak observed at 2θ = 28.45°, 33.31°, 47.79° a,nd 56.21° could be assigned to (112), (200), (220), and (312) planes of kesterite phase of CZTS, respectively. The lattice parameters calculated for CZTS nanoparticle considering (112) XRD peak are found to be a = 5.391 Å and c = 10.798 Å. The crystallite size estimated from the Scherrer formula is found to be ~ 15 nm. From the XRD pattern, it is seen that the intensity of the (112) peak is dominant in all the CZTS samples. It is to be noted that, there are no impurity peaks observed in the XRD pattern. The XRD pattern of some binary and ternary phase compounds is [34, 35] similar to that of CZTS. Therefore, to identify the kesterite phase CZTS compound, the only XRD pattern is not sufficient. Therefore, to confirm the phase purity, the Raman spectrum CZTS sample was recorded. The Raman spectrum showed three major peaks at positions 287, 338, and 368 cm−1 as depicted in Fig. 2b. The XRD pattern of CZTS thin film deposited on FTO substrate is shown in Fig. 2c. These observations are in good agreement with reported values for kesterite phase CZTS [36, 37].
3.2 Compositional and morphological studies
In order to estimate the elemental composition, the energy-dispersive spectroscopy (EDS) technique was employed. In the synthesis process, the ideal stoichiometric composition of Cu/(Zn + Sn) = 1 and that of Zn/Sn = 1 [36] of metal precursors was chosen. The EDS analysis showed a slight change in the composition of CZTS nanoparticles. The observed composition ratio of Cu/(Zn + Sn) was 0.86 and Zn/Sn ratio was 0.918. For high-performance CZTS-based solar cell, the optimum composition ratio of Cu/(Zn + Sn) and Zn/Sn was reported to be about 0.75 ~ 0.95 and 1.1 ~ 1.4, respectively [36, 37].
Figure 3a–d shows the SEM image of CZTS nanoparticles synthesized using the hydrothermal method. From the SEM images, it is seen that the grown CZTS nanoparticles exhibit coagulation of nanospheres. The morphology of CZTS nanoparticles was further investigated by HRTEM analysis. Figure 4a shows HRTEM images of the CZTS nanoparticles that reveal the relatively small particles of ~ 15 nm size. Figure 4b shows the lattice fringe pattern of crystalline CZTS nanoparticles. The interplanar spacing corresponding to (112) peak is observed to be 0.318 nm, which is identical to the theoretical value of 0.31 nm. The SAED pattern shown in Fig. 4c indicates the polycrystalline nature of CZTS nanoparticles. The particle size distribution of CZTS nanoparticles from HRTEM images is depicted in Fig. 4d. The average particle size is 16.89 nm. The result of the HRTEM image indicates that the (112) plane is the preferred growth plane of CZTS nanoparticles.
3.3 Optical studies
The room temperature optical absorbance was recorded in the wavelength range of about 350–1100 nm. The bandgap energy was estimated by plotting the graph of (αhν)2 versus (hν) and extra plotting with the energy axis as shown in Fig. 5. The direct bandgap of spherical CZTS nanoparticles was estimated to be 1.52 eV which is in good agreement with earlier reported values [23, 24, 38, 39].
3.4 BET analysis
The CZTS nanoparticles were further analyzed by measuring N2 adsorption–desorption isotherm to elucidate the surface area and pore volume. Figure 6 depicts the isotherm of CZTS nanoparticles displaying a hysteresis in the relative pressure range from 0.4 to 0.9. The surface area of CZTS nanoparticles estimated using BET analysis was found to be 25.086 m2/g. The corresponding pore volume and pore size estimated were observed to be 0.029 cm3/g and 6.87 nm, respectively. The catalyst with a higher surface area showed enhanced catalytic activity [40, 41]. Also, the bandgap of photocatalyst should be compatible with the visible region, to improve the light absorption ability. The CZTS nanoparticles showed the highest surface area (25.086 m2/g) and showed a direct optical bandgap of around 1.52 eV. Therefore, CZTS nanoparticles are used to study the photocatalytic activity to degrade RhB dye.
3.5 Visible light-driven photocatalytic activity of CZTS nanoparticles
The CZTS nanoparticles were employed in the photocatalytic reactor and analyzed for degradation of RhB dye. The results indicating the variation of absorbance against the time of degradation of RhB dye in presence of CZTS nanoparticles are depicted in Fig. 7a. In the results, at around 554 nm, a gradual decrease in absorption intensity of RhB dye was observed over a given period [42]. A decrease in the absorption intensity indicates the degradation of RhB dye by CZTS under indoor light illumination. The percentage degradation efficiency of RhB dye was estimated using Eq. 1 and found to be 55% within 240 min. Yu et al. [43] reported 60%, 88%, and 95% degradation of RhB dye using silver and platinum-doped CZTS photocatalyst. The rate constant k was calculated using Eq. 2 and was found to be 0.0046 min−1 as shown in Fig. 7c.
a Absorbance vs wavelength spectra of degradation of RhB dye by CZTS photocatalyst, b plot of At/Ao vs reaction time, b plot of the linear fit of ln[At/Ao] vs reaction time for evaluation of a reaction rate constant of RhB dye degradation, d schematic illustration of photocatalytic activity by CZTS nanocrystals for the degradation of RhB dye under visible light
The CZTS nanoparticles absorb light photons of energy greater than the bandgap energy (Eg > hν). The additional energy is transferred to the excited electrons and therefore electrons migrate from the valence band to the conduction band of CZTS, which results in the formation of electron–hole pairs (Eq. 3). The water molecules on the surface of CZTS nanoparticles get oxidized by positively charged holes and results in OH* free radicals (Eq. 4). At the same time, the electrons from the conduction band are donated to molecular oxygen to form anionic superoxide radical \({\mathrm{O}}_{2 }^{-*}\), which further helps in the dissociation of water molecules and the formation of highly reactive OH* hydroxyl radicals (Eqs. 5–7). The hydroxyl OH* radicals generated are responsible for the photodegradation of RhB dye in an aqueous solution (Fig. 7d). The possible reaction mechanism of photodegradation is described in the following equations [44,45,46]:
The hydroxyl radicals OH* are highly reactive, which would react with RhB dye to degrade it and produce non-toxic byproducts. The CZTS nanoparticles have been successfully used for photocatalytic degradation of methylene blue (MB), Rhodamine B (RhB), and trinitrotoluene (TNT) [46, 47]. Phaltane et al. reported 50% degradation of methylene blue (MB) by CZTS nanoparticle synthesized using the solvothermal method [48]. Apostolopoulou et al. studied the degradation of RhB dye using nanocrystalline CZTS thin films and reported 97.5% and 70% degradation of basic blue 41 (BB-41) and acidic orange 8 (AO-8) organic azo dye, respectively [49].
3.6 Photoelectrochemical (PEC) performance of CZTS thin films
The photoelectrochemical (PEC) properties were investigated using CH electrochemical analyzer instrument, with a three-electrode system consisting of CZTS as a photoelectrode, Pt as a counter electrode, and Ag/AgCl as a reference electrode. Three electrodes were immersed in the redox mediator containing 0.5 M aqueous solution of Na2SO4 (pH = 6) solution under illumination. The sample was irradiated from the semiconductor electrolyte interface with a solar simulator with AM 1.5 G illumination (100 mW/cm2). Various cell parameters like short-circuit current density (Jsc), open circuit voltage (Voc), and photovoltaic conversion efficiency (η) of the cell were estimated using the following equations:
where FF is the fill factor, η is photoconversion efficiency, Imax is maximum current density, Vmax is maximum voltage, Jsc is the short-circuit current density, and Voc is the open circuit potential. The Pin is input power in watt/cm2.
From the J–V plot shown in Fig. 8, it is observed that the sample showed 2.98% of photoconversion efficiency with a current density (Jsc) = 8.87 mA/cm2, open circuit voltage (Vov) = 0.58 V, and fill factor (FF) = 58%. The composition of CZTS nanoparticles is slightly copper poor and zinc rich. The reduction in Cu concentration below the stoichiometric value results in the removal of Cu-based binary phases and leads to the optimized [Cu/(Zn + Sn)] composition ratio. The existence of the Cu vacancy (VCu) and Cu-substituted Zn sites (CuZn) [42] at transition energies of 0.02 and 0.10 eV above the valance band maxima greatly influence the power conversion efficiency of the cell. The Cu poor composition of the film enhances the Cu vacancy (Vcu) formation and suppresses the copper-substituted Zn sites (CuZn), resulting in a higher short-circuit current density (Jsc). The results showed that CZTS can be the significant candidate for application in solar cell devices [50, 51].
4 Conclusion
In this study, kesterite-structured CZTS nanoparticles were successfully synthesized by facile hydrothermal route. The analysis of XRD data showed formation of tetragonal kesterite phase of CZTS. The SEM images showed dense and compact coagulation of nanospheres. The HRTEM images showed the CZTS nanoparticles with the average size around ~ 15 nm and (112) orientation is dominant. UV–Vis absorption spectra showed the direct bandgap-type CZTS with a bandgap energy of about 1.52 eV, which is optimum for the absorber layer of the solar energy. The CZTS nanoparticles were used as photocatalysts for the degradation of RhB dye. The CZTS nanoparticles as a photocatalyst displayed 55% degradation of RhB dye under visible light illumination for 4 h. The PEC device fabricated using CZTS photoelectrode has shown 2.98% of photoconversion efficiency. The results verify that the structural and optical properties make CZTS a potential candidate for photocatalyst as well as photovoltaic applications. Further research is underway to improve the photocatalytic and photovoltaic properties of CZTS compound semiconductors.
References
J.R. Aspland, Textile dyeing and coloration. (American Association of Textile Chemists and Colorists, Research Triangle Park, 1997), p. 354
Q. Husain, Crit. Rev. Biotechnol. 26, 201 (2006)
M. Muruganandham, N. Sobana, M. Swaminathan, J. Hazard. Mater. 137(3), 1371 (2006)
F. Han, V.S.R. Kambala, M. Srinivasan, D. Rajarathnam, R. Naidu, Appl. Catal. A 359(1–2), 25 (2009)
N. Barka, S. Qourzal, A. Assabbane, Y. Ait-itchou, A. Nounah, H. Lachheb, A. Houas, Arab. J. Sci. Eng. 35, 131 (2009)
T. Ali, Y.M. Hunge, A. Venkatraman, J. Mater. Sci.: Mater. Electron. 29, 1209 (2018)
H. Zhou, W.C. Hsu, H.S. Duan, B. Bob, W. Yang, T.B. Somg, C.J. Hsu, Y. Yang, Energy Environ. Sci. 6, 2822 (2013)
S.R. Hall, J.T. Szymanski, J.M. Stewart, Can. Mineral. 16, 131 (1978)
H. Katagity, Thin Solid Films 480, 426 (2005)
A. Hiller, B. Miller, Electrochem. Acta 25, 29 (1980)
C. Dun, W. Huang, H. Huang, J. Xu, N. Zhou, Y. Zheng, H. Tsai, W. Nie, D. Onken, Y. Li, D.L. Carroll, J. Phy. Chem. C 118, 30302 (2014)
C. Shi, G. Shi, Z. Chen, P. Yang, M. Yao, Mater. Lett. 73, 89 (2012)
S.J. Patil, R.N. Bulakhe, C.D. Lokhande, J. Anal. Appl. Pyrolysis 117, 310 (2016)
S. Jung, J. Gwak, H.H. Yun, S. Ahn, D. Nam, H. Cheong, S. Ahn, A. Cho, K. Shin, K. Yoon, Thin Solid Films 535, 52 (2013)
J. Seol, S. Lee, J. Lee, H. Nam, K. Kim, K. Sol, Energy Mater. Sol. Cells 75, 155 (2003)
A. Weber, H. Krquth, S. Pertl, B. Schubert, I. Kotschau, S. Schorr, H. Schock, Thin Solid Films 517, 2524 (2009)
K. Wang, O. Gunawan, T. Tordov, B. Shin, S.J. Chey, N.A. Bojarczuk, D. Mitzi, S. Guha, Solar Cells 97, 143508 (2010)
C.P. Chan, H. Lam, C. Surya, Energy Mater. Sol. Cells 94, 207 (2010)
N. Kamoun, H. Bouzouita, B. Rezig, Thin Solid Films 515, 5949 (2007)
J.P. Sawant, H.M. Pathan, R.B. Kale, ES Energy Environ. (2020). https://doi.org/10.30919/esee8c933
J. Kavalakkatt, X. Lin, K. Kornhuber, P. Kusch, A. Ennaoui, S. Reich, M.C. Lux-Steiner, Thin Solid Films 535, 380 (2013)
M.T. Wang, O. Winkler, T. Gunawan, T.K. Gokmen, Y. Todorov, D.B. Zhu, D. Mitzi, Adv. Energy Mater. 4, 1301465 (2014)
J.P. Sawant, R.B. Kale, Mater. Lett. 265, 127407 (2020)
J.P. Sawant, R.B. Kale, J Solid State Electrochem. 24, 461 (2020)
Q.B. Wei, P. Xu, X.P. Ren, F. Fu, J. Alloys Compd. 770, 424 (2019)
S.M. Camara, L. Wang, X. Zhang, Nanotechnology 24, 495401 (2013)
A.G. Kannana, T.E. Manjulavallia, J. Chandrasekaran, Proced. Eng. 141, 15 (2016)
Y.M. Hunge, A.A. Yadav, S. Liu, V.L. Mathe, Ultrason. Sonochem. 56, 284 (2019)
X. Hou, Y. Li, J.J. Yan, C.W. Wang, Mater. Res. Bull. 60, 628 (2014)
S.A. Phaltane, S.A. Vanalakar, T.S. Bhat, P.S. Patil, S.D. Sartale, L.D. Kadam, J. Mater. Sci.: Mater. Electron. 28, 11 (2017)
S.S. Shinde, J. Semicond. 36, 073003 (2015)
J.M. Buriak, P.M. Kamat, K.S. Schanze, ACS Appl. Mater. Interfaces 6, 118515 (2014)
Z. Zhou, P. Zhang, Y. Lin, E. Ashalley, H. Ji, J. Wu, H. Li, Z. Wang, Nanoscale Res. Lett. 9, 477 (2014)
J. Kong, Z. Zhou, M. Li, W.H. Zhou, S.J. Yuan, R. Yao, Y. Zhao, S. Wu, Nanoscale Res. Lett. 8, 464 (2013)
M. Li, W.H. Zhou, J. Guo, Y.L. Zhou, Z.L. Hou, J. Jiao, Z.J. Zhou, Z. Du, S. Wu, J. Phys. Chem. C 116(50), 26507 (2012)
W.C. Jin, I. Agus, S.J. Park, W. Kim, S. Yoon, B.K. Min, ACS Appl. Mater. Interfaces 5, 4162 (2013)
C. Zou, L.J. Zhang, D.S. Lin, Y. Yang, Q. Li, X.J. Xu, X. Chen, S.M. Huang, CrystEngComm 13, 3310 (2011)
C. Persson, J. Appl. Phys. 107, 053710 (2010)
Q. Tian, X. Xu, L. Han, M. Tang, R. Zou, Z. Chen, M. Yu, J. Yang, J. Hu, CrystEngComm 14, 3847 (2014)
Y. Xia, B. Wang, X. Zhao, G. Wang, H. Wang, Electrochim. Acta 187, 55 (2016)
G. Gogoi, S. Arora, N. Vinothkumar, M. De, M. Quereshi, RSC Adv. 5, 40475 (2015)
M. Basu, A.K. Sinha, M. Pradhan, S. Sarkar, Y. Negishi, T. Govind, Environ. Sci. Technol. 44, 6313 (2010)
X. Yu, A. Shavel, X. An, Z. Luo, M. Ibaneez, A. Cabot, J. Am. Chem. Soc. 136, 9236 (2014)
Z. Chen, D. Li, W. Zhang, Y. Shao, T. Chen, M. Sun, X. Fu, J. Phys. Chem. C 113, 4433 (2009)
A. Houas, H. Lachheb, M. Ksibi, M. Elaloui, C. Guillard, J.M. Herrmann, Appl. Catal. B 31, 145 (2001)
P. Kush, Mater. Chem. Phys. 162, 608 (2015)
S.A. Phaltane, S.A. Wanalakar, T.S. Bhat, P.S. Patil, S.D. Sartale, L.D. Kadam, J. Mater. Sci. Mater. Electron. 28, 8186 (2017)
A. Apostolopoulou, S. Mahajan, R. Sharma, E. Stathatos, J. Phys. Chem. Solids. 112, 37 (2018)
S. Chen, J.H. Yang, X.G. Gong, A. Walsh, S.H. Wei, Phys. Rev. B 81, 245204 (2010)
A.G. Kannan, T.E. Manjulavalli, J. Chandrasekaran, Proced. Eng. 141, 15 (2016)
S.M. Camara, L. Wang, X. Zhang, Nanotechnology 24(49), 495401 (2013)
Acknowledgements
This research work is supported by the Department of Science and Technology, India under FIST (SR/FST/PSI-173/2012) program. The authors are thankful to the Director, The Institute of Science, Fort, Mumbai, for the encouragement and providing the necessary facility. Authors are also thankful to INUP, as a part of the reported work (characterization) was carried out at IITBNF, and IITB under INUP which is sponsored by deity, MCIT, Government of India.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sawant, J.P., Rajput, R., Patil, S. et al. Photocatalytic activities of hydrothermal synthesized copper zinc tin sulfide nanostructures. J Mater Sci: Mater Electron 32, 22803–22812 (2021). https://doi.org/10.1007/s10854-021-06759-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-021-06759-9